Abstract
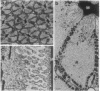
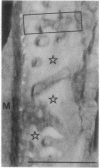
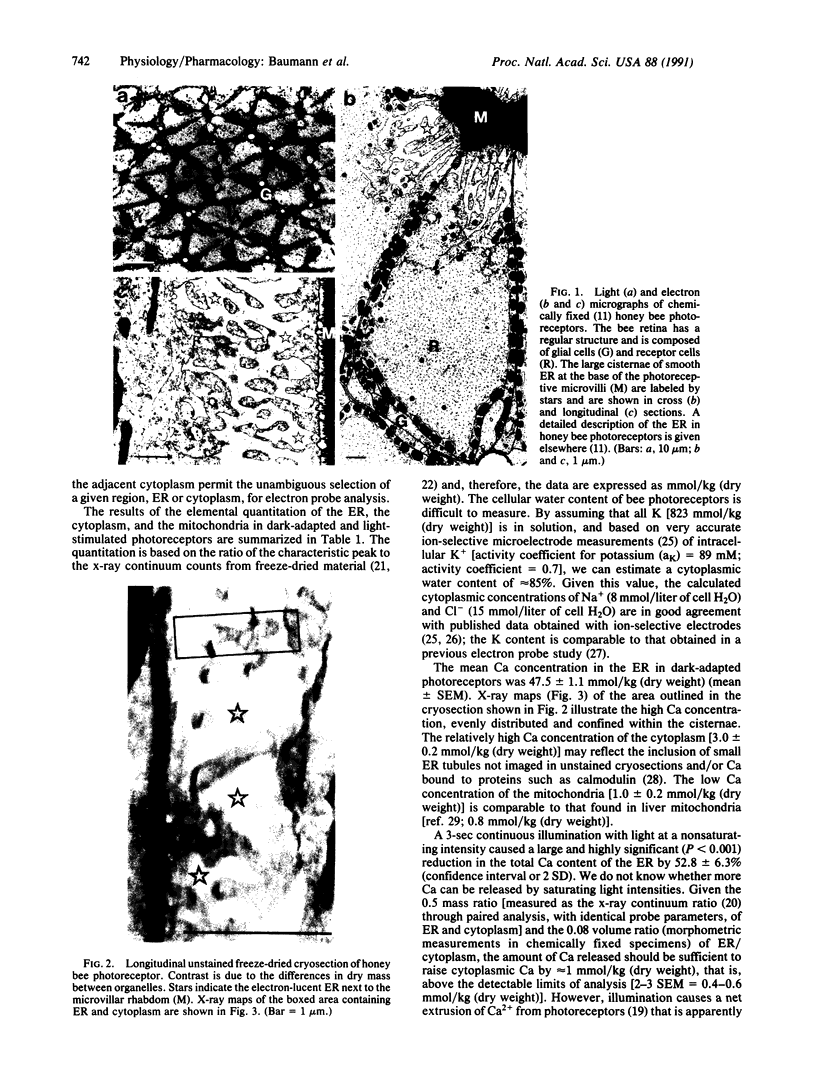
Honey bee photoreceptors contain large sacs of endoplasmic reticulum (ER) that can be located unequivocally in freeze-dried cryosections. The elemental composition of the ER was determined by electron probe x-ray microanalysis and was visualized in high-resolution x-ray maps. In the ER of dark-adapted photoreceptors, the Ca concentration was 47.5 +/- 1.1 mmol/kg (dry weight) (mean +/- SEM). During a 3-sec nonsaturating light stimulus, approximately 50% of the Ca content was released from the ER. Light stimulation also caused a highly significant increase in the Mg content of the ER; the ratio of Mg uptake to Ca released was approximately 0.7. Our results show unambiguously that the ER is the source of Ca2+ release during cell stimulation and suggest that Mg2+ can nearly balance the charge movement of Ca2+.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Fuortes G., Muri R. Pigment transformation and electrical responses in retinula cells of drone, Apis mellifera male. J Physiol. 1979 Nov;296:431–441. doi: 10.1113/jphysiol.1979.sp013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Vadasz G., Somlyo A. V., Somlyo A. P. Subcellular calcium and magnesium mobilization in rat liver stimulated in vivo with vasopressin and glucagon. J Biol Chem. 1987 Nov 15;262(32):15630–15636. [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Coles J. A., Orkand R. K. Changes in sodium activity during light stimulation in photoreceptors, glia and extracellular space in drone retina. J Physiol. 1985 May;362:415–435. doi: 10.1113/jphysiol.1985.sp015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Orkand R. K., Yamate C. L. Chloride enters glial cells and photoreceptors in response to light stimulation in the retina of the honey bee drone. Glia. 1989;2(5):287–297. doi: 10.1002/glia.440020502. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Hall T. A., Gupta B. L. The localization and assay of chemical elements by microprobe methods. Q Rev Biophys. 1983 Aug;16(3):279–339. doi: 10.1017/s0033583500005114. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Krause K. H., Pittet D., Volpe P., Pozzan T., Meldolesi J., Lew D. P. Calciosome, a sarcoplasmic reticulum-like organelle involved in intracellular Ca2+-handling by non-muscle cells: studies in human neutrophils and HL-60 cells. Cell Calcium. 1989 Jul;10(5):351–361. doi: 10.1016/0143-4160(89)90061-4. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Payne R., Fein A. Inositol 1,4,5 trisphosphate releases calcium from specialized sites within Limulus photoreceptors. J Cell Biol. 1987 Apr;104(4):933–937. doi: 10.1083/jcb.104.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P. Cell physiology: cellular site of calcium regulation. Nature. 1984 Jun 7;309(5968):516–517. doi: 10.1038/309516b0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Bond M., Broderick R., Goldman Y. E., Shuman H., Walker J. W., Trentham D. R. Calcium and magnesium movements in cells and the role of inositol trisphosphate in muscle. Soc Gen Physiol Ser. 1987;42:77–92. [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Broderick R., Shuman H., Buhle E. L., Jr, Somlyo A. P. Atrial-specific granules in situ have high calcium content, are acidic, and maintain anion gradients. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6222–6226. doi: 10.1073/pnas.85.16.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977 Aug 11;268(5620):556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A., Walz B. Evidence for light-induced release of Ca2+ from intracellular stores in bee photoreceptors. Neurosci Lett. 1990 Mar 26;111(1-2):87–91. doi: 10.1016/0304-3940(90)90349-e. [DOI] [PubMed] [Google Scholar]