Abstract
Nutritional abnormalities are common in patients with gastroparesis (Gp), a disorder that may affect gastric motility and may delay emptying. The aim of this work was to identify relationships between serum nutrition markers including 25-OH vitamin D and gastric motility measures in Gp patients. We enrolled 59 consecutive gastric motility clinic patients (48 females, 11 males; mean age 44 years; 42 idiopathic; 17 diabetes mellitus) with Gp symptoms. The 25-OH vitamin D levels, for most patients slightly above the lower limit of normal (96.98 nmol/l ± 60.99), were lowest in diabetic range (DM) (75.68 nmol/l ± 34.22) vs. idiopathic (ID) (105.03 nmol/l ± 67.08) gastroparesis patients. First hour GET: one unit increase in 25-OH vitamin D level was associated 0.11 % improvement (95 % CI − 0.22, 0.01 p = 0.056) in gastric motility in all patients; this association, although marked in ID Gp patients, (− 0.13, CI − 0.25, − 0.01 p = 0.034), was not seen in DM Gp, (0.2, CI − 0.45, 0.87, p = 0.525). Fourth hour GET: Every unit increase of 25-OH vitamin D was associated with significant improvement in all patients, (0.11 % CI − 0.23, 0.01, p = 0.053), and some weak improvement in ID group, (0.11 % − 0.24, 0.01, p = 0.076) and absent in patients with DM (0.03, CI − 0.66, 0.72, p = 0.932). It is concluded that 25-OH vitamin D levels may influence gastric emptying. Underlying mechanisms for this observation might include the impact of 25-OH vitamin D on the health of the enteric nervous system. 25-OH vitamin D contributions to enteric nerve functions should be explored, particularly where autonomic nervous system comorbidities exist.
Keywords: gastroparesis, low vitamins D, gastric emptying test (GET), autonomic nervous system (ANS), diabetes
Introduction
Gastric motility disorders, often characterized by impaired gastric emptying in the absence of obstruction, may stem from enteric nervous system dysfunction. The symptoms of this debilitating disorder include nausea, vomiting, bloating, early satiety, dehydration, and abdominal pain, any of which can severely compromise a patient’s ability to manage nutrition, sustain health, and engage in social interactions. These symptoms can also lead to permanent disability, vulnerability to thrombosis [1], and greater mortality.
One report recently estimated the age-adjusted prevalence of gastroparesis in the United States to be 10 men and 38 women per 100 000 persons [2]. However, establishing the disorder’s actual prevalence remains controversial, owing to under-reporting. Until quite recently, delayed gastric emptying provided the primary, if not sole, criterion for a differential diagnosis of gastroparesis. An appreciation of the greater range of pathological effects occasioned by this disease, however, has rendered the diagnostic contribution of GET less definitive [3], with an interpretive dilemma arising in cases for which GET is normal, but Gp symptoms (e. g., abdominal pain) persist [4].
The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health suggests a compensatory diet for impaired gastric emptying; this diet consists of food low in fiber and fat, which may cause gastric retention in patients with gastroparesis [5]. Rising interest in dietary intake for Gp patients has led to dramatic improvement in their nutritional intake [5], but dietary effects on gastric motility remain under-recognized for patients with DM Gp and ID Gp. A recent Gastroparesis Clinical Research Consortium (GpCRC) study has indicated that nutritional consults were obtained for only 32 % of Gp patients after symptom onset; such studies were more likely for patients with longer duration of symptoms, more hospitalizations, and diabetes [5]. This fact suggests that dietary history and treatment are neglected and incomplete for patients with gastroparesis, especially those in the idiopathic subgroup [5].
The etiology of gastroparesis is unknown for 70 % of Gp patients, who are currently classified as having idiopathic gastroparesis. Interestingly, this Gp subgroup presents with multiple complex comorbidities, many of them neuromuscular and autonomic nervous system dysfunction disorders [6]. The exact mechanism of enteric autonomic nervous system (ANS) pathophysiology and gastric dysmotility is unknown; however, the association of micronutrient deficiencies with motility may help improve our understating. 25-OH vitamin D, is obtained from skin in the hormonal form 25-hydroxy (25-OH) vitamin D, is an important regulator of immune functions [7]. Most of the nucleated cells have 25-OH vitamin D receptors (VDR) and it is widely believed that T cells, which regulate autoimmune response, have both direct and indirect VDR targets. Recent publications suggest that the CD8 and NKT T cell groups express VDR to protect against the generation of autoantibodies during selection, especially in the gut [8]. Although associations of 25-OH vitamin D with various disease states including blood pressure regulation have been extensively studied in obese, nonobese, and nondiabetic individuals [7, 9, 10], there is limited data on gastric motility and low levels in obese, nonobese and nondiabetic individuals.
About 60 % of long standing diabetics develop gastrointestinal tract difficulties during their lifetime. Maintaining euglycemic states along with administration of prokinetics drugs, has been reported to help improve gastric motility or relieve gastric stasis [11]. Investigations of GET and its association with such macronutrients as glucose suggest that daily hyperglycemic states in Type 1 diabetics (16–20 mmol/l) are associated with delayed gastric emptying, while daily euglycemic states (4–8 mmol/l) are associated with normal gastric emptying [12]. Further, Reddy et al. showed that the 3-month measure of glycemic control in patients with diabetes fails to provide the specificity needed for correlation with precisely timed GET measures. Neither Reddy nor Fraser observed a significant correlation between gastric emptying and HbA1c in enteric nerve health, corroborating Fraser’s view that daily glycemic control may be of greater consequence than previously recognized [13]. A recent review by Koch and Uwaifo [14], emphasized the importance of good glycemic control to avoid gastrointestinal complication of diabetes independent of disease duration.
Diabetic patients who develop gastroparesis may provide an ideal group through which to examine nutritional factors important in gastric dysmotility. Patients with hyperinsulinemia show visceral sensory system alterations at a peripheral level presenting with gastrointestinal symptoms such as pain, bloating, nausea, and vomiting which are more frequent in prediabetic states [15]. Here, we obtained serum micronutrient levels and gastric emptying time measures from a consecutive cohort of patients for whom a diagnosis of drug-refractory diabetic and idiopathic gastroparesis had previously been established. We assessed and compared these measures to determine any relationships between abnormalities in vitamin levels and gastric delay.
Methods
From January 2009 through August 2009, we evaluated 59 sequential patients seeking treatment for symptoms of gastroparesis at their baseline visit to the University of Mississippi Medical Center (UMMC) gastroparesis clinic.
Experimental design
We obtained baseline serum nutrient levels of vitamin A, B2, B6, B12, D, E, folate, ferritin, and homocysteine; gastric emptying was evaluated via standardized scintigraphy at 1, 2, and 4 h, followed by summed ‘Total GET’. As we have observed low 25-OH vitamin D levels in all our clinical patients, we were particularly interested in this parameter. All findings, as well as symptoms and gastric emptying test (GET) recordings, were examined for potential clinical and statistical relationships.
Study patients
We evaluated a total of 59 patients (mean age; 44 years; 17 DM Gp, 42 ID Gp), 46 of whom were Caucasian and 13 of whom were African Americans. The male-to-female ratio of our study population differed by ethnicity at 1:4 for the Caucasian group and 1:3 for the African American group.
Clinical assessments
At the baseline clinic visit, historical data was obtained by the evaluating physician through patient interviews. A retrospective study of patient charts was also performed to help determine any use of nutritional supplements (prescription and over the counter) at the time of blood sample collection.
Symptom score
Each patient recorded Gp symptoms on a simple, 5-item, Patient Reported Outcome (PRO) diary which provided an easy method for noting the daily absence, or presence and severity, of vomiting, nausea, anorexia/early satiety, bloating/distension, and abdominal pain. Symptom severity was recorded on a 0–4 scale, with 0 = no symptom, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe (debilitating) symptoms. Study staff summed the 5 daily ratings (1 per symptom) to calculate each patient’s Total Symptoms Score (TSS) [16].
Blood sample collection
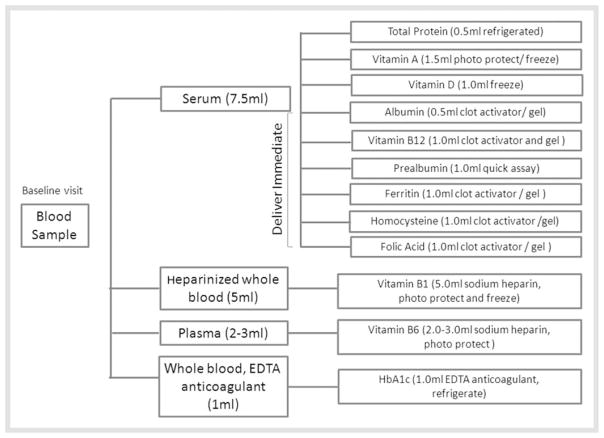
Upon completion of the clinical assessment by the attending physician, patient consent was obtained for the study blood draw protocol so as to permit micronutrient and macronutrient assays (Fig. 2).
Fig. 2.
Sample collection, processing, and storage logistics for analysis of serum micronutrition levels for this study.
Micronutrient laboratory assessments
Serum ferritin, vitamin B12, and folate
A Roche Cobas e601 immunoassay analyzer (Roche Diagnostics, Indianapolis, Indiana) was used to analyze serum samples via an electrochemiluminescent assay technique [17], based on the antibody sandwich principle.
Prealbumin, albumin, total proteins, and HbA1c
A Roche Cobas c501 chemistry analyzer (Roche Diagnostics) was used to perform immunoturbidimetric [18]and photometric assays [19], so as to quantify prealbumin and albumin and to perform TP2 assays by Biuret reaction for total protein [20].
Homocysteine
Chemiluminescent enzyme immunoassays of homocysteine were performed on serum samples using a Siemens Immulite 2500 analyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois) [21].
Vitamin B6 and 25-OH vitamin D
Liquid chromatography and tandem mass spectrometry assays [22]were performed using a 4000 QTRAP® LC/MS/MS System (Quest Diagnostics, Madison, New Jersey).
Gastric emptying test (GET)
A multicenter GET protocol [23, 24]was utilized. Patients, who had followed a ‘nothing by mouth’ diet after midnight prior to the exam were given 1.0 m Ci technetium 99m sulfur colloid in a standard egg beater meal. A single head gamma camera was used to obtain anterior and posterior abdominal planar images of the patient in an upright position at 1, 2, and 4 h, with one minute of data collection for each image. Data, expressed as percent (%) of the isotope remaining in the stomach were considered delayed if > 60 % of the isotope was seen at the 2nd hour, and > 10 % at 4th hour. Total gastric emptying times were calculated for each patient by summation of gastric retention observed at 1, 2, and 4 h, with findings interpreted by comparison with normal values (< 160 % in healthy individuals).
Statistical Analysis
Descriptive statistics were computed as means and standard deviations. Etiologic differences at baseline were examined by Student’s t-test for continuous variables and by Fisher’s exact test for categorical variables. Multiple linear regression models were used to examine associations between serum nutritional markers and gastric motility measures, as well as the effect modification of these associations by diabetic etiology. Diagnostic checks towards linearity assumptions were conducted by examining nonparametric lowess smoothers [25], and no violations were seen.
Results
Baseline measures
Serum proteins
For all patients and both groups, serum proteins fell well within the normal range (50.0–86.00 g/l) with the mean for all patients at 75.34 g/l ± 6.53; for the diabetic group at 75.73 g/l ± 8.25, and for the idiopathic group at 75.17 g/l ± 5.78.
Adequate protein intake was indicated by the overall serum albumin and prealbumin levels of both groups; these levels were within the normal range for both diabetic and idiopathic patients as reported in Table 1.
Table 1.
Baseline descriptive statistics.
| Variable | Normal range | All patients | Diabetics | Idiopathics | Group diff (p-value) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std Dev. | Mean | Std Dev. | Mean | Std Dev. | ||||
| Serum Nutrition Levels | Total protein | 50.00–86.00g/l | 75.34 | 6.53 | 75.73 | 8.25 | 75.17 | 5.78 | 0.818 |
|
| |||||||||
| Albumin | 35.0–52.0g/l | 44.57 | 3.51 | 44.08 | 4.7 | 44.76 | 2.99 | 0.560 | |
|
| |||||||||
| Prealbumin | 150–370mg/l | 275.27 | 59.39 | 275 | 60.72 | 275.36 | 60.09 | 0.988 | |
|
| |||||||||
| Vitamin A | 1.33–3.42μmol/l | 1.99 | 0.74 | 1.75 | 0.93 | 2.07 | 0.66 | 0.245 | |
|
| |||||||||
| Vitamin D | 50–250nmol/l | 96.98 | 60.99 | 75.68 | 34.22 | 105.03 | 67.08 | 0.126 | |
|
| |||||||||
| Vitamin B6 | 20.0–120nmol/l | 43.95 | 42.59 | 68.86 | 63.3 | 35.06 | 28.95 | 0.03 | |
|
| |||||||||
| Vitamin B12 | 155–700pmol/l | 539.19 | 312.82 | 650.22 | 346.98 | 494.77 | 290.88 | 0.09 | |
|
| |||||||||
| Ferritin | 40–990pmol/l | 185.73 | 201.72 | 295.87 | 304.94 | 141.67 | 121.04 | 0.01 | |
|
| |||||||||
| Homocysteine | 5–15μmol/l | 8.93 | 4.40 | 10.45 | 6.01 | 8.24 | 3.31 | 0.097 | |
|
| |||||||||
| Folate | 7.0–40.0nmol/l | 34.72 | 18.73 | 30.69 | 13.6 | 36.13 | 20.18 | 0.354 | |
|
| |||||||||
| HbA1c | (0.046–0.065mmol/mol) | 0.063 | 0.0137 | 0.0799 | 0.0153 | 0.0572 | 0.0051 | 0.010 | |
|
| |||||||||
| Gp Symptoms Scores | Vomiting | 0–4 | 1.61 | 1.42 | 1.8 | 1.49 | 1.53 | 1.4 | 0.534 |
|
| |||||||||
| Nausea | 0–4 | 2.77 | 1.14 | 3.03 | 1.01 | 2.66 | 1.19 | 0.294 | |
|
| |||||||||
| Anorexia early satiety | 0–4 | 2.38 | 1.32 | 2.23 | 1.39 | 2.43 | 1.3 | 0.626 | |
|
| |||||||||
| Bloating | 0–4 | 2.43 | 1.35 | 2.6 | 1.45 | 2.36 | 1.32 | 0.563 | |
|
| |||||||||
| Abdominal pain | 0–4 | 2.50 | 1.36 | 2.97 | 1.03 | 2.31 | 1.44 | 0.115 | |
|
| |||||||||
| Total Symptoms Score | 0–20 | 11.75 | 4.62 | 12.63 | 3.41 | 11.4 | 5.03 | 0.388 | |
|
| |||||||||
| Enteric Physiologic Measures | Gastric emptying test (1 h retention) | >90% | 59.51 | 23.52 | 59.71 | 23.49 | 59.42 | 23.91 | 0.97 |
|
| |||||||||
| Gastric emptying test (2 h retention) | >60% | 38.82 | 25.76 | 39.27 | 29.25 | 38.63 | 24.58 | 0.938 | |
|
| |||||||||
| Gastric emptying test (4 h retention) | >10% | 19.94 | 24.17 | 25.93 | 28.67 | 17.32 | 21.91 | 0.271 | |
|
| |||||||||
| Gastric emptying (total retention time) | <160% | 118.13 | 70.26 | 124.08 | 79.95 | 115.52 | 66.8 | 0.708 | |
Summary of study population demographics, stratified by diabetic and idiopathic etiologies describing serum nutrition levels, severity of Gp symptoms, and gastric emptying test. Values reported as means ± standard deviation
Serum micronutrients
Our retrospective review of clinical charts for supplement use revealed an unsurprising between-group inconsistency in the data obtained: all patients in the diabetic group (n = 17/17) had far more clinic visits and follow-up than did idiopathic patients (n = 10/42); patients with diabetic gastroparesis also had a better characterized disease profile. Moreover, all diabetic patients were using multivitamin therapies, many with high doses of vitamin B12, while fewer idiopathic patients (n = 10/42) had such nutritional supplementation.
25-OH vitamin D levels in all patients, at 96.98 nmol/l ± 60.99, were on the lower side of expected range of 50–250 nmol/l. The idiopathic group, however, had slightly higher mean 25-OH vitamin D levels, at 105.03 nmol/l ± 67.08, than did the diabetic group, at 75.68 nmol/l ± 34.22, with a between group difference of p = 0.126. A similar trend was seen for vitamin A, for which the overall mean was 1.99 μmol/l ± 0.74 (range, 1.33–3.42 μmol/l). The mean for vitamin A in the idiopathic group was slightly higher, 2.07 μmol/l ± 0.66, than in the diabetic group, at 1.75 μmol/l ± 0.93, with a between-group difference of p = 0.245 (see Table 1).
Two components of the vitamin B complex, vitamin B6 and vitamin B12, were of particular interest. The diabetic group had significantly higher levels of vitamin B6, at 68.86 nmol/l ± 63.3, than did the idiopathic group, at 35.06 nmol/l ± 28.95, p = 0.030. This trend was also true for ferritin, for which the mean level in diabetics was 295.87 pmol/l ± 304.94, but in idiopathics was 141.67 pmol/l ± 121.04, with a group difference of p = 0.010 (see Table 1).
As the widespread use of vitamin B12 therapy in patients with diabetes would suggest, vitamin B12 levels were higher in the diabetic group, at 650.22 pmol/l ± 346.98, than in the idiopathic group, at 494.77 pmol/l ± 290.88. However, this finding was not significant (p = 0.090) (see Table 1).
Serum homocysteine and ferritin levels were within the expected range for all study patients, but were significantly higher (p = 0.097, p = 0.010, respectively) in the diabetic group. For diabetics, mean homocysteine was 10.45 μmol/l ± 6.01, p = 0.097 and mean ferritin was 295.87 pmol/l ± 304.94, p = 0.01, while for the idiopathic group, these means were 8.24 μmol/l ± 3.31 and 141.67 pmol/l ± 121.04, respectively. HbA1c was expectedly higher in the diabetic group, at (0.079 mmol/mol ± 0.015), than in idiopathic patients, at (0.057 mmol/mol ± 0.01), with p < 0.010. By contrast, idiopathic patients had higher serum folate levels, at 36.13 nmol/l ± 20.18, than did diabetic patients, at 30.69 nmol/l ± 13.6; however, this difference was not significant, at p = 0.354 (See Table 1).
Gastroparesis symptoms index
The diabetic group had comparatively higher individual symptom scores for nausea (3.03 ± 1.01 vs. 2.66 ± 1.19), vomiting (1.80 ± 1.49 vs. 1.53 ± 1.40), bloating (2.60 ± 1.45 vs. 2.36 ± 1.32), and abdominal pain (2.97 ± 1.03 vs. 2.31 ± 1.44) than did the idiopathic group, which had higher scores for early satiety/anorexia (2.43 ± 1.30 vs. 2.23 ± 1.39) (see Table 1).
Gastric emptying test (GET)
Overall gastric emptying at the 4th hour was significantly delayed, with > 10 % retention in all patients (19.94 % ± 24.17). This delay was somewhat higher for diabetic patients (25.93 % ± 28.67) than for idiopathic patients (17.32 % ± 21.91). Gastric delay at the 1st and 2nd hour measures [26]were almost identical for both groups (see Table 1).
Gastric emptying and vitamins
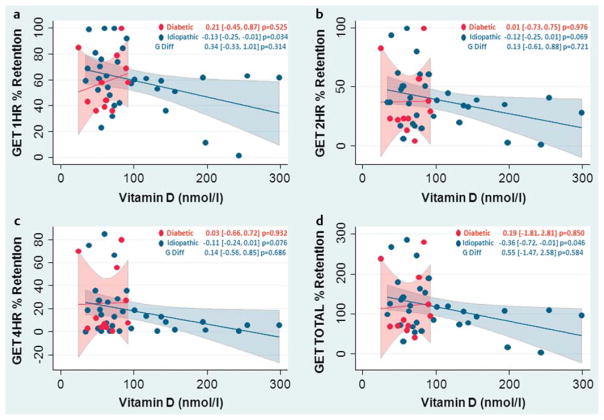
In idiopathic patients a varying degrees of improvement in gastric emptying time accompanied increased micronutrient levels for almost all vitamins were assessed. A weak association with 25-OH vitamin D levels was seen during the first 2 h of the 4-h GET testing period, as measured at the 1st and 2nd hour (Fig. 1, Table 2). However, an association between increased 25-OH vitamin D levels and improved GET measures for these patients approached statistical significance at the 4th hour, at (− 0.11, CI − 0.24, 0.01, p = 0.076). Vitamin B12 had differential group effects on gastric motility. Diabetic patients, all of whom used a vitamin B complex or vitamin B12 supplements and/or injections, had higher levels of serum B12 that were related to worsened mean 2nd and 4th hour GET measures, at (0.04, CI 0.01, 0.08, p = 0.057) and (0.04, CI 0.01, 0.08, p = 0.031) respectively. By contrast, in the idiopathic group, increasing vitamin B12 was associated with a mild improvement in 2nd d and 4th hour GET values at (− 0.02, CI − 0.05, 0.01, p = 0.144) and (− 0.02, CI − 0.05, 0.01, p = 0.140), respectively (see Table 2).
Fig. 1.
Increasing 25-OH vitamin D levels in the serum show an association with improving gastric emptying throughout the 4-h study period in the idiopathic Gp population. There is an association seen at the 2nd hour b, p = 0.069 and total test period (summation of all 4 h GET recordings), p = 0.046 d being statistically significant. At the 1st and 4th hour, however, this relationship is very strong at p = 0.034 a and p = 0.076 c. No associations were observed in the diabetic population for gastric emptying and 25-OH vitamin D levels.
Table 2.
Vitamin complex and other nutritional parameters investigated.
| All patients (n = 59) | ||||
|---|---|---|---|---|
| GET 1 h | GET 2 h | GET 4 h | ||
| Vitamin B6 | All patients | −0.17 (− 0.42, 0.09), p = 0.194 | −0.04 (− 0.32, 0.25), p = 0.795 | 0.00 (− 0.26, 0.26), p = 0.985 |
|
| ||||
| Diabetics | 0.29 (−0.10, 0.68), p = 0.144 | 0.38 (− 0.08, 0.84), p = 0.098 | 0.29 (− 0.14, 0.73), p = 0.175 | |
|
| ||||
| Idiopathic | −0.42 (− 0.72,− 0.12), p = 0.007 | −0.25 (− 0.60, 0.09), p = 0.146 | −0.16 (− 0.49, 0.16), p = 0.316 | |
|
| ||||
| Difference of slopes | 0.71 (0.22, 1.21), p=0.006 | 0.64 (0.06, 1.21), p=0.031 | 0.46 (− 0.08, 1.00), p = 0.095 | |
|
| ||||
| Vitamin B12 | All patients | 0.01 (−0.02, 0.02), p = 0.811 | 0.01 (− 0.02, 0.02), p = 0.975 | 0.01 (− 0.02, 0.03), p = 0.680 |
|
| ||||
| Diabetics | 0.03 (−0.01, 0.06), p = 0.161 | 0.04 (0.01, 0.08), p = 0.057 | 0.04 (0.01, 0.08), p = 0.031 | |
|
| ||||
| Idiopathic | −0.02 (− 0.05, 0.01), p = 0.149 | −0.02 (− 0.05, 0.01), p = 0.144 | −0.02 (− 0.05, 0.01), p = 0.140 | |
|
| ||||
| Difference of slopes | 0.05 (0.01, 0.09), p = 0.050 | 0.06 (0.01, 0.11), p = 0.018 | 0.06 (0.01, 0.10), p = 0.010 | |
|
| ||||
| Vitamin A | All patients | −2.14 (− 15.88, 11.59), p = 0.753 | 4.82 (− 10.10, 19.73), p = 0.515 | 1.69 (− 11.69, 15.07), p = 0.799 |
|
| ||||
| Diabetics | 9.81 (−14.76, 34.38), p = 0.421 | 20.37 (− 6.01, 46.75), p = 0.125 | 19.31 (− 3.95, 42.58), p = 0.100 | |
|
| ||||
| Idiopathic | −8.22 (− 25.14, 8.69), p = 0.421 | −3.03 (− 21.19, 15.14), p = 0.736 | −6.57 (− 22.58, 9.45), p = 0.409 | |
|
| ||||
| Difference of slopes | 18.03 (−11.80, 47.87), p = 0.227 | 23.40 (− 8.63, 55.43), p = 0.146 | 25.88 (− 2.37, 54.13), p = 0.071 | |
|
| ||||
| 25-OH Vitamin D | All patients | −0.11 (− 0.22, 0.00), p = 0.056 | −0.11 (− 0.23, 0.02), p = 0.088 | −0.11 (− 0.23, 0.01), p = 0.053 |
|
| ||||
| Diabetics | 0.21 (−0.45, 0.87), p = 0.525 | 0.01 (− 0.73, 0.75), p = 0.976 | 0.03 (− 0.66, 0.72), p = 0.932 | |
|
| ||||
| Idiopathic | −0.13 (− 0.25,− 0.01), p = 0.034 | −0.12 (− 0.25, 0.01), p = 0.069 | −0.11 (− 0.24, 0.01), p = 0.076 | |
|
| ||||
| Difference of slopes | 0.34 (−0.33, 1.01), p = 0.314 | 0.13 (− 0.61, 0.88), p = 0.721 | 0.14 (− 0.56, 0.85), p = 0.686 | |
|
| ||||
| HbA1c | All patients | 0.74 (−14.15, 15.63), p = 0.921 | 2.02 (− 3.12, 7.17), p = 0.433 | 4.16 (− 0.66, 8.97), p = 0.089 |
|
| ||||
| Diabetics | 5.74 (−2.77, 14.26), p = 0.181 | 5.59 (− 3.74, 14.92), p = 0.233 | 6.66 (− 2.04, 15.36), p = 0.130 | |
|
| ||||
| Idiopathic | 0.74 (−14.15, 15.63), p = 0.921 | −1.48 (− 17.79, 14.83), p = 0.856 | −2.92 (− 18.12, 12.29), p = 0.701 | |
|
| ||||
| Difference of slopes | 5.01 (−12.15, 22.16), p = 0.559 | 7.07 (− 11.72, 25.86), p = 0.452 | 9.57 (− 7.95, 27.09), p = 0.277 | |
|
| ||||
| Folic acid | All patients | 0.04 (−0.32, 0.40), p = 0.812 | −0.04 (− 0.43, 0.35), p = 0.836 | −0.16 (− 0.53, 0.21), p = 0.384 |
|
| ||||
| Diabetics | 0.53 (−0.58, 1.65), p = 0.342 | 0.76 (− 0.45, 1.96), p = 0.211 | 0.66 (− 0.46, 1.77), p = 0.241 | |
|
| ||||
| Idiopathic | −0.01 (− 0.40, 0.38), p = 0.974 | −0.13 (− 0.55, 0.29), p = 0.541 | −0.23 (− 0.62, 0.16), p = 0.238 | |
|
| ||||
| Difference of slopes | 0.54 (−0.64, 1.72), p = 0.364 | 0.88 (− 0.39, 2.16), p = 0.168 | 0.89 (− 0.29, 2.07), p = 0.136 | |
|
| ||||
| Homocysteine | All patients | −1.63 (− 3.06,− 0.21), p = 0.026 | −1.31 (− 2.96, 0.34), p = 0.118 | −0.82 (− 2.43, 0.79), p = 0.309 |
|
| ||||
| Diabetics | −0.77 (− 2.65, 1.11), p = 0.413 | −0.43 (− 2.64, 1.77), p = 0.695 | −0.88 (− 3.03, 1.26), p = 0.412 | |
|
| ||||
| Idiopathic | −3.01 (− 5.22,− 0.80), p = 0.009 | −2.70 (− 5.29,− 0.11), p = 0.041 | −1.22 (− 3.74, 1.30), p = 0.336 | |
|
| ||||
| Difference of slopes | 2.24 (−0.67, 5.14), p = 0.128 | 2.27 (− 1.13, 5.67), p = 0.185 | 0.34 (− 2.97, 3.64), p = 0.839 | |
|
| ||||
| Ferritin | All patients | 0.01 (−0.03, 0.03), p = 0.957 | 0.01 (− 0.04, 0.03), p = 0.913 | −0.01 (− 0.04, 0.03), p = 0.682 |
|
| ||||
| Diabetics | −0.01 (− 0.05, 0.03), p = 0.708 | 0.01 (− 0.05, 0.04), p = 0.837 | −0.01 (− 0.06, 0.03), p = 0.498 | |
|
| ||||
| Idiopathic | 0.02 (−0.05, 0.08), p = 0.596 | 0.01 (− 0.07, 0.07), p = 0.985 | −0.02 (− 0.08, 0.05), p = 0.592 | |
|
| ||||
| Difference of slopes | −0.02 (− 0.10, 0.05), p = 0.517 | 0.01 (− 0.09, 0.08), p = 0.926 | 0.01 (− 0.08, 0.08), p = 0.922 | |
Serum micronutrients, vitamin B6, B12, A, and D and association with gastric emptying test measures and group differences in this measure in diabetic and idiopathic Gp groups are shown. Results reported as slope (95 % CI) p-value. Other serum markers like HbA1c, folic acid, ferritin, and homocysteine were investigated in this study for an association with gastric dysmotility in diabetic and idiopathic Gp groups
For diabetic patients, serum vitamin B6 levels did not show any association with gastric motility functions. However, an increasing level of vitamin B6 in the idiopathic group was related to improved 1st hour gastric emptying measures, at (− 0.42, CI − 0.72, − 0.12, p = 0.007). This association was weaker at both 2nd hour and 4th hour measures, as reported in Table 2. However, these results are limited by the fact that only 50 % of all idiopathic patients received nutrional supplements compared to 100 % in the diabetic group.
Gastric emptying and serum proteins
A statistically significant group difference was seen for the DM Gp and ID Gp cohorts between prealbumin levels and 2nd hour GET, at (40.40, CI 0.04, 0.76, p = 0.029), and these levels and 4th hour GET, at (0.43, CI 0.06, 0.81, p = 0.023).
Albumin and prealbumin levels fell within the expected physiological range of 35.0–52.0 g/l and 150–370 g/l, respectively for all patients in our study. No associations were observed between micronutrient levels for HbA1c, folate, homocysteine, and ferritin and gastric emptying values, nor between these micronutrient levels and patient reported values for symptoms of gastroparesis.
Discussion
Our investigation of gastric emptying time and vitamin levels sought to identify any relationships between nutritional status and impaired motility in patients with gastroparesis. Nutritional deficiencies have been observed for these patients, but not well described in relation to GET.
In this study, we were able to identify associations between micronutrient deficiencies and impaired gastric motility. Low serum levels observed in our study could be due to dietary deficiency and/or possible 25-OH vitamin D sequestration in obese conditions [27], or volumetric dilution [28]of this fat soluble vitamin suggesting a potential risk of developing diabetes which may be reduced by the intake of dietary fiber in whole grain products [29]. A recent report on increasing fasting glucose levels in metabolic syndrome patients with low 25-OH vitamin D, suggests a potential risk of developing diabetes [30]. Preliminary studies from our lab of full thickness stomach biopsies obtained from body antral junction region demonstrate immune filtration of the myenteric plexus and presence of inflammatory T cells (CD4, CD8, and CD68) in this region affecting gastric motor neurons and Cajal cell counts [31]in all patients with gastroparesis. A low serum 25-OH vitamin D level and its association with delayed gastric transit at the 1st, 2nd, and 4th hour recording in the idiopathic GP population could be a reflection of worsening enteric nervous function and pathophysiology. Understanding the exact mechanism by which 25-OH vitamin D improves gastric transit may also help provide the missing links in understanding the pathophysiology of idiopathic Gp. High dose treatment with 25-OH vitamin D reportedly improves the immune response via immunomodulation and decreases inflammation surrounding the motor neurons in addition to increasing neurotrophic factors like glial cell line-derived neurotrophic factor (GDNF), and insulin-like growth factor-I (IGF-1) [32, 33].
25-OH vitamin D’s role as a hormone with potent immunomodulatory effects has been demonstrated in various monocytes, T- and B-lymphocytes and macrophages which not only express VDR receptors, but also possess required enzyme 25-hydroxyvitamin D3-1α-hydroxylase, to synthesize 25-OH vitamin D locally [34]. Similar immunomodulation effects of 25-OH vitamin D on the enteric nervous system may be responsible for improving gastric motility and should be explored.
More recent studies recommend high serum levels of 25-OH vitamin D in all diabetics [35, 36]due to its beneficial effects by improving B-cell function and peripheral insulin sensitivity. However, we did not see any association between serum 25-OH vitamin D levels and gastric motility (GET), suggesting a different mechanism of pathophysiology for gastric motility impairment in this cohort. Nonetheless, the role of 25-OH vitamin D and its beneficial effects in improving glycemic control cannot be overlooked and therapeutic supplementation is highly recommended in all diabetic patients with serum 25-OH vitamin D levels less than 50 nmols/l. Our results indicate that adjuvant nutritional supplementation therapy with a multivitamin complex, and especially with 25-OH vitamin D, may help normalize delays in gastric emptying, and thereby improve chronic Gp symptoms.
However, our unexpected finding in patients with diabetic gastroparesis of a correlation between GET and B12 levels suggests that attention to the nutritional status of these patients, and caution in prescribing supplements. Elevated B12 levels in patients with diabetes may worsen a preexisting enteric nervous system vulnerability leading to declining gastric motility functions. Moreover, Yang and Cook [37]reported cases of false normal results of vitamin B12 levels that were generated by automated analyzers mandating further investigations on abnormal serum B12 levels in these patients
Serum proteins in our study also showed an unexpected association with gastric motility in the diabetic cohort, with increasing levels appearing to worsen gastric emptying.
Future Studies
Our findings suggest that further studies are needed to assess mechanisms through which 25-OH vitamin D may improve enteric neuropathy in patients with gastroparesis [38].
The contribution of 25-OH vitamin D to improved contractility of bronchial smooth muscle cells has been recently demonstrated [39]. Further, a similar role in smooth muscular coupling and contraction mechanisms to that observed in smooth muscle contractions of the bladder, which occur via L-type calcium channels, has been suggested for 25-OH vitamin D [40].
Conclusion
Our study findings indicate that an association between low 25-OH vitamin D levels and impaired motility exists in gastroparesis. These findings further argue for an assessment of serum 25-OH vitamin D levels in conjunction with GET for all Gp patients at baseline clinic visits. We would further suggest that all patients with gastroparesis and particularly those with chronic and medically refractory Gp, should receive a nutritional assessment, followed by appropriate supplementation to ensure adequate micronutrient levels.
Future studies assessing the effects of 25-OH vitamin D on gastric smooth muscle contractility are urgently needed.
Acknowledgments
The authors would like to thank Olivia R Henry, MS Ph.D., Valerie M McNaire, LPNII and Margaret Smith RN II. for patient recruitment and sample collection. We would also like to thank the staff of the GI Division, GI Laboratory, Department of Nuclear Medicine and Department of Pathology laboratory at the University of Mississippi Medical Center for their help with this study.
Footnotes
Conflict of Interest
No conflicts of interest exist; Dr. Thomas Abell is a licensee, consultant, and investigator for Medtronic, Inc.
References
- 1.Creel WB, Abell TL, Lobrano A, Deitcher SR, Dugdale M, Smalley D, Johnson WD. To clot or not to clot: are there predictors of clinically significant thrombus formation in patients with gastroparesis and prolonged IV access? Dig Dis Sci. 2008;53:1532–1536. doi: 10.1007/s10620-007-0040-x. [DOI] [PubMed] [Google Scholar]
- 2.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in. Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson PH. Diabetic gastroparesis: a review. J Diabetes Complications. 1996;10:113–122. doi: 10.1016/1056-8727(96)00001-3. [DOI] [PubMed] [Google Scholar]
- 4.Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676–681. doi: 10.1016/j.cgh.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Parkman HP, Yates KP, Hasler WL, Nguyan L, Pasricha PJ, Snape WJ, Farrugia G, Calles J, Koch KL, Abell TL, McCallum RW, Petito D, Parrish CR, Duffy F, Lee L, Unalp-Arida A, Tonascia J, Hamilton F NIDDK Gastroparesis Clinical Research Consortium. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141:486–498. doi: 10.1053/j.gastro.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 7.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees (N) population. Horm Metab Res. 2009;41:402–407. doi: 10.1055/s-0028-1128131. [DOI] [PubMed] [Google Scholar]
- 8.Cantorna MT, Zhao J, Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc. 2012;71:62–66. doi: 10.1017/S0029665111003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah MI, Uwaifo GI, Nicholas WC, Koch CA. Does vitamin d deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol. 2010;2010:579640. doi: 10.1155/2010/579640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsammak MY, Al-Wosaibi AA, Al-Howeish A, Alsaeed J. Vitamin D deficiency in Saudi Arabs. Horm Metab Res. 2010;42:364–368. doi: 10.1055/s-0030-1248296. [DOI] [PubMed] [Google Scholar]
- 11.Perusicová J. Gastrointestinal complications in diabetes mellitus. Vnitr Lék. 2004;50:338–343. [PubMed] [Google Scholar]
- 12.Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 13.Reddy S, Ramsubeik K, Vega KJ, Federico J, Palacio C. Do HbA1C Levels correlate with delayed gastric emptying in diabetic patients? J Neurogastroenterol Motil. 2010;16:414–417. doi: 10.5056/jnm.2010.16.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch CA, Uwaifo GI. Are gastrointestinal symptoms related to diabetes mellitus and glycemic control? Eur J Gastroenterol Hepatol. 2008;20:822–825. doi: 10.1097/MEG.0b013e3282f5f75e. [DOI] [PubMed] [Google Scholar]
- 15.Søfteland E, Dimcevski G, Graversen C, Nedrebø BG, Drewes AM, Frøkjær JB. Effects of isolated hyperinsulinaemia on sensory function in healthy adults. Exp Clin Endocrinol Diabetes. 2011;119:604–609. doi: 10.1055/s-0031-1286316. [DOI] [PubMed] [Google Scholar]
- 16.Daram SR, Tang SJ, Abell TL. Video: temporary gastric electrical stimulation for gastroparesis: endoscopic placement of electrodes (ENDOstim) Surg Endosc. 2011;25:3444–3445. doi: 10.1007/s00464-011-1710-5. [DOI] [PubMed] [Google Scholar]
- 17.Yong QW, Chan SP, Cheng A. Homocysteine, folate and vitamin B12 as risk factors for acute myocardial infarction in a Southeast Asian population. Ann Acad Med Singapore. 2002;31:636–640. [PubMed] [Google Scholar]
- 18.Kleine TO, Merten B. Rapid manual immunoturbidimetric and immunonephelometric assays of prealbumin, albumin, IgG, IgA and IgM in cerebrospinal fluid. J Clin Chem Biochem. 1980;18:245–254. doi: 10.1515/cclm.1980.18.4.245. [DOI] [PubMed] [Google Scholar]
- 19.Gibson RB, Carr TL, Green S, Fowler WM. Photometric assay of plasma heparin. Proc Soc Exp Biol Med. 1952;79:577–580. doi: 10.3181/00379727-79-19452. [DOI] [PubMed] [Google Scholar]
- 20.Hatcher DW, Anderson NG. GeMSAEC: a new analytic tool for clinical chemistry total serum protein with the biuret reaction. Am J Clin Pathol. 1969;52:645–651. doi: 10.1093/ajcp/52.6.645. [DOI] [PubMed] [Google Scholar]
- 21.Brown CR, Higgins KW, Frazer K, Schoelz LK, Dyminski JW, Marinkovich VA, Miller SP, Burd JF. Simultaneous determination of total IgE and allergen-specific IgE in serum by the MAST chemiluminescent assay system. Clin Chem. 1985;31:1500–1505. [PubMed] [Google Scholar]
- 22.Midttun O, Hustad S, Solheim E, Schneede J, Ueland PM. Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin Chem. 2005;51:1206–1216. doi: 10.1373/clinchem.2005.051169. [DOI] [PubMed] [Google Scholar]
- 23.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Jr, Ziessman HA American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 24.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Rydén J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Smoothing. In: Cox DR, Hinkley DV, Rubin D, Silverman BW, editors. Generalized Additive Models. New York: Chapman and Hall; 1990. pp. 9–38. [Google Scholar]
- 26.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Jr, Ziessman HA American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 27.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–396. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 28.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 29.Kaline K, Bornstein SR, Bergmann A, Hauner H, Schwarz PE. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Horm Metab Res. 2007;39:687–693. doi: 10.1055/s-2007-985811. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, Jialal G, Cook T, Siegel D, Jialal I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm Metab Res. 2011;43:72–74. doi: 10.1055/s-0030-1268485. [DOI] [PubMed] [Google Scholar]
- 31.Kedar A, Vedanarayanan V, Subramony C, Lahr CJ, Sunesara I, Griswold ME, Marshall GD, Abell TL. Quantification of Inflammation in the Enteric Plexus: Differences in Patients With Gastroparesis Who Have Acute vs. Non-Acute Symptom Onset. Gastroenterology. 2011:Sa2032.373. [Google Scholar]
- 32.Karam C, Scelsa SN. Can vitamin D delay the progression of ALS? Med Hypotheses. 2011;76:643–645. doi: 10.1016/j.mehy.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Mason RS. Vitamin D: a hormone for all seasons. Climacteric. 2011;14:197–203. doi: 10.3109/13697137.2010.514366. [DOI] [PubMed] [Google Scholar]
- 34.Reichrath J, Lehmann B, Carlberg C, Varani J, Zouboulis CC. Vitamins as hormones. Horm Metab Res. 2007;39:71–84. doi: 10.1055/s-2007-958715. [DOI] [PubMed] [Google Scholar]
- 35.Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, Rissanen H, Montonen J, Reunanen A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 36.Blanton D, Han Z, Bierschenk L, Linga-Reddy MV, Wang H, Clare-Salzler M, Haller M, Schatz D, Myhr C, She JX, Wasserfall C, Atkinson M. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes. 2011;60:2566–2570. doi: 10.2337/db11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang DT, Cook RJ. Spurious elevations of vitamin B12 with pernicious anemia. N Engl J Med. 2012;366:1742–1743. doi: 10.1056/NEJMc1201655. [DOI] [PubMed] [Google Scholar]
- 38.Bossé Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 39.Morelli A, Squecco R, Failli P, Filippi S, Vignozzi L, Chavalmane AK, Fibbi B, Mancina R, Luciani G, Gacci M, Colli E, Francini F, Adorini L, Maggi M. The vitamin D receptor agonist elocalcitol upregulates L-type calcium channel activity in human and rat bladder. Am J Physiol Cell Physiol. 2008;294:C1206–C1214. doi: 10.1152/ajpcell.90634.2007. [DOI] [PubMed] [Google Scholar]
- 40.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10:377–385. [PubMed] [Google Scholar]