Abstract
Mutants in presenilins (PS1 or PS2) is the major cause of Familial Alzheimer’s disease (FAD). FAD causing PS mutants affect intracellular Ca2+ homeostasis by enhancing the gating of inositol trisphosphate (IP3) receptor (IP3R) Ca2+ release channels on the endoplasmic reticulum, leading to exaggerated Ca2+ release into the cytoplasm. Using experimental IP3R-mediated Ca2+ release data, in conjunction with a computational model of cell bioenergetics, we explore how the differences in mitochondrial Ca2+ uptake in control cells and cells expressing FAD-causing PS mutants affect key variables such as ATP, reactive oxygen species (ROS), NADH, and mitochondrial Ca2+. We find that as a result of exaggerated cytosolic Ca2+ in FAD-causing mutant PS-expressing cells, the rate of oxygen consumption increases dramatically and overcomes the Ca2+ dependent enzymes that stimulate NADH production. This leads to decreased rates in proton pumping due to diminished membrane potential along with less ATP and enhanced ROS production. These results show that through Ca2+ signaling disruption, mutant PS leads to mitochondrial dysfunction and potentially to cell death.
Introduction
Alzheimer’s disease (AD) is a form of dementia that is characterized by extensive synaptic and neuronal loss which leads to impaired memory and cognitive decline. Most AD cases are sporadic (SAD) and account for a large population of AD patients, but about 10% are inherited (FAD) and develop as early as the age of 30 as a result of mutations in amyloid precursor protein (APP) or presenilins (PS1, PS2) [5, 20]. Both SAD and FAD share the features of accumulated extra-and intracellular β-amyloid plaques, intracellular neurofibrillary tangles composed mostly of hyperphosphorylated tau protein, and cell atrophy and death in multiple brain regions [18, 25, 38]. Both types of AD share similar phenotypes, suggesting a common pathogenic origin. However, mechanisms for how these proteins create such devastating effects in the neuron are still unclear.
PS are transmembrane proteins that are synthesized on the endoplasmic reticulum (ER) [1]. PS forms a protein complex that is transported to the cell surface where it functions as a γ-secretase that cleaves type 1 transmembrane proteins, including APP [10]. The cleavage of APP releases Aβ peptides, which is a major component of amyloid plaques in the brains of patients with AD. These mutant PSs are thought to affect APP processing by increasing the production of amyloidogenic Aβ. Besides the disruption of APP processing, FAD-linked PS mutants dysregulate intracellular Ca2+ homeostasis [5, 6, 24]. PS mutants inuence intracellular Ca2+ signaling by means of exaggerated Ca2+ release from the ER through the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R), the main intracellular Ca2+ release channel in non-excitable cells [5, 6, 19, 26, 39–41]. The Ca2+ hypothesis of brain aging and AD first proposed by Khatchaturian [23] suggests that over time, Ca2+ handling of neurons becomes compromised, leading to excessive free intracellular Ca2+ concentration ([Ca2+]i) and reactive oxygen species (ROS), mitochondrial dysfunction, and eventually apoptosis. Increasing data suggests that neuronal Ca2+ dysregulation plays a critical role in AD pathogenesis [3, 42].
Mitochondria is responsible for the production of ATP, which is driven by the TCA cycle and oxidative phosporylation. It is generally believed that mitochondria can function as a dynamic Ca2+ sequestration system [36]. Ca2+ plays a crucial role in facilitating the communication between mitochondria and other components of the cell [34]. Ca2+ has been shown to activate the mitochondrial matrix dehydrogenases in the TCA cycle [12, 30] and inuence the supply of NADH and FADH2, thereby affecting the oxidative phosphorylation and the amount of ATP produced in the matrix. Thus, irregularities in mitochondrial Ca2+ homeostasis can cause severe complications to cellular physiological functions including bioenergetics.
Cheung et al. [5] observed that FAD-causing PS mutants lead to gain-of-function enhancement of IP3R Ca2+ release channels. This gain-of-function enhancement at the single channel level leads to high-frequency, high-amplitude whole-cell Ca2+ oscillations in FAD-causing PS mutants-expressing cells as compared to control cells expressing wild-type (WT) PS1.
Here we investigate how these high frequency and high amplitude [Ca2+]i oscillations impair mitochondrial function in cells expressing PS mutants. We achieve this by feeding experimental whole cell [Ca2+]i time traces directly into a computational model that takes into consideration the TCA cycle, oxidative phosphyorilation, Ca2+ dynamics, and ROS production. We compare the mitochondrial function in a cell with PS mutant-induced Ca2+ signals to that in a control cell. We show that the increased Ca2+ uptake in the mitochondria due to exaggerated Ca2+ release through IP3R in FAD causing PS1 mutant-expressing cells leads to decreased ATP production, increased ROS in mitochondria ( ) and [H2O2], which over time can lead to cell death. The cells that we study are representatives of all the observations in [5] and the conclusions apply to all experiments in that study.
Materials and Methods
Experimental Methods
Intracellular Calcium Recordings
Whole-cell recordings obtained using fluorescence Ca2+ imaging of intracellular Ca2+ signals in human B lymphocytes elicited by exposure to human IgM, as reported in [5], were used in this study. We compare the Ca2+ signals in human lymphocytes expressing FAD-causing M146L mutant PS derived from FAD patients to signals in control lymphocytes expressing WT PS derived from normal individuals to investigate the impact of mutation in PS on various mitochondrial functions.
Computational Methods
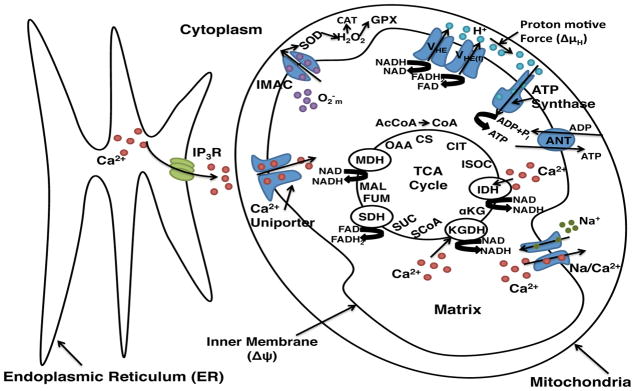
Our model incorporates mitochondrial physiological processes such as the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, Ca2+ signaling pathways, and ROS dynamics. We adopted the computational model from Cortassa et al. [7], which was modified from the work done by Magnus and Keizer [28]. The main components in our model are shown in Figure 1. Rate equations for variables are given in Table S1 and their corresponding parameters and fluxes are described in the supporting information.
Figure 1.
Schematic representation of the mitochondrial physiological processes, metabolic energetics, ROS production, transport and scavenging incorporated in our model. The model takes into account TCA cycle dehydrogenases and enzymes. The TCA cycle of the mitochondrial matrix is fed by acetyl CoA (AcCoA), which oxidize fatty acids and glucose. The TCA cycle completes the oxidation of AcCoA to CO2 and produces NADH and FADH2, which are the driving force for the oxidative phosphorylation process. Activities of TCA cycle enzymes IDH and KGDH are explicitly dependent on Ca2+ in the matrix, which allows for the rate of Ca2+ uptake by mitochondria to be involved in membrane energization through the TCA cycle and the oxidative phosphorylation. As the NADH and FADH2 are oxidized by the respiratory chain, they drive the proton pumping across the inner membrane (VHE and VHE(f)) to establish an electrochemical gradient, or proton motive force (ΔμH) composed of the electrical gradient (ΔΨm) and proton concentration gradient (ΔpH). The proton motive force drives phosphorylation of matrix ADP to ATP by the F1F0 – ATPase (ATP synthase). The ΔΨm governs the electrogenic transport of ions, which includes the influx of Ca2+ through the uniporter, the Na+/Ca2+ exchanger and the adenine nucleotide translocator (ANT). The schematic shows the inner membrane ROS coupled kinematics. Superoxide anion is transported through the inner membrane anion channel (IMAC) and [H2O2] is produced. See supporting information text for model equations and abbreviations not shown here.
The model developed by Magnus and Keizer describes Ca2+ handling by mitochondria in the pancreatic β-cell. The Magnus and Keizer model includes 6 transport mechanisms in the inner mitochondrial membrane: proton pumping due to respiration, proton pumping uptake by the F1F0 – ATPase, a proton leak, the adenine nucleotide exchanger, the Ca2+ uniporter, and the Na+/Ca2+ exchanger. The original Magnus and Keizer model also includes dynamic changes in mitochondria membrane potential (ΔΨm), ADP ([ADP]m), and Ca2+ concentration ([Ca2+]m) by means of 3 rate equations. The Magnus and Keizer model was expanded by Fall and Keizer [16] to include coupling with Ca2+ release through IP3R based on the De Young-Keizer model that describes the properties of Ca2+ release from the ER through IP3R channels [11]. In the Fall and Keizer model, Ca2+ released from the ER is sequestered into mitochondria through the Ca2+ uniporter [16]. The Fall and Keizer model was then expanded to include the mitochondria permeability transition pore (PTP) [13, 34].
Missing in these studies is a mechanism through which Ca2+ regulates the TCA cycle and ROS production dynamics that is dependent upon oxygen consumption of the mitochondria. The work done by Cortassa et al. [7, 8] includes TCA cycle dehydrogenases that drive NADH production, and ROS production due to a shunt of electrons from the electron transport chain. The Cortassa et al. model is a 16 variable model and shows that when Ca2+-sensitive dehydrogenases are the main rate-controlling steps of respiratory flux, there are significant increases in oxygen consumption (Vo2), proton efflux, NADH, and ATP synthesis. The Cortassa et al. model is able to reproduce experimental data concerning mitochondrial bioenergetics, Ca2+ dynamics, and respiration control [7]. Thus, we choose this model for our study although other expansions of the Magnus-Keizer model are also available in the literature [22, 31, 32].
Our main goal in this study, is to combine experimental Ca2+ release from whole-cell cytosolic data from [5] and the Cortassa et al. model to compare the differences in mitochondrial bioenergetics in the absence and presence of PS mutant-induced exaggerated IP3R-mediated cytosolic Ca2+ release. We remark that our conclusions about the impairment of mitochondrial function in the presence of PS-mutants qualitatively remain the same irrespective of the model selection. In the following we describe the key components of the model. The full details and equations of the model are given in supporting information.
TCA Cycle
Isocitrate ([ISOC]), α-ketoglutarate ([αkg]), succinyl CoA ([SCoA]), succinate ([Suc]), fumarate ([FUM]), malate ([MAL]), and oxalacetate ([OAA]) are all chemical intermediates involved in the TCA cycle. The enzymes generating or processing these entities in the TCA cycle are citrate synthase (CS), aconitase (ACO), isocitrate dehydrogenase (IDH), alpha-ketoglutarate dehydrogenase (KGDH), succinyl CoA lyase (SL), succinate dehydrogenase (SDH), fumarate hydratase (FH), malate dehydrogenase (MDH), and aspartate amino transferase (AAT). The rate equations of the reactions catalyzed by these enzymes are shown in the supporting material. The TCA cycle provides a pathway for substrate oxidation. The cycle completes the oxidation of AcCoA to CO2 and produces NADH and FADH2. The TCA cycle enzymes isocitrate dehydrogenase (IDH) and alpha ketoglutarate dehydrogenase (KGDH) have explicit dependence on [Ca2+]m. The coefficients of the TCA cycle equations were determined in [7, 28], and can be viewed in Table S2 and S3.
Oxidative Phosphorylation
The oxidative phosphorylation equations are based on Cortassa et al. [7] and Magnus and Keizer model [28]. Both models describe the NADH-driven electron transport, proton efflux, F1F0 – ATPase activity, and the influx of protons. Ares, AF1, and ΔμH are driving forces for the oxidative phosphorylation that correspond to redox potential, phosphorylation potential, and proton motive force. The coefficients in the equations are given in Table S4 and S5.
Mitochondrial Ca2+ Dynamics
Ca2+ influx and efflux are conducted mainly by Ca2+ uniporter and the Na+/Ca2+ exchanger respectively [7, 28]. The Na+/Ca2+ exchanger is set to be electrogenic based on the parameters given by [28]. Equations governing the influx and efflux rates can be found in the supporting material and rate parameters for the equations are given in Table S6.
ROS Production
ROS is modeled as a shunt of electrons from the electron transport chain (ETC) into the matrix. Mitochondrial ROS generation is considered a side path for electrons diverging from the ETC [8]. Rate equations governing the production of ROS are given in Table S7 and their corresponding fluxes are given in the supporting material.
Numerical Methods
Numerical integration of the model equations was performed with Intel Fortran compiler (Intel Corporation, Santa Clara, CA). ODEs were solved using RK4 method. Code producing key results in the paper is available upon request from authors.
Results
In the results we compare mitochondrial TCA cycle, oxidative phosporylation, Ca2+ dynamics, and ROS production derived from whole-cell uorescence Ca2+ imaging measurements from control cells (CTL) and cells expressing FAD-causing PS mutant- M146L [5]. The parameters for Figures 2–4 can be found in Tables S2–S8. We then show how the total amount of mitochondrial ATP ([ATP]), [Ca2+]m, [H2O2], ROS in cytoplasm , and [NADH] change as we vary rates of adenine nucleotide translocator (VmaxANT), the mitochondrial uniporter (Vmuni), and mitochondria Na+/Ca2+ exchanger rate (Vnacamax). We choose these rates because they directly inuence mitochondrial Ca2+ dynamics and ATP production. Other rates did not significantly change mitochondrial bioenergetics and are not shown.
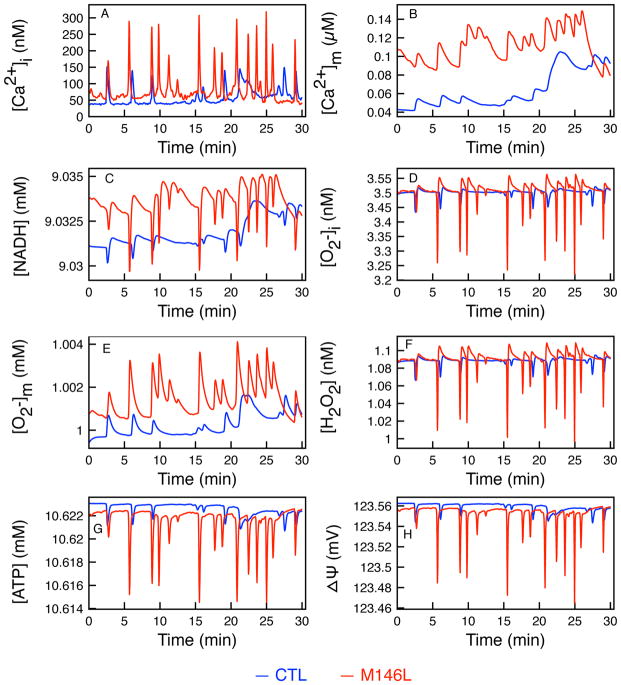
Figure 2.
Time evolution of state variables. Showing the effects of [Ca2+]i uptake into the mitochondria matrix. Control (blue) and M146L (red) time traces of (A) experimentally measured [Ca2+]i, (B) [NADH], (C) [Ca2+]m, (D) [H2O2], (E) mitochondrial ROS ( ), (F) Cytoplasmic ROS ( ), (G) [ATP], and (H) ΔΨm.
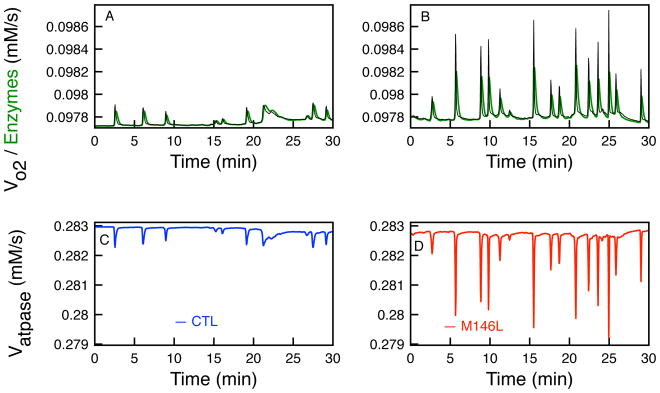
Figure 4.
The rates of the oxygen consumption (Vo2) and the total rate of the three enzymes (VIDH, VKGDH, and VMDH) that determine the concentration of NADH in the TCA cycle. Effects on oxygen consumption (black) and total enzyme rate (green) in control cells (A) and M146L-expressing cells (B). Rate of Vatpase as the changes in NADH occur due to uctuations in [Ca2+]i in CTL (C) and M146L-expressing cells (D).
Inuence of cytoplasmic Ca2+ on mitochondria over time
In Cheung et al. [5], IP3R-mediated Ca2+ release in human B-lymphoblats cells from individuals with various PS mutants (FAD lymphoblasts) and individuals with wild type PS (control lymphoblasts) were compared. IP3R in control lymphoblasts had low channel open-probability (Po) ranging from 0.18±0.02 to 0.23±0.03 [5]. These channel activities are associated with brief openings and relatively long closings. Po of IP3R channels in FAD B-lymphoblats was substantially higher at 0.81±0.02.
To determine if this observed higher Po affects [Ca2+]i signals, IP3R-mediated whole-cell Ca2+ signals in B-lymphoblasts were measured with uorescence Ca2+ imaging. Control B-lymphoblasts showed spontaneous low frequency and low amplitude Ca2+ oscillations. In contrast, FAD lymphoblasts exhibited exaggerated spontaneous Ca2+ signals showing high frequency and high amplitude Ca2+ oscillations. Both the fraction of active cells exhibiting Ca2+ oscillations and the frequency of the oscillations doubled in FAD cells relative to control cells [5]. In this study, we selected a representative control (CTL) cell and a cell expressing the M146L mutant of PS (M146L-expressing) studied in [5] to evaluate the effects of this exaggerated Ca2+ signaling on mitochondrial function. However, the main conclusions in this paper should be applicable to all the observations in [5].
The influence on mitochondrial physiological processes due to changes in [Ca2+]i was examined by combining the experimental time traces for [Ca2+]i with our computational model as described in the supporting material. Figure 2 shows the time trace of all the state variables tracked in the model, excluding the TCA cycle which is shown in Figure 3. Figure 2 shows the time series of the CTL (blue lines) and M146L-expressing cells (red) [Ca2+]i and its effect on [Ca2+]m, [NADH], [H2O2], , [ATP], and ΔΨm. As observed previously [5], the M146L-expressing cell shows elevated Ca2+ signals that oscillate at a higher frequency and amplitude than the control cells (Figure 2A). The observed [Ca2+]i shows how the higher Po of IP3R due to PS mutants affects whole cell Ca2+ signals as compared to the control cell. The mitochondrial Ca2+ uptake simulated using our model is shown in Figure 2B. Mitochondria buffers [Ca2+]i as [Ca2+]i oscillates, and the M146L-expressing cells show an elevated increase in total [Ca2+]m over time as compared to the control cell. Total amount of Ca2+ in the cytoplasm and Ca2+ uptake by mitochondria over the 30 min simulation was calculated by integrating over the full duration of the experiment. In the cytoplasm, there is 33% more IP3R-mediated Ca2+ release which leads to 66% more Ca2+ uptake by the mitochondria in M146L-expressing cell compared to the control cell according to our simulations.
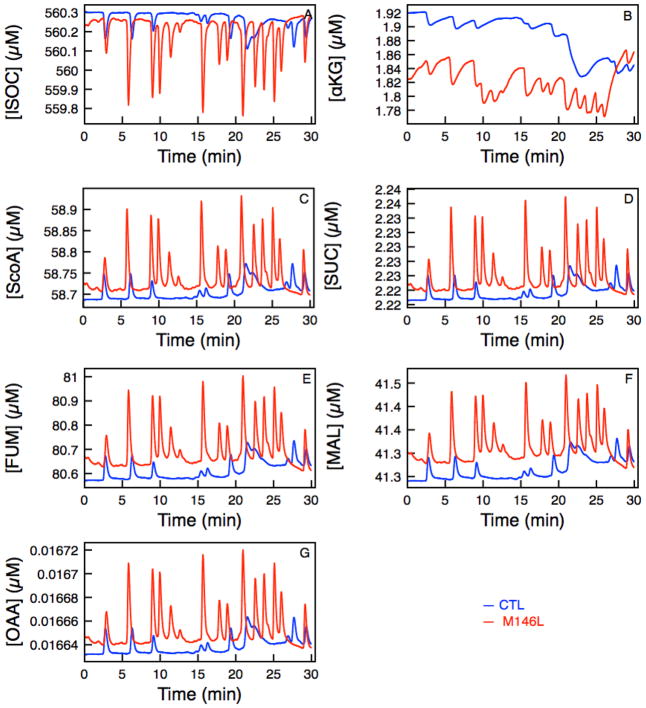
Figure 3.
Time traces of various products in the TCA cycle as a function of Ca2+ uptake into the mitochondria matrix Control (blue) and M146L-expressing cell (red). (A) [ISOC], (B) [αkg], (C) [SCoA], (D) [Suc], (E) [FUM], (F) [MAL], and (G) [OAA].
Figure 2C shows the simulated NADH production over the 30 minutes for both cell types. As we discuss further in Figure 4, the sharp decreases in the [NADH] correspond to the high amplitude oscillations in [Ca2+]i because Vo2 exceeds the NADH production rates of the three enzymes (VIDH, VKGDH, and VMDH).
In Figures 2D–F we show the variables related to the ROS production in the model. In this case, all parameters in the tables were kept such that the would not exceed the critical value beyond which the VIMAC would open, releasing ROS to the cytoplasm and depolarizing ΔΨm. The limit on was imposed to keep the IMAC channel closed because we are interested in the total amount of , rather than its downstream effects. As a consequence, [H2O2] and stay relatively low throughout the duration of the simulation. Though the [H2O2] and show little differences between the two cell types because of the reasons noted above, the shows a noticeable difference following the spiking of [Ca2+]i. The is positively correlated with Vo2 based on the percentage of oxygen consumption that is partitioned for producing . In this case, 4.8% of total oxygen consumption is used for , as shown by the shunt parameter in Table S8. Thus, as Vo2 increases with the high concentration of [Ca2+]m, the increases along with it. Figure 2G shows the [ATP] production over 30 min of simulation. The sharp declines in ATP production that correspond to the oscillations in [Ca2+]i are due to the decreases in NADH production during oscillations. Figure 2H shows depolarization of ΔΨm due to increases in oxygen consumption that decreases the rate of H+ transport across the membrane.
Ca2+ driven TCA cycle
We are interested in how the perturbations in the cytosolic Ca2+ signals affect the bioenergetics of the mitochondria. Denton and McCormack demonstrated that Ca2+ stimulated mitochondrial matrix dehydrogenase, which increased electron supply to the respiratory chain and thus the rates of the respiration [12, 30]. To analyze the complete picture and the time evolution of the levels of intermediate products of the TCA cycle, we evaluated the dehydrogenase activity throughout the duration of the experiment (Figure 3). In this model, KGDH and IDH are directly stimulated by Ca2+ imported into the matrix through uniporter. Thus in Figure 3A, the production of ISOC is negatively affected by Ca2+ because it is regulated by IDH, which is responsible for catalyzing the oxidative decarboxylation of ISOC into αkg. As ISOC catalyzes the third step of the TCA cycle and converts NAD to NADH, [αkg] is formed. [αkg] is regulated by enzymes IDH, KGDH and AAT. KGDH is much more dependent on the influx of Ca2+ and since KGDH catalyzes the conversion between αkg and SCoA, the higher [Ca2+]m (Figure 2B) leads to a steeper drop in [αkg] (Figure 3B). Figure 3C–G shows the rest of the TCA cycle dehydrogenases activity. The rates of key enzymes (VIDH, VKGDH, and VMDH) are what drive the production of NADH and hence the oxidative phosphorylation of the mitochondria shown in Figure 4.
Response of NADH and ATP production
Two interesting observations can be made from Figure 2. The total [NADH] (integrated over 30 minutes) produced in the M146L-expressing cell during the 30 min experiment is greater than the CTL cell by about 3 mM but the [ATP] produced is 1 mM less than the control cell. Over time this can add up to catastrophic differences. What is most interesting is that while the PS mutant-expressing cell produces a larger amount of [NADH], which drives oxidative phosphorylation, it produces less [ATP]. In Figure 2, at the troughs of [Ca2+]i oscillations, there are rapid declines in [NADH] and [ATP], as much as 5 μM and 6 μM, respectively. The reason behind this behavior, is given below.
Ca2+ affects the bioenergetics of the mitochondria in two opposing ways. Ca2+ influx diminishes ΔΨm, which will decrease the proton motive force. While this is happening, Ca2+ stimulates the production of NADH, which increases the proton motive force (ΔμH) and ATP production. In the following, we show that the influx of Ca2+ drives ΔμH down, while the rise in Vo2 overcomes the rates of the enzymes producing NADH that drive oxidative phosphorylation. As shown in Figure 4, when the rate of oxygen consumption and NADH driving enzymes oscillate at high amplitude according to the influx of Ca2+, there are times when Vo2 surpasses the enzymes which saturate according to Michaelis-Menten kinetics. This will decrease the production of NADH as seen in Figure 2. Since the production of ATP is heavily dependent upon the NADH production, we see a decline in ATP at these instances according to the rate of the F1F0 – ATPase. This is observed in Figure 4C–D where the rate of F1F0 – ATPase decreases when the Vo2 surpasses the rate of the NADH producing enzymes. This occurrence is much more pronounced in the M146L-expressing cells as [Ca2+]i oscillates at a higher amplitude and frequency, driving the [NADH] lower more frequently, subsequently leading to decreased ATP production by the mitochondria.
Changing maximal rates of enzymes involved in NADH production increases the difference of overall production of key variables considerably
In the following, we investigate the maximum rates of VmaxANT, Vunimax, and Vnacamax and their affects on the total [ATP], [Ca2+]m, [H2O2], , and [NADH] in both CTL and M146L-expressing cells. The results were obtained by determining the total amount of these entities by integrating the relevant variable over 30 minutes in CTL cells (TotalCTL) and M146L-expressing cells (TotalML). The differences (TotalML-TotalCTL) are plotted against each of the maximum rates as shown in Figures 5–7. Apart from VmaxANT, Vunimax, or Vnacamax, all other parameters were held fixed as given in Tables S2–S8.
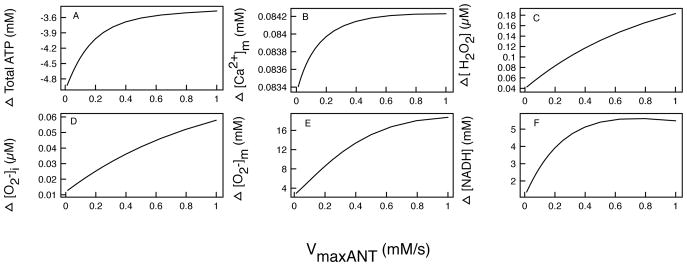
Figure 5.
The difference in the mitochondrial main state variables in control and M146L- expressing cells as VmaxANT is varied. Difference (Δ) is defined as TotalML-TotalCTL. Difference in total: (A) ATP production, (B) Mitochondrial [Ca2+]m, (C) [H2O2], (D) , (E) , and (F) [NADH].
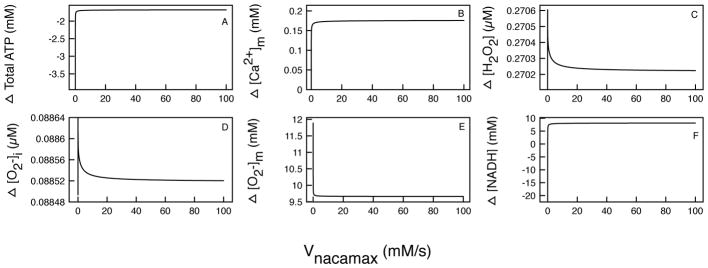
Figure 7.
The difference in the mitochondrial main state variables in control and M146L- expressing cells as VNaCaMax is varied. Difference in total: (A) ATP production, (B) Mitochondrial [Ca2+]m, (C) [H2O2], (D) , (E) , and (F) [NADH].
The rate of the adenine nucleotide translocator (ANT)
The adenine nucleotide translocator (ANT) is responsible for the transfer of [ATP] and cytosolic ADP ([ADP]i) across the inner membrane. As in [4, 7, 28], we consider the ANT to be electrogenic and dependent on the gradient of ATP and ADP. It is important to understand how the rate of ANT serves the mitochondria in transporting ADP and ATP across the inner membrane. This exchange is not only important to the mitochondria as it brings in ADP, but also for the whole cell as it releases ATP to energize various cellular physiological processes.
Figure 5A shows the difference in total ATP production as a function of VmaxANT. The mitochondria in CTL cell produce more ATP than those in M146L-expressing cell and the difference increases with decreasing VmaxANT. At VmaxANT close to 0, representing a severe dysfunction in the mitochondrial ANT, the difference in production is nearly 5 mM; and as the VmaxANT increase to a normal rate, there is still a difference of 3.5 mM in total ATP production over a 30 minute duration. As the ANT rate slows down, no ATP can escape from the matrix, leading to an ATP build up in the mitochondria and lower values of ADP influx to drive the proton pumping across the membrane. Figure 5B shows how mitochondrial Ca2+ buffering changes as we increase VmaxANT. As VmaxANT increases, increasing the influx of ADP, the total difference in amount of Ca2+ in the matrix changes over a range of 83.4μM-84.2μM in 30 minutes. This is because increasing VANT makes ΔΨm more negative, thereby allowing more Ca2+ into the matrix. Figures 5C–E show the differences in total [H2O2], , and , and all of them increase in M146L-expressing cells over a 30 minute experiment. As the ANT rate increases, the [ADP]i/[ATP] ratio increases, which increases the respiration rate Vo2 [7], allowing more oxygen consumption for ROS production in the matrix. The difference of total reaches 18mM for a fully functional ANT (Figure 5E). This constitutes a large difference in ROS production and has high potential for causing complications for the mitochondria in M146L-expressing cells. The [H2O2] and difference continues to increase with increasing VmaxANT. This is because as the production increases, the rate of transport of ROS across the inner mitochondrial membrane increases, which facilitates the transport of ROS to the cytoplasm. This increases the production of superoxide dismutase (SOD) which in turn increases [H2O2]. Figure 5F, which shows the difference in total [NADH] production, is somewhat misleading at first. It shows that mitochondria in the the M146L-expressing cell produces more NADH than those in CTL cells. This should lead to more ATP being produced by the M146L-expressing cell. But as Figure 5A shows, this is clearly not the case. The reason for this is the same as described in the previous section. Overall, more NADH may be produced because of the peaks shown in Figure 2C. But the rapid declines in NADH production are more pronounced because of the increased respiration rate, which lowers the rate of the F1F0 – ATPase, and in turn lowers the ATP production by as much as 5mM.
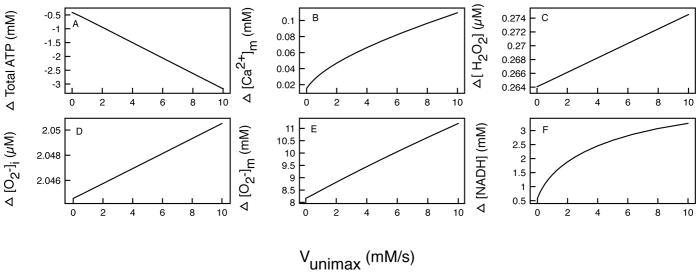
Changing the rate of uniporter
The maximal rate of the mitochondrial Ca2+ uniporter (Vunimax) determines the rate of Ca2+ uptake into the matrix. Abnormal uniporter activity can alter Ca2+ buffering by mitochondria and lead to overall cell dysfunction.
Figure 6 shows that when Vunimax is close to 0, the uniporter is unable to bring much Ca2+ into the matrix to stimulate key driving factors in the matrix. This leads to small differences in [Ca2+]m, [ATP], [H2O2], , and [NADH] between CTL and M146L-expressing cells. However, even with a slow uniporter, a significant difference of over 8 mM in is still observed. As the rate through the uniporter increases, we observe, for the most part, a near-linear increase in the difference between all the key variables. With a maximal uniporter rate of 10mM/s, we observe that M146Lexpressing cells produce 3mM more NADH but 3mM less ATP than CTL cells. Also, the difference in reaches 11mM.
Figure 6.
The difference in the mitochondrial main state variables in control and M146L- expressing cells as Vmuni is varied. Difference in total: (A) ATP production, (B) Mitochondrial [Ca2+]m, (C) [H2O2], (D) , (E) , and (F) [NADH].
The rate of Na+/Ca2+ exchanger
The mitochondrial Na+/Ca2+ exchanger (VNaCa) is the main mechanism for Ca2+ efflux from the mitochondrial matrix and therefore plays a crucial role in maintaining Ca2+ homeostasis in the mitochondrial matrix.
As the maximal rate of the exchanger increases, thereby extruding more Ca2+ from the mitochondrial matrix, the difference in ATP production rapidly plateaus to about 1mM. This is because the exchanger uses a three step Altman-King-Hill process of binding 1 Ca2+ and 3 Na+ for every exchange across the membrane, and [Na+] is constant. This is evident in Figure 7B, where the difference in [Ca2+]m rapidly saturates to a fixed value after VNaCa is increased beyond a certain value. As the exchanger rate decreases, the efflux of Ca2+ to the cytoplasm decreases, leaving more Ca2+ inside the matrix. The Vo2 rate then increases (see Figure 4) and ATP and NADH production declines as evident in Figure 7A and F, respectively. This explains why the values in Figure 7F becomes negative. The CTL cell’s mitochondria is able to produce more NADH as Ca2+ is unable to escape due to decreasing VNaCa rate. Since the [Ca2+]m difference increases, the [H2O2] and ROS production difference increases and eventually plateaus to and 270 μM ([H2O2]).
Discussion
In the AD brain, several pathophysiological events such as disruptions in Ca2+ homeostasis and mitochondrial bioenergetics resulting in cell dysfunction and apoptosis are known to occur [2, 9, 33]. Mitochondria is responsible for generating large amounts of ATP required for neurons to function properly, buffering Ca2+ in the synapses due to the abundance of Ca2+ channels and glutamatergic receptors, rapid uptake of Ca2+ released from the ER, and proper neurotransmission [9]. Mitochondria can also unleash a cascade leading to cell death [14]. Mitochondria dysfunction in AD is characterized by decreased cytochrome c oxidase (COX), increased ROS generation, and altered activity of the TCA cycle enzymes [14, 27]. Thus, elucidating key pathways leading to mitochondrial dysfunction in AD is crucial.
The main goal of this study is to fuse whole cell [Ca2+]i time traces from previous experiments [5] with a detailed computational model to investigate mitochondrial bioenergetics and how the differences in Ca2+ signals in control cells and cells expressing FAD-causing PS mutants affect several key variables regulated by mitochondria such as Ca2+ uptake, ROS, NADH, and ATP production. Our simulations show that mitochondria produces significantly less ATP and more ROS as a result of exaggerated [Ca2+]i in PS mutant-expressing cells. Furthermore, we tested a wide range of values of key parameters in the model (VmaxANT, Vunimax, and Vnacamax) that govern the ATP production and Ca2+ fluxes across the mitochondrial membrane for their effects on mitochondrial function in control and FAD cells.
Our model provides key insights into the relationship between ΔΨm, Vo2, and F1F0 – ATPase activity and allows us to better understand how overload of Ca2+ into the mitochondria leads to detrimental effects on cellular functions that are not easy to study experimentally. It has been established that accumulation of Ca2+ in the mitochondria can cause membrane potential collapse, a rapid drop in ATP levels, and increase in ROS [17]. We show that high influx of Ca2+ into the mitochondria as a result of PS mutant-induced enhanced Ca2+ release through IP3Rs can diminish ATP production significantly. This is because IP3R-mediated Ca2+ release from the ER raises [Ca2+]i, thereby drive more Ca2+ influx into the mitochondrial matrix [7]. This has two opposing effects on mitochondria: (1) the activity of the TCA cycle dehydrogenases is stimulated (Figure 3), and (2) ΔΨm is dissipated. Our simulation (Figure 2H) clearly demonstrate that the dissipative effect is the predominate one in PS mutant-expressing cells.
In isolated mitochondria, Ca2+ stimulation can increase NADH production [30, 44], although the magnitude of this increase also depends on NADH oxidation [7]. Thus, there is an ongoing competition between the two opposing effects in the production of NADH. In both CTL and PS mutant-expressing cells, when the total enzyme rate overcomes Vo2, the flux of F1F0 – ATPase stays constant. However, during times of high Ca2+ influx, ΔμH is dissipated and Vo2 is faster, thereby reducing NADH production. When NADH production is diminished along with ΔμH, the redox potential that drives NADH-mediated proton pumping falls. Also, the increase in ADP contributes negatively to the phosphorylation potential and lowers the rate of F1F0 – ATPase. All these effects resulting from the sharp increases in [Ca2+]m stemming from high frequency, high amplitude [Ca2+]i oscillations contribute to diminished ATP production by the mitochondria.
ROS production in the mitochondria is considered a side path to diverge electrons from the normal electron transport chain [8], (see Figure 1). In the rate equation governing mitochondrial ROS production, it is clear that depends on a shunt of the oxygen consumption. This together with the dissipative effects on ΔμH and higher Vo2 lead to more production. Although ROS has been shown to have beneficial biological effects [29], over-production of ROS is detrimental due to its toxicity and ability to open the mitochondrial PTP [45]. In most cases, difference of several mM in has been observed between the M146L-expressing and CTL cells (see Figures 5–7). Over extended periods, this difference will lead to damaging effects such as exacerbated Ca2+ homeostasis dysregulation and potentially cell death in M146L-expresing cells.
We took advantage of the robustness of the computational model to explore how changes in three main fluxes affect the overall amount of key variables of the mitochondria. By varying maximal flux rates of ANT, uniporter, and Na+/Ca2+ exchanger in this study we are able to see how the detrimental effects on mitochondria could be further exacerbated due to a range of normal to abnormal flux rates. The ANT, which controls the rate of ATP efflux from and ADP influx into the mitochondria shows interesting results as the rate is increased from a close-to-zero to a normally operational value. The PS mutant-expressing cell shows a 5 fold increase in the difference in ROS production. Also, the ATP in PS mutant-expressing cells shows as much as 4.8mM deficiency compared to control mitochondria at the same near zero ANT flux. The difference in [Ca2+]m shows little dependence on the rate of ANT, but still shows a significant difference in the two cases.
By changing Ca2+ flux through uniporter from almost zero to 10mM/s, we observe a 5 fold increase in the difference between the total [Ca2+]m and a near 38% increase in the difference between the total among control and M146L-expressing cells. At high Vunimax, the PS mutation causes a near 3mM deficiency in [ATP] compared to the CTL cells. On the other hand, changing the ability of mitochondria to release Ca2+ through Na+/Ca2+ exchanger causes the difference between different variables in CTL and M146L-expressing cells to saturate because of the fixed Na+ concentration in the model. Even so, we see a near 12mM difference in the total between the PS mutant-expressing and control cells. We also observe 4mM deficiency in [ATP] and 0.17mM higher total [Ca2+]m in M146L-expressing cells.
Dragicevic et al. [14] studied the effects of PS and APP mutations in multiple regions of the mice’s brain including cortical, hippocampal, stratal, and amygdalae. They measured respiration rates, ROS level, ΔΨm, and Cytochrome C oxidase activity and showed that mitochondria from cells expressing PS mutations were more impaired than those from mutant APP-ecpressing mice. Furthermore, mitochondria from mutant APP-expressing mice produced higher levels of ROS than those from control mice, but the highest levels of ROS production came from PS-mutant expressing mice. They observed double the amount of ROS in PS-mutant expressing mice from the cortex and hippocampus [14]. They also observed decreased ΔΨm in all 4 regions of the brain and higher Ca2+ uptake by mitochondria. These observations agree well with conclusions from our modeling study.
The close proximity of IP3R Ca2+ release channels at the ER to the uniporter in the mitochondria creates a local Ca2+ microdomain [35], in which Ca2+ concentrations can rise to a substantially higher level than in general cytosol during IP3R-mediated Ca2+ release. Evidence for fast Ca2+ uptake by the mitochondria have been reported in [15, 37]. It has also been reported that 90% of IP3R are located close to mitochondria and the majority of the uniporters sense the high Ca2+ in the microdomain [43]. Thus, the ER and mitochondria are spatially linked and their proximity to each other will dictate the influx of Ca2+ into the mitochondrial matrix. The higher open-probability of the IP3R in FAD-causing PS mutant-expressing cells will lead to a significant [Ca2+]i increase in the Ca2+ microdomain. Higher IP3R Ca2+ release resulting in higher microdomain Ca2+ in FAD-causing PS-mutant-expressing cells will subsequently exacerbate the impairment of mitochondrial function, which will be the subject of our future research.
Conclusion
In summary, we successfully fused experimental whole cell intracellular free Ca2+ concentration time traces recorded from cells expressing WT and mutant M146L PS1- expressing cells with a computational model to elucidate that how mitochondrial bioenergetics is affected in FAD cells. We show that the higher [Ca2+]i in M146L-expressing cells can lead to drastic differences between the overall production of key variables governing ATP and ROS production as compared to control cells, which could lead to cell death.
Supplementary Material
Acknowledgments
This work is supported by a start up grant from college of Arts and Sciences, University of South Florida awarded to Ghanim Ullah and National Institutes of Health grant number GM065830 awarded to Don-on Daniel Mak. The authors would like to thank Sonia Cortassa and Casey Diekman for providing the codes for the models and Patrick Bradshaw for useful discussions about this work.
References
- 1.Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1998;1366:211–223. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pugers Arch, EJP. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 4.Bohnensack R. The role of the adenine nucleotide translocator in oxidative phosphorylation: A theoretical investigation on the basis of a comprehensive rate law of the translocator. J Bioenerg Biomembr. 1982;14:45–61. doi: 10.1007/BF00744078. [DOI] [PubMed] [Google Scholar]
- 5.Cheung KH, Mei L, Mak DOD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3R receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung KH, Shineman D, Müller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of IP3R receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortassa S, Aon MA, Marbán E, Winslow RL, O’Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David G, Barrett EF. Stimulation-evoked increases in cytosolic Ca2+ in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 11.De Young GW, Keizer J. A single-pool inositol 1, 4, 5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci. 1992;89:9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denton R, McCormack J. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol Endocrinol Metab. 1985;249:E543– E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- 13.Diekman CO, Fall CP, Lechleiter JD, Terman D. Modeling the neuroprotective role of enhanced astrocyte mitochondrial metabolism during stroke. Biophys J. 2013;104:1752–1763. doi: 10.1016/j.bpj.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC. Mitochondrial amyloid-β levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J Alzheimers Dis. 2010;20:S535–S550. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- 15.Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fall CP, Keizer JE. Mitochondrial modulation of intracellular Ca2+ signaling. J Theor Biol. 2001;210:151–165. doi: 10.1006/jtbi.2000.2292. [DOI] [PubMed] [Google Scholar]
- 17.Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- 18.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J Biol Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 20.Hutton M. The presenilins and Alzheimer’s disease. Hum Mol Gen. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 21.Ionescu L, Cheung KH, Vais H, Mak DOD, White C, Foskett JK. Graded recruitment and inactivation of single IP3R receptor Ca2+-release channels: implications for quartal Ca2+ release. J Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri MS, Kotulska M. Modeling the mechanism of metabolic oscillations in ischemic cardiac myocytes. J Theor Biol. 2006;242:801–817. doi: 10.1016/j.jtbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Khachaturian ZS. Calcium, membranes, aging, and Alzheimer’s-disease - introduction and overview. Ann N Y Acad Sci. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- 24.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 25.LaFerla FM, Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer’s presenilin-1 mutation potentiates inositol 1, 4, 5-trisphosphatemediated calcium signaling in xenopus. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 28.Magnus G, Keizer J. Minimal model of beta-cell mitochondrial Ca2+ handling. Am J Physiol - Cell Physiol. 1997;273:C717–C733. doi: 10.1152/ajpcell.1997.273.2.C717. [DOI] [PubMed] [Google Scholar]
- 29.Martin K, Barrett J. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 30.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen MHT, Dudycha SJ, Jafri MS. Effect of Ca2+ on cardiac mitochondrial energy production is modulated by Na+ and H+ dynamics. Am J Physiol - Cell Physiol. 2007;292:C2004–C2020. doi: 10.1152/ajpcell.00271.2006. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen MHT, Jafri MS. Mitochondrial calcium signaling and energy metabolism. Ann N Y Acad Sci. 2005;1047:127–137. doi: 10.1196/annals.1341.012. [DOI] [PubMed] [Google Scholar]
- 33.Offen D, Elkon H, Melamed E. Advances in Research on Neurodegeneration. Springer; 2000. Apoptosis as a general cell death pathway in neurodegenerative diseases; pp. 153–166. [DOI] [PubMed] [Google Scholar]
- 34.Oster AM, Thomas B, Terman D, Fall CP. The low conductance mitochondrial permeability transition pore confers excitability and CICR wave propagation in a computational model. J Theor Biol. 2011;273:216–231. doi: 10.1016/j.jtbi.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Qi H, Li L, Shuai J. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep. 2015;5:7984. doi: 10.1038/srep07984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566– 578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 37.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 38.Shilling D, Müller M, Takano H, Mak DOD, Abel T, Coulter DA, Foskett JK. Suppression of IP3R receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial alzheimer’s disease pathogenesis. J Neurosci. 2014;34:6910–6923. doi: 10.1523/JNEUROSCI.5441-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffieine-induced Ca2+ release in the 3xtg-ad mouse model of Alzheimer’s disease. J Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 40.Stutzmann GE. Calcium dysregulation, IP3R signaling, and Alzheimer’s disease. Neuroscientist. 2005;11:110–115. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- 41.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3R signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Supnet C, Bezprozvanny I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2010;20:487–498. doi: 10.3233/JAD-2010-100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szopa P, Dyzma M, KaŸmierczak B. Membrane associated complexes in calcium dynamics modelling. PB. 2013;10:035004. doi: 10.1088/1478-3975/10/3/035004. [DOI] [PubMed] [Google Scholar]
- 44.Territo P, French S, Balaban R. Simulation of cardiac work transitions, in vitro: effects of simultaneous Ca2+ and ATPase additions on isolated porcine heart mitochondria. Cell calcium. 2001;30:19–27. doi: 10.1054/ceca.2001.0211. [DOI] [PubMed] [Google Scholar]
- 45.Vercesi A, Kowaltowski A, Grijalba M, Meinicke A, Castilho R. The role of reactive oxygen species in mitochondrial permeability transition. Biosci Rep. 1997;17:43–52. doi: 10.1023/a:1027335217774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.