Abstract
Background
The optimal systolic blood pressure (SBP) treatment goal is in question, with the SBP intervention trial (SPRINT) suggesting benefit for 120mmHg. However, achieving SBP this low may reduce diastolic BP (DBP) to levels that could compromise myocardial perfusion.
Objectives
To examine the association of DBP with prevalent and progressive myocardial damage (using high-sensitivity cardiac Troponin-T, hs-cTnT). We also examined prospective associations between DBP and coronary heart disease (CHD), stroke, or death over 21 years; overall and stratified by subgroups of interest.
Methods
We studied 11,565 adults from the Atherosclerosis Risk in Communities (ARIC) study. We evaluated cross-sectional DBP and hs-cTnT (dichotomized at 14 ng/L) associations with logistic regression, longitudinal associations between DBP and hs-cTnT change using generalized linear models adjusted for attrition, and prospective associations between DBP and events with Cox regression.
Results
Mean baseline age was 57 years, 57% were female and 25% were black. Relative to persons with DBP 80–89mmHg, those with DBP 60–69mmHg and <60mmHg had higher prevalence of baseline hs-cTnT ≥14ng/L (OR 1.5 [95%CI 1.0–2.3] and 2.2 [1.2–4.1]). Participants with DBP 60–69mmHg and <60mmHg also had relatively larger increases in hs-cTnT over the initial 6 years of follow-up (β +6 [95%CI 2–10] and +9 [3–14] ng/L). DBP <60mmHg (vs. 80–89mmHg) was associated with incident CHD (HR 1.5 [1.2–1.9]) and mortality (HR 1.3 [1.1–1.6]), but not with stroke. The DBP and incident CHD association was strongest when baseline hs-cTnT ≥14ng/L (p-value-for-interaction <0·001). Associations of low DBP with prevalent hs-cTnT and incident CHD were most pronounced among those with baseline SBP ≥120mmHg.
Conclusion
Particularly among adults with SBP ≥120mmHg, and thus elevated pulse-pressure, low DBP was associated with subclinical myocardial damage and CHD events. When titrating treatment to SBP <140mmHg, it may be prudent to ensure DBP levels do not fall below 70mmHg, and particularly below 60mmHg.
Keywords: Diastolic BP, Hypertension, High-sensitivity Troponin, Coronary Heart Disease
INTRODUCTION
Diastolic blood pressure (DBP) was historically thought to be the main driver of adverse cardiac outcomes in adults with hypertension. (1) While initially overlooked, seminal work from Framingham and other observational cohorts subsequently demonstrated the importance of systolic BP (SBP)(2,3), leading to a paradigm shift whereby SBP became the focus of modern risk assessment and treatment. However, to this day, there is uncertainty about the optimal SBP goal.(4–7) For example, the Systolic Blood Pressure Intervention Trial (SPRINT)(8) reported reductions in cardiovascular disease (CVD) death and heart failure, among high-risk non-diabetic adults treated to a SBP target of 120 mmHg or less. In contrast, BP lowering among intermediate risk adults was not beneficial, and showed a trend for harm among those with baseline SBP levels <130 mmHg, in the Heart Outcomes Prevention Evaluation (HOPE)-3 trial. (7)
Achieving intensive SBP reductions will inevitably also lower DBP. For example, in a secondary analysis of elderly SPRINT participants, the authors reported that diastolic BP in the intensive therapy arm fell from a mean of 71.5 mmHg at baseline to 62 mmHg during active treatment. (9) This is of potential concern due to the known J-curve for DBP and CHD events. (10–12) Particularly among persons obstructive coronary artery disease (CAD) or left ventricular hypertrophy (LVH), a fall in DBP has been shown to reduce coronary perfusion pressure (coronary blood flow occurs primarily in diastole), which can result in ischemia and myocardial damage. (13)
High-sensitivity cardiac troponin assays can detect asymptomatic myocardial damage and have been strongly predictive of fatal and non-fatal coronary heart disease (CHD) events in numerous observational studies, including among primary prevention populations. (14–17) As such, high-sensitivity troponin may be of value in understanding whether lower achieved BP, in particular low DBP levels, are associated with myocardial damage.
Therefore, the aim of this analysis from the Atherosclerosis Risk in Communities (ARIC) cohort study was to determine whether low DBP was associated with either cross-sectional (measured with high-sensitivity cardiac Troponin-T [hs-cTnT] at baseline) or progressive (measured with trajectories of temporal hs-cTnT change over follow-up) subclinical myocardial damage. We also evaluated whether low DBP increases the risk for future adverse outcomes, including CHD, stroke, and all-cause death, in the overall study sample as well as after stratification by baseline SBP and baseline hs-cTnT (levels ≥14 ng/L are associated structural heart disease [e.g. LVH] and subclinical macro/microvascular coronary disease).
METHODS
Study Population
The ARIC Study is a prospective observational cohort of 15,792 adults sampled from four U.S. communities (Forsyth Country, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland). Details of the study design have been published. (18) Institutional review boards at each study site approved the study and written informed consent was obtained from all participants. Of the 14,348 persons who attended ARIC visit 2 (1990–1992), we excluded those who had known CVD or HF at or prior to visit 2 (n=1,651) and those who were missing other variables of interest (n=1,132). Thus, 11,565 persons were included in our main analytic sample (eTable 1). For supplemental analyses we generated a secondary subsample of 1,403 visit 2 participants who met SPRINT enrollment criteria. (8) (Online Supplement)
Measurement of hs-cTnT and other exposure variables
Measurement of hs-cTnT occurred at three time points over a span of 21 years- visit 2 (1990–1992), visit 4 (1996–1998) and visit 5 (2011–2013). The measurement range of the assay is 3–100,000 ng/L. Values ≥14ng/L represent the 90th percentile in the ARIC sample and the 99th percentile value for a “healthy” reference group aged 20–70 years. (19) More details on hs-cTNT measurements at each visit is available in the online supplement.
Demographic and cardiovascular risk factors were assessed at visit 2, with measurements obtained using standardized protocols. (18) Participants self-reported race, alcohol use and smoking status. Body mass index (BMI) was calculated from measured weight and height. After 5 minutes of seated rest, we recorded BP as the mean of the last 2 of 3 measurements collected over 5 minute intervals using a random zero sphygmomanometer. Hypertension was defined as SBP ≥140mmHg, DBP ≥90mmHg, or the use of antihypertensive medications. Antihypertensive drug use was assessed with a medication inventory. Diagnosed diabetes was defined as a self-reported physician diagnosis of diabetes or current use of diabetic medications. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride measurements were obtained after a 12-h fast. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.
Follow-up for clinical outcomes of interest
Clinical endpoints included CHD, stroke, and mortality. Of note, stroke was used as a ‘negative control’ because we hypothesized that low DBP would not be adversely associated with this outcome as the physiologic relationship between DBP and coronary perfusion should have no bearing on stroke risk. We also conducted a sensitivity analysis for incident heart failure (supplement). Visit 2 was baseline for analysis of incident events.
The ascertainment of deaths and classification of CHD and stroke in ARIC have been described. (20,21) Briefly, hospitalizations and deaths were reported annually by study participants or their proxy and also identified through death certificates from state vital statistics offices and surveillance of hospitals within each ARIC community. CHD events were adjudicated by an ARIC end points committee and defined as a definite or probable myocardial infarction, death from CHD, or cardiac procedure. (20) Stroke signs, symptoms, neuroimaging (CT or magnetic resonance), and other diagnostic reports were recorded if the list of discharge diagnoses included a cerebrovascular disease code (International Classification of Diseases, 9th Revision, code 430–437), if a cerebrovascular condition or procedure was mentioned in the discharge summary, or if a cerebrovascular finding was noted on a CT or magnetic resonance imaging report. Each eligible case was classified according to criteria adapted from the National Survey of Stroke. (22)
Statistical Analyses
Baseline characteristics were compared across 6 categories of DBP (<60, 60–69, 70–79, 80–89, 90–99, and ≥100 mmHg) using ANOVA for continuous variables and chi-square test for proportions.
To model the cross-sectional association between DBP categories and baseline hs-cTnT, we defined the outcome of “elevated hs-cTnT" (≥14 ng/L, yes, no). (19) We constructed logistic models with robust standard errors adjusted for: age (years), race-center, gender, body mass index in kg/m2, smoking (current; former; never), alcohol intake (current; former; never), systolic BP (in mmHg), hypertension medication use (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes, no), and estimated glomerular filtration rate in mL/min/1.73m2. We repeated these logistic models after stratification by baseline systolic BP category (<120, 120–139, ≥140 mmHg), with SBP removed from the adjustment terms. We also modeled DBP as a continuous variable and graphed the adjusted odds ratio (OR) using restricted cubic splines with knots at 57, 68, 75 and 90 mm Hg (the 5th, 35th, 65th, and 95th percentiles), using 85 mmHg as the reference value, and truncated at the 1st and 99th percentiles.(23)
To model the longitudinal association between baseline DBP categories and temporal change in hs-cTnT, we constructed linear models fitted with generalized estimating equations. We used unstructured correlation matrices and robust variance estimation. Persons with hs-cTnT <3ng/L had values imputed at 1·5ng/L. Time since baseline was modeled using a linear spline with a knot at 6 years (mean duration between visits 2 and 4). Coefficients of interest were the interactions between DBP categories and time spline terms, which address differences in annual hs-cTnT change according to DBP, after adjusting for variables listed in the model above. We used inverse probability of attrition weighting to account for informative missingness due to differential withdrawal across baseline DBP categories (i.e. different proportions of subjects who died, were lost, or had missing hs-cTnT data over follow-up). (24)
To model the prospective association between baseline DBP categories and clinical outcomes, we constructed Cox models, adjusted for the above variables. We verified the proportionality of the hazards visually and with Schoenfeld residuals. We also modeled DBP as a continuous variable and graphed the adjusted Hazard Ratio (HR) using restricted cubic splines. (23) We repeated the models described above in the SPRINT-eligible subsample. We also conducted a sensitivity analysis in the primary study sample with DBP as a time-varying exposure using updated DBP values at visits 2, 3 and 4. This time varying outcomes analysis also included adjustment for SBP, hypertension medication use and estimated glomerular filtration rate as time-varying covariables. Furthermore in a supplemental analysis, we repeated the categorical Cox models in the primary sample after stratification by the following variables of interest, 1) baseline anti-hypertensive treatment status (with hypertension medication use [yes, no] removed from the adjustment terms), 2) baseline hs-cTnT category (<14, ≥14 ng/L), 3) baseline presence of LVH by EKG, and 4) baseline SBP category (<120, 120–139, and ≥140 mmHg, with SBP removed from the adjustment terms). In these stratified analyses we compressed the DBP categories to preserve power (<60, 60–79, 80–89, >90 mmHg).
Finally, we conducted a number of sensitivity analyses exploring the continuous relationships of, 1) DBP with elevated hs-cTnT and incident CHD with and without additional adjustment for SBP, 2) SBP with elevated hs-cTnT and incident CHD with and without additional adjustment for DBP, and 3) Pulse pressure (SBP minus DBP) with elevated hs-cTnT and incident CHD after adjustment for confounders. All models were tested for interaction by age, sex, and race. The level of significance was defined as p<0.05 (2-sided).
RESULTS
Characteristics of the sample by baseline DBP are presented in Table 1. Individuals with lower DBP tended to be older, female, white, have lower BMI, and have healthier lipid profiles. As expected, persons with higher DBP tended to have higher SBP and more frequent use of antihypertensive medications. With the exception of sex and BMI, similar differences according to DBP category were noted in the SPRINT-eligible subsample (eTable 2). Interaction terms for age, sex, and race were non-significant in all models.
Table 1.
Characteristics of the Study Sample, Overall and According to categories of diastolic blood pressure (mmHg) at baseline (ARIC visit 2, 1990–1992)
| Overall | DBP <60 |
DBP 60–69 |
DBP 70–79 |
DBP 80–89 |
DBP 90–99 |
DBP ≥100 |
P Value | |
|---|---|---|---|---|---|---|---|---|
| Number, % | 11565 | 1087 (9.4%) |
3728 (32.2%) |
4249 (36.7%) |
1902 (16.4%) |
487 (4.2%) |
112 (1.0%) |
|
| Age, years | 56.7 (5.7) | 57.7 (6.0) | 56.9 (5.8) | 56.5 (5.6) | 56.5 (5.6) | 56.3 (5.7) | 55.1 (5.4) | <0.001 |
| Female, % | 57.3 | 72.5 | 63.7 | 54.5 | 47.8 | 41.1 | 39.3 | <0.001 |
| Black, % | 24.5 | 13.2 | 20 | 24.5 | 32.9 | 41.3 | 64.3 | <0.001 |
| Systolic BP, mmHg | 121.0 (18.5) |
103.4 (14.5) |
112.1 (13.2) |
122.4 (13.6) |
135.4 (15.0) |
149.5 (17.2) |
167.7 (22.7) |
<0.001 |
| Antihypertensive Medication Use, % |
28 | 18.2 | 22.4 | 28.2 | 37.9 | 47.6 | 53.6 | <0.001 |
| Left Ventricular Hypertrophy by EKG, % |
2.2 | 1.1 | 1.2 | 2.0 | 3.2 | 7.0 | 17.0 | <0.001 |
| Smoking status, % | <0.001 | |||||||
| Never smoking | 41.2 | 34 | 41.3 | 42 | 44.3 | 38.2 | 42.9 | |
| Current smoker | 21.8 | 32.4 | 24.1 | 18.9 | 17.8 | 20.7 | 29.5 | |
| Former smoker | 36.9 | 33.6 | 34.6 | 39.2 | 37.9 | 41.1 | 27.7 | |
| Drinking status, % | 0.51 | |||||||
| Never drinking | 22.7 | 24.7 | 23 | 23.1 | 21 | 20.5 | 21.4 | |
| Current drinker | 57.6 | 55.7 | 57.2 | 57.7 | 59.1 | 57.5 | 58.9 | |
| Former drinker | 19.6 | 19.6 | 19.7 | 19.2 | 19.9 | 22 | 19.6 | |
| Diagnosed diabetes, % | 7.8 | 8.8 | 8.3 | 7.3 | 7.7 | 5.7 | 5.4 | 0.163 |
| BMI, kg/m2 | 27.8 (5.3) | 25.7 (4.8) | 27.1 (5.0) | 28.2 (5.2) | 28.9 (5.4) | 29.7 (6.2) | 30.6 (7.4) | <0.001 |
| LDL-cholesterol, mg/dL | 133.1 (36.6) |
131.3 (37.0) |
131.4 (36.0) |
133.5 (36.6) |
135.3 (37.2) |
136.7 (38.3) |
141.7 (36.2) |
<0.001 |
| HDL-cholesterol, mg/dL | 50.6 (16.8) |
52.3 (16.8) |
51.6 (16.9) |
49.7 (16.4) |
50.0 (17.5) |
49.0 (15.8) |
49.2 (14.4) |
<0.001 |
| Triglycerides, mg/dL | 127.2 (64.5) |
120.1 (60.9) |
123.2 (61.9) |
129.6 (65.5) |
132.2 (67.2) |
133.8 (69.6) |
126.6 (67.0) |
<0.001 |
| Lipid medication, % | 5.2 | 4.8 | 5.4 | 5.8 | 4.5 | 2.3 | 0.9 | 0.002 |
| eGFR, ml/min/1.73 m2 |
96.8 (15.2) |
97.2 (13.5) |
96.7 (14.8) |
96.7 (15.2) |
97.2 (16.0) |
96.0 (17.5) |
95.2 (20.4) |
0.469 |
Estimates are mean (SD) or %.
BMI= body mass index, DBP= Diastolic Blood Pressure, LDL= Low Density Lipoprotein, HDL= High Density Lipoprotein, eGFR= estimated Glomerular Filtration Rate.
Compared to persons with baseline DBP between 80–89 mmHg, the adjusted odds of having hs-cTnT ≥14ng/L at baseline was 2.2 (95%CI 1.2–4.1) and 1.5 (95%CI 1.0–2.3) in those with DBP <60 mmHg and 60–69 mmHg, respectively (Table 2). When DBP was modeled continuously using linear splines, we observed a linear inverse relationship between DBP and hs-cTnT when DBP was below 65 mmHg (Figure 1). There appeared to be similar associations among the SPRINT-eligible subsample (e.g. OR of 1.7 for DBP<60 and 1.2 for DBP 60–69 mmHg, relative to 80–89 mmHg); however, these findings were not statistically significant (eTable 3).
Table 2.
Adjusted* Odds Ratios† (95% confidence intervals) for prevalent baseline Elevated Hs-cTnT and Adjusted* Beta-Coefficients‡ for Expected Annual Change in Hs-cTnT, according to baseline categories of diastolic blood pressure (mmHg) (N=11,565)
| Cross-Sectional Analysis Elevated Hs-cTNT (≥14 ng/L) |
Longitudinal Analysis Estimated Additional Annual Change (95% CI) in Hs-cTNT (ng/L) |
||||||
|---|---|---|---|---|---|---|---|
| Visit 2 Diastolic BP |
n/N | Odds Ratio (95% CI) |
p-value | Annual Change Between Visits 2 and 4 |
p-value | Annual Change Between Visits 4 and 5 |
p-value |
| <60 mm Hg | 39/1087 |
2.24 (1.22, 4.10) |
0.01 |
1.46 (0.51, 2.40) |
0.002 | −0.09 (−0.69, 0.51) |
0.77 |
| 60–69 mm Hg | 120/3728 |
1.52 (1.00, 2.32) |
0.05 |
0.95 (0.28, 1.61) |
0.005 | 0.32 (−0.69, 1.34) |
0.54 |
| 70–79 mm Hg | 144/4249 | 1.02 (0.71, 1.47) |
0.90 |
0.85 (0.27, 1.44) |
0.004 | 0.02 (−0.26, 0.31) |
0.86 |
| 80–89 mm Hg | 102/1902 | 1 (reference) | . | 0 (reference) | 0 (reference) | ||
| 90–99 mm Hg | 36/487 | 1.06 (0.61, 1.83) |
0.84 | −0.73 (−1.47, 0.01) |
0.06 | 0.26 (−0.07, 0.60) |
0.13 |
| ≥ 100 mm Hg | 14/112 | 1.54 (0.63, 3.78) |
0.34 | −0.99 (−2.58, 0.58) |
0.21 | 0.43 (−0.32, 1.18) |
0.26 |
Adjusted for: age (years), race-center, gender, body mass index in kg/m2, smoking (current; former; never), alcohol intake (current; former; never), systolic BP (in mmHg), hypertension medication use (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes or no), and estimated glomerular filtration rate in mL/min/1.73m2.
Logistic model for cross-sectional association between diastolic BP and baseline elevated hs-cTnT
Linear model with generalized estimating equations and inverse probability of attrition weighting
Significant values in bold.
Hs-cTnT= high-sensitivity Troponin T, other abbreviations as per Table 1.
Figure 1. Adjusted* Odds Ratio (95% confidence interval) for Prevalent Elevated hs-cTnT (≥14 ng/L), according to baseline Diastolic Blood Pressure (DBP) with background histogram of DBP distribution in the study sample.
*Adjusted for: age (years), race-center, gender, body mass index in kg/m2, smoking (current; former; never), alcohol intake (current; former; never), systolic BP (in mmHg), hypertension medication use (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes or no), and estimated glomerular filtration rate in mL/min/1.73m2.
Restricted Cubic Spline for odds of elevated hs-cTnT with background distributional histogram of baseline Diastolic BP. Note that the "frequency" axis label identifies the number ARIC participants at each point on this background histogram. Splines are centered at 85 mmHg, have knots at 57, 68, 75 and 90 mm Hg, and are truncated at the 1st and 99th percentiles. The shaded area around the regression line represents the 95% confidence interval
Hs-cTnT= high-sensitivity Troponin T, other abbreviations as per Table 1
Low DBP at baseline was also independently associated with progressive myocardial damage, as assessed by estimated annual change in hs-cTnT over the 6 years between visits 2 and 4. The estimated annual change in hs-cTnT was +1.5 (95% CI 0.5–2.4) ng/L per year higher in the DBP <60 mmHg group and +1.0 (95% CI 0.3–1.6) ng/L per year higher in the DBP 60–69 mmHg group, compared to DBP 80–89 mmHg. However, visit 2 DBP was not associated with higher annual hs-cTNT change in the follow-up period occurring after visit 4 (i.e., from 1996–1998 to visit 5 in 2011–2013) (Table 2).
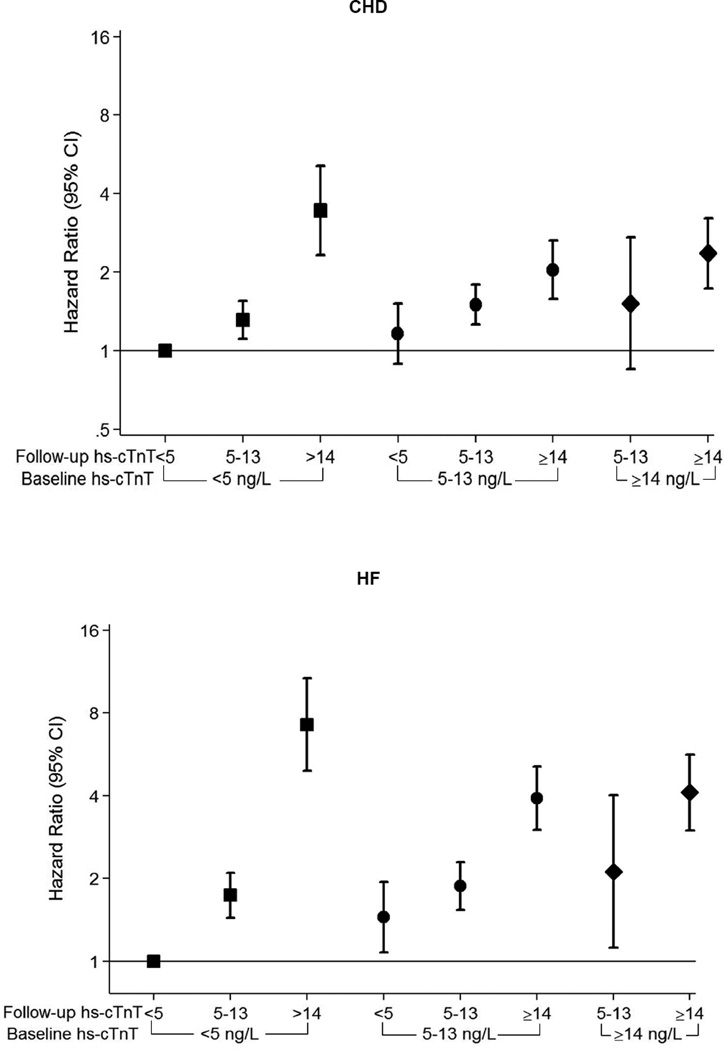
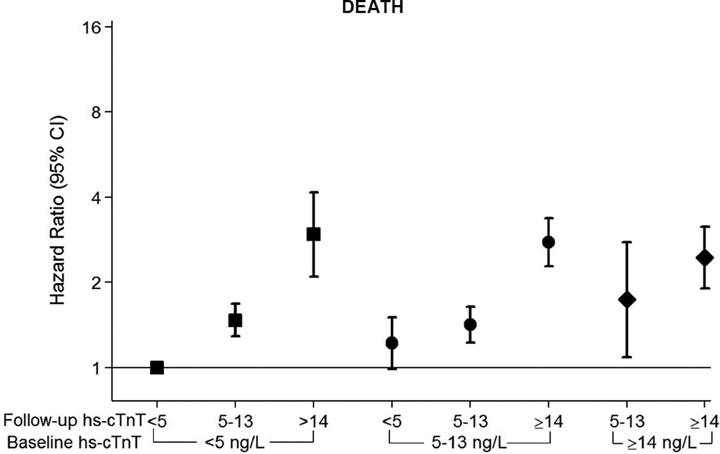
Consistent with results for hs-cTnT, low DBP was associated with subsequent CHD and mortality over a median follow-up of 21 years. The highest relative hazard for events was among persons with DBP <60 mmHg for both CHD (HR 1.5, 95% CI 1.2–1.9) and for all-cause death (HR 1.3, 95% CI 1.1–1.6), compared to DBP 80–89 mmHg. Unlike mortality, there was also increased CHD risk among persons with DBP of 60–69 mmHg (HR 1.23, 95% CI 1.05–1·44) and 70–79 mmHg (HR 1.20, 95% CI 1.05–1.37) (Table 3). When evaluating the sub-components of the CHD outcome, this association appeared stronger for fatal CHD and myocardial infarction, relative to revascularization (eTable 4). As expected, there was no association between DBP and stroke after accounting for SBP and clinical confounders (Table 3). The results of our supplemental analysis for incident heart failure were similar to those for CHD, with a trend for increased risk at low DBP (eTable 5). We also found similar associations between low DBP and CHD events in the SPRINT-eligible subsample (eTable 6). Furthermore, DBP <60 mmHg was consistently associated with excess risk for events in our sensitivity analysis evaluating DBP as a time-varying exposure and with adjustment for SBP, antihypertensive medication use and renal function as time-varying confounders (eTable 7).
Table 3.
Adjusted* Hazards Ratios (95% confidence intervals) for Incident Coronary Heart Disease (CHD), Stroke, or Mortality events over prospective follow-up (N=11,565)
| Visit 2 Diastolic BP |
CHD | Stroke | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n/N | HR (95% CI) |
p- value |
n/N | HR (95% CI) |
p- value |
n/N | HR (95% CI) |
p- value |
|
| <60 mm Hg | 165/ 1087 |
1.49 (1.20–1.85) |
<0.001 | 56/ 1084 |
1.13 (0.79–1.61) |
0.52 | 345/ 1087 |
1.32 (1.13–1.55) |
<0.001 |
| 60–69 mm Hg | 547/ 3728 |
1.23 (1.05–1.44) |
0.01 | 197/ 3722 |
1.03 (0.80–1.32) |
0.83 | 1017/ 3727 |
1.10 (0.98–1.23) |
0.12 |
| 70–79 mm Hg | 752/ 4247 |
1.20 (1.05–1.37) |
0.01 | 271/ 4234 |
1.07 (0.86–1.32) |
0.55 | 1142/ 4247 |
0.99 (0.89–1.10) |
0.89 |
| 80–89 mm Hg | 350/ 1902 |
1 (reference) | . | 143/ 1894 |
1 (reference) |
. | 597/ 1902 |
1 (reference) |
. |
| 90–99 mm Hg | 104/ 487 |
0.93 (0.74–1.16) |
0.52 | 53/ 484 |
1.20 (0.87–1.66) |
0.27 | 189/ 487 |
1.01 (0.85–1.19) |
0.92 |
| ≥ 100 mm Hg | 25/ 112 |
0.76 (0.50–1.17) |
0.21 | 19/ 112 |
1.50 (0.90–2.50) |
0.12 | 49/ 112 |
1.03 (0.76–1.40) |
0.84 |
Cox Model adjusted for: age (years), race-center, gender, body mass index in kg/m2, smoking (current; former; never), alcohol intake (current; former; never), systolic BP (in mmHg), hypertension medication use (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes or no), and estimated glomerular filtration rate in mL/min/1.73m2.
Significant values in bold.
Abbreviations as per Table 1.
When our primary sample was stratified by baseline antihypertensive treatment status, the association between DBP categories and CHD events was qualitatively similar as in the sample overall (eTable 8). However, when the sample was stratified by baseline hs-cTnT (<14 ng/L or ≥14 ng/L), the risk for subsequent CHD was highest among those with both low DBP and baseline myocardial damage (HR 2.6, 95% CI 1.3–5.0, for DBP of <60mmHg among persons with hs-cTnT ≥14 ng/L, compared to 1.3, 95% CI 1.1–1.7 in those with hs-cTnT <14 ng/L, p-value for interaction <0.001). Similarly, there was a trend towards excess hazard for CHD among persons with low DBP and baseline LVH by EKG (although results were underpowered due to low numbers of participants with LVH).
Finally, Table 4 shows results for both the myocardial damage and clinical event outcomes according to baseline DBP levels, after stratifying the study sample by SBP categories. The association of low DBP (specifically DBP <60 mmHg) with both prevalent myocardial damage and incident CHD appeared to be primarily driven by excess risk among those with SBP of ≥120 mmHg (in order words, pulse pressure >60 mmHg). These results were consistent in a number of sensitivity analyses, demonstrating that, 1) low DBP, modeled continuously, is a risk factor for elevated hs-cTnT and incident CHD (particularly after adjusting for SBP, eFigure 1), 2) despite the adverse associations with low DBP, high SBP is also a risk factor for elevated hs-cTnT and incident CHD (eFigure 2), 3) as such, pulse-pressure >60 mmHg appears to be an important driver of these results (eFigure 3), and 4) consistent with this, the association of low DBP with hs-cTnT and incident CHD is most evident among those with SBP ≥120 (eFigure 4).
Table 4.
Adjusted* Odds Ratios† (95% confidence intervals) for prevalent baseline Elevated Hs-cTnT and Adjusted* Hazard Ratios‡ for Events, according to categories of diastolic BP after stratification by baseline systolic BP (mmHg) (N=11,565)
| Cross-Sectional Analysis for Elevated Hs-cTNT (≥14 ng/L) |
Prospective Proportional Hazards Analysis for Incident Outcomes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit 2 Diastolic BP |
Visit 2 Systolic BP |
n/N | Odds Ratio (95% CI) |
p- value |
n/N | CHD HR (95% CI) |
p- value |
n/N | Mortality HR (95% CI) |
p- value |
| <60 mm Hg |
<120 mm Hg |
26/ 957 |
1.16 (0.47–2.86) |
0.74 | 130/ 957 |
1.05 (0.71–1.54) |
0.81 | 281/ 957 |
1.23 (0.89–1.70) |
0.22 |
| 60–79 mm Hg | 109/ 4891 |
0.86 (0.40–1.88) |
0.71 | 637/ 4891 |
0.97 (0.69–1.36) |
0.85 | 1061/ 4891 |
1.07 (0.79–1.45) |
0.66 | |
| 80–89 mm Hg | 9/227 | 1 (reference) |
. | 37/ 227 |
1 (reference) |
. | 45/ 227 |
1 (reference) |
. | |
| ≥90 mm Hg | 0/7 | --- | --- | 0/7 | --- | --- | 1/7 | 0.91 (0.12–6.60) |
0.94 | |
| <60 mm Hg |
120–139 mm Hg |
9/101 |
2.49 (1.06–5.84) |
0.03 | 26/ 101 |
1.71 (1.11–2.63) |
0.01 | 49/ 101 |
1.25 (0.91–1.71) |
0.17 |
| 60–79 mm Hg | 98/ 2507 |
0.90 (0.59–1.36) |
0.61 | 497/ 2505 |
1.17 (0.97–1.40) |
0.09 | 800/ 2505 |
0.99 (0.85–1.14) |
0.85 | |
| 80–89 mm Hg | 41/ 1033 |
1 (reference) |
. | 176/ 1033 |
1 (reference) |
. | 275/ 1033 |
1 (reference) |
. | |
| ≥90 mm Hg | 7/144 | 1.15 (0.50–2.64) |
0.75 | 31/ 144 |
1.19 (0.81–1.75) |
0.36 | 37/ 144 |
1.00 (0.71–1.41) |
0.99 | |
| <60 mm Hg |
≥ 140 mm Hg |
4/29 | 1.45 (0.38–5.53) |
0.59 | 9/29 | 1.46 (0.73–2.92) |
0.29 | 15/29 | 0.97 (0.57–1.65) |
0.90 |
| 60–79 mm Hg | 57/ 579 |
0.94 (0.60–1.46) |
0.77 | 165/ 579 |
1.31 (1.03–1.66) |
0.03 | 298/ 579 |
1.02 (0.86–1.21) |
0.78 | |
| 80–89 mm Hg | 52/ 642 |
1 (reference) |
. | 137/ 642 |
1 (reference) |
. | 277/ 642 |
1 (reference) |
. | |
| ≥90 mm Hg | 43/ 448 |
0.87 (0.54–1.41) |
0.58 | 98/ 448 |
1.02 (0.78–1.33) |
0.89 | 200/ 448 |
1.02 (0.85–1.24) |
0.81 | |
Adjusted for: age (years), race-center, gender, body mass index in kg/m2, smoking (current; former; never), alcohol intake (current; former; never), hypertension medication use (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes or no), and estimated glomerular filtration rate in mL/min/1.73m2.
Logistic model for cross-sectional association between diastolic BP and baseline elevated hs-cTnT
Cox model for prospective association between diastolic BP and incident events
Significant values in bold.
CHD=coronary heart disease, Hs-cTnT= high-sensitivity Troponin T, other abbreviations as per Table 1.
DISCUSSION
Our results have a number of potential implications, particularly in the post-SPRINT era where the threshold for diagnosing and treating hypertension could be redefined. (25) Despite the undeniable clinical benefits reported in SPRINT, one of many concerns relating to aggressive SBP reduction with pharmacotherapy is the possibility of myocardial ischemia by lowering DBP. This concern is based on strong physiologic rationale and a wealth of prior observational data. Indeed, there was a trend to harm with BP treatment among participants enrolled in HOPE-3 who had baseline SBP <131.5 mmHg. (7) We extend these findings by demonstrating that, at any given SBP, 1) low DBP is cross-sectionally associated with prevalent myocardial damage, 2) low DBP is prospectively associated with near-term progression of myocardial damage, 3) low DBP is prospectively associated with incident CHD events (and mortality), but, as expected, not with incident stroke, and 4) the association between low DBP and incident CHD appears to be strongest among those with evidence of preceding myocardial damage at baseline. Considered in isolation, each of these 4 findings are of clinical importance; however, taken together, they form a compelling argument that excessively low DBP may directly harm the myocardium.
A J-curve has been repeatedly demonstrated for DBP and coronary events. (13) For example, in a study of 902 hypertensive patients, Cruickshank et al. found a J-relationship between death from CHD and treated DBP in patients with CAD. (10) The nadir of the J-curve in DBP was at 85 to 90 mm Hg, with an increase of CHD mortality on either side of this range. Farnett et al. confirmed this J-shaped relationship in their meta-analysis. (11) The INVEST study enrolled 22,576 patients with CAD and hypertension and found that the primary outcome doubled when DBP was below 70 mm Hg and quadrupled when it was below 60 mm Hg. (12,26) Our findings support these data, particularly by demonstrating increased risk for CHD events at DBP levels below 80 mmHg in the main sample and at DBP levels below 60 mmHg in the smaller SPRINT-eligible subsample (the latter sensitivity analysis lacked power to demonstrate increased risk for CHD at DBP levels between 60–80 mmHg). This finding was independent of baseline anti-hypertensive treatment, suggesting that both ‘native’ and ‘on-treatment’ DBP lowering may have the same effect on the myocardium (hence, the presence of low DBP may be more important than the cause, whether that cause be native vascular stiffness or drug treatment, for example).
We also found weaker associations with mortality at the lowest DBP levels. Given the results for CHD, the association between low DBP and incident heart failure demonstrated in our sensitivity analysis may represent ischemic heart failure events. In contrast, there were no associations found for stroke, our ‘negative control’, which lacks biologic plausibility for increased risk according to DBP. Furthermore, a novel feature of this analysis is that our results suggest the association between DBP and CHD events may relate to subclinical myocardial injury at lower perfusion pressures, as implicated by our findings of higher hs-cTnT levels at baseline and over follow-up among participants with low DBP (Central Illustration). We note that the association between visit 2 DBP and temporal change in hs-cTnT was most pronounced over the period when hs-cTnT was next measured (6 years later at visit 4) and had little effect on hs-cTnT change between visits 4 and 5. That the association between DBP and temporal change in hs-cTnT was strong for proximate hs-cTnT measurements and weaker for distal measurements is not surprising given that BP levels are highly labile over time.
Longstanding hypertension and LVH have been shown to narrow the range of coronary perfusion auto-regulation, especially in the subendocardium. (27) Thus, in patients with hypertension and LVH, ischemia can occur with low DBP even in the absence of coronary stenosis. For example, Lindblad et al. reported that lowering of DBP in 1,121 hypertensive men with LVH by ECG increased the risk for CHD events. (28) This result is compatible with our finding that persons with subclinical myocardial damage at baseline (as indicated by hscTnT ≥14 ng/L) appear to have the highest risk of future CHD when DBP is low.
It is important to note that all of the above findings represent the results of statistical models that consider DBP in isolation. However, DBP is inextricably related to SBP. Therefore, we also evaluated the association between DBP and outcomes within sub-categories of SBP. This analysis demonstrated that the association of low DBP with both subclinical myocardial damage and incident CHD was strongest among persons with SBP ≥120–139 mmHg range. There was also a trend towards higher risk of progression of subclinical myocardial damage and incident CHD among those with DBP<60 and SBP >140 mmHg, however, due to low numbers of events in this group, our estimates were imprecise. In contrast, no trend towards myocardial damage or CHD was noted among those with DBP <60 and SBP <120 mmHg.
These results suggest that discordance between SBP and DBP (i.e., elevated pulse pressure) may be an important factor linking low DBP to myocardial outcomes. (29) Indeed, because systolic pressure is the main determinant of cardiac afterload and, thus, a primary driver of myocardial energy requirements(30), it is not surprising that our results appear to demonstrate that adverse myocardial outcomes appear most likely when both DBP is low (when myocardial energy supply is reduced due to lower coronary perfusion pressure) and SBP is above 120 mmHg (when myocardial energy demand is higher).
There are limitations to our analysis. This is an observational study and our inferences may not reflect direct causal effects. For example, we cannot know for sure whether the association between low diastolic BP and outcomes in our analysis is due to low diastolic BP from drug treatment, from arterial stiffness, or from a combination of both. The sensitivity analysis evaluating a SPRINT-eligible subsample was underpowered due to small sample size. We note that SPRINT investigators used automated oscillometric meters (8), which tend to report similar or slightly lower SBP readings than manual random zero sphygmomanometers and higher DBP readings (the latter being usually around 2.5 mmHg higher). (31,32) The longitudinal analysis of DBP and temporal change in hs-cTnT may have been influenced by significant drop-out between visits 4 and 5, despite our use of inverse-probability of attrition weighting to account for this.
In conclusion, our results suggest that low DBP levels, particularly below 60 mmHg, may harm the myocardium and are associated with subsequent CHD. However, this phenomenon appears to be most likely in clinical settings where SBP is above 120 mmHg and pulse pressure is higher. Thus, among patients who are being treated to SBP goals of 140 mmHg or lower, attention may need to be placed not only on SBP, but also, importantly, on achieved DBP. Diastolic and systolic BP are inextricably married and our results highlight the importance of not ignoring the former and focusing only on the latter, instead emphasizing the need to consider both in the optimal treatment of adults with hypertension.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Using novel high sensitivity troponin assays, this is the first study to suggest that intensive diastolic BP lowering may directly harm the myocardium. We found that diastolic BP levels <70 mmHg were independently associated with prevalent and prospective increases in high-sensitivity Troponin-T. This translated into increased coronary heart disease, heart failure and mortality among those with low diastolic BP. The adverse findings for low diastolic BP were most pronounced when systolic BP was greater than 120mmHg, and, hence, when pulse pressure was elevated.
Competency in Patient Care
Among patients who are being treated to intensive systolic BP goals of 140 mmHg or lower, attention should also be placed on achieved diastolic BP. Diastolic and systolic BP are inextricably married and our results highlight the importance of not ignoring the former and focusing only on the latter, instead emphasizing the need to consider both in the optimal treatment of adults with hypertension.
Translational Outlook
An examination of the SPRINT and HOPE-3 trial datasets to evaluate for an association between achieved diastolic BP and adverse outcomes is highly desirable, both to confirm our findings and to guide clinical practice.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Dr Ballantyne has received support from Roche Diagnostics, Abbott Diagnostics, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Pfizer, Otsuka, Regeneron, Sanofi-Synthelabo, and Takeda. Drs. Ballantyne is a co-investigator on a provisional patent filed by Roche for use of biomarkers in heart failure prediction. Drs. Ballantyne and Selvin have served on an advisory board for Roche Diagnostics.
Funding: This research was supported by NIH/NIDDK grants R01DK089174 and K24DK106414 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Abbreviations
- BMI
Body Mass Index
- CAD
Coronary Artery Disease
- CHD
Coronary Heart Disease
- CVD
Cardiovascular Disease
- DBP
Diastolic Blood Pressure
- Hs-cTnT
High Sensitivity cardiac Troponin-T
- HDL-c
High Density Lipoprotein Cholesterol
- LDL-c
High Density Lipoprotein Cholesterol
- LVH
Left Ventricular Hypertrophy
- SBP
Systolic Blood Pressure
Footnotes
Supplementary information is provided in an Online Appendix.
Disclosures: The other authors declare no commercial conflicts of interest (but receive National Institutes of Health grant funding).
References
- 1.Hay J. A British Medical Association Lecture on THE SIGNIFICANCE OF A RAISED BLOOD PRESSURE. British medical journal. 1931;2:43–47. doi: 10.1136/bmj.2.3679.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. The American journal of cardiology. 1971;27:335–346. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenman RH, Sholtz RI, Brand RJ. A study of comparative blood pressure measures in predicting risk of coronary heart disease. Circulation. 1976;54:51–58. doi: 10.1161/01.cir.54.1.51. [DOI] [PubMed] [Google Scholar]
- 4.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA : the journal of the American Medical Association. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 5.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Annals of internal medicine. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 6.Kovell LC, Ahmed HM, Misra S, et al. US Hypertension Management Guidelines: A Review of the Recent Past and Recommendations for the Future. Journal of the American Heart Association. 2015;4 doi: 10.1161/JAHA.115.002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonn EM, Bosch J, Lopez-Jaramillo P, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. The New England journal of medicine. 2016 doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 8.Group SR, Wright JT, Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2016 doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1:581–584. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 11.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA : the journal of the American Medical Association. 1991;265:489–495. [PubMed] [Google Scholar]
- 12.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Annals of internal medicine. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 13.Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? Journal of the American College of Cardiology. 2009;54:1827–1834. doi: 10.1016/j.jacc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 14.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA : the journal of the American Medical Association. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA : the journal of the American Medical Association. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers KM, Al-Shakarchi J, Berglund L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. American heart journal. 2013;166:541–548. doi: 10.1016/j.ahj.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical chemistry. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 20.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. Journal of clinical epidemiology. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke; a journal of cerebral circulation. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 22.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke; a journal of cerebral circulation. 1981;12:I1–I91. [PubMed] [Google Scholar]
- 23.Herndon JE, 2nd, Harrell FE., Jr The restricted cubic spline as baseline hazard in the proportional hazards model with step function time-dependent covariables. Statistics in medicine. 1995;14:2119–2129. doi: 10.1002/sim.4780141906. [DOI] [PubMed] [Google Scholar]
- 24.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkovic V, Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. The New England journal of medicine. 2015;373:2175–2178. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 26.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 27.Harrison DG, Florentine MS, Brooks LA, Cooper SM, Marcus ML. The effect of hypertension and left ventricular hypertrophy on the lower range of coronary autoregulation. Circulation. 1988;77:1108–1115. doi: 10.1161/01.cir.77.5.1108. [DOI] [PubMed] [Google Scholar]
- 28.Lindblad U, Rastam L, Ryden L, Ranstam J, Isacsson SO, Berglund G. Control of blood pressure and risk of first acute myocardial infarction: Skaraborg hypertension project. Bmj. 1994;308:681–686. doi: 10.1136/bmj.308.6930.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaraj S, Steg PG, Elbez Y, et al. Pulse Pressure and Risk for Cardiovascular Events in Patients With Atherothrombosis: From the REACH Registry. Journal of the American College of Cardiology. 2016;67:392–403. doi: 10.1016/j.jacc.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 30.Katz LN, Feinberg H. The relation of cardiac effort to myocardial oxygen consumption and coronary flow. Circulation research. 1958;6:656–669. doi: 10.1161/01.res.6.5.656. [DOI] [PubMed] [Google Scholar]
- 31.Stang A, Moebus S, Mohlenkamp S, et al. Algorithms for converting random-zero to automated oscillometric blood pressure values, and vice versa. American journal of epidemiology. 2006;164:85–94. doi: 10.1093/aje/kwj160. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson M, Carlberg B, Jansson JH. Comparison of blood pressure measurements between an automated oscillometric device and a Hawksley random-zero sphygmomanometer in the northern Sweden MONICA study. Blood pressure monitoring. 2012;17:164–170. doi: 10.1097/MBP.0b013e328356ef58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.