Abstract
Objective
This study evaluates treatment of gastroparesis patients refractory to gastric electrical stimulation (GES) therapy with surgical replacement of the entire GES system.
Summary Background Data
Some patients who have symptomatic improvement with GES later develop recurrent symptoms. Some patients improve by simply altering pulse parameter settings. Others continue to have symptoms with maximized pulse parameters. For these patients, we have shown that surgical implantation of a new device and leads at a different gastric location will improve symptoms of gastroparesis.
Methods
This study evaluates 15 patients with recurrent symptoms after initial GES therapy who subsequently received a second GES system. Positive response to GES replacement therapy is evaluated by symptoms scores for vomiting, nausea, epigastric pain, early satiety, and bloating using a modified Likert score system, 0 to 4.
Results
Total symptom scores improved for 12 of 15 patients with GES replacement surgery. Total score for the replacement group decreased from 17.3 ± 1.6 to 13.6 ± 3.7 with a difference of 3.6 (P value = .017). This score is compared with that of the control group with a preoperative symptom score of 15.8 ± 3.6 and postoperative score of 12.3 ± 3.5 with a difference of 3.5 (P value = .011). The control group showed a 20.3% decrease in mean total symptoms score, whereas the study group showed a 22.5% decrease in mean with an absolute reduction of 2.2.
Conclusion
Reimplantation of a GES at a new gastric location should be considered a viable option for patients who have initially failed GES therapy for gastroparesis.
Keywords: gastric electrical stimulation, gastroparesis, Enterra therapy
Introduction
Gastroparesis is a disabling condition characterized by decreased gastric motility, producing symptoms such as nausea, vomiting, early satiety, bloating, and epigastric pain. Gastroparesis is defined as delayed gastric emptying in the absence of mechanical obstruction.1–9 In mild disease, treatment can be achieved with dietary management, including following a low-fiber and low-fat diet, making food particle sizes small, and having frequent and small meals.10,11 In moderate to severe disease, medical therapy is added. Common pharmaceuticals include pro-kinetic medications (metoclopramide, domperidone, erythromycin, and azithromycin), antiemetics (ondansetron and mirtazapine), tricyclic antidepressants, and the peptide hormone ghrelin.12–21 Gastroparesis symptoms refractory to dietary and pharmaceutical modifications may respond to surgical therapy with gastric electrical stimulation (GES). GES is used to treat gastroparesis by delivering high-energy depolarizing stimuli to the stomach above the physiological slow-wave frequency of 3 cpm. This promotes gastric emptying and reduces symptoms of gastroparesis. GES therapy with the Enterra system has been shown to (1) reduce vomiting; (2) improve nausea and vomiting better than placebo; (3) reduce total symptom severity scores from severe to mild-moderate ranges; (4) increase quality of life; (5) increase weight; (6) decrease use of medications; (7) decrease the use of J-tubes; and (8) decrease medical billing costs compared with medical therapy alone.22–29,32
Although it is uncommon, some patients who have symptomatic improvement with GES later develop recurrent symptoms. Sometimes, this can be corrected by altering device settings such as voltage, amplitude, and impedance to the external GES controller. Other complications with GES include lead displacement, dead battery, defective device, or infection around the device. These complications are easily corrected with an additional procedure to replace the dysfunctional component of the device. However, even without these complications, some patients still fail to respond to a functioning stimulator with correct placement of leads. This study evaluates treatment of GP patients refractory to GES therapy by replacing the entire GES system. We expect that the symptom scores of patients who undergo replacement GES therapy will significantly decrease.
Methods
Study Design
Data were retrospectively collected on all patients.
Patients
Study Group
A total of 15 patients with recurrent symptoms after initial GES therapy received a second implantation of a new GES system. Of these, 12 patients had a preoperative diagnosis of idiopathic gastroparesis, whereas 3 patients had diabetic gastroparesis. Of the 15 who underwent replacement GES surgeries, 11 were women and 4 were men, with an average age of 44 (range = 30–63) years. Recurrent symptoms developed on average 48 months (range = 9–130 months) after initial GES placement.
Control Grou
For the control arm, 15 patients with positive response to GES therapy without developing recurrent symptoms were selected from a pool of 87 patients matched by the 3 variables of investigator-derived independent outcome score (IDIOMS), baseline symptom scores before initial GES implantation, and etiology of disease (ie, diabetic or idiopathic).
Criteria
Each patient met specific indications to receive GES replacement surgery. All patients had a preoperative diagnosis of drug-refractory diabetic, idiopathic, or postsurgical gastroparesis and disordered gastric emptying with or without significant weight change. Patients who initially had symptomatic improvement with GES and later developed recurrent symptoms were evaluated for GES replacement therapy. They were evaluated over a 1- to 2-week period with insertion of a temporary endoscopic gastric stimulator. Patients with a positive response to temporary stimulation received GES replacement surgery. Common gastroparesis symptoms were evaluated before and after GES replacement using the Likert score system (0 to 4). These symptoms include vomiting, nausea, epigastric pain, early satiety, and bloating. Additionally, preoperative gastric emptying times (GETs) and serosal electrogastrogram (EGG) recordings for frequency and amplitude at the time of GES placement were evaluated to reinforce physiological similarity between the 2 groups.
Procedure
Prior to the replacement GES procedure, patients undergo a trial, temporary GES to assess if the replacement procedure is warranted. During trial GES, a temporary cardiac pacing lead is placed endoscopically through the nose and inserted into the gastric mucosa in the middle of the stomach. After 1 to 2 weeks of temporary stimulation, patients are revaluated for symptom improvement. Patients with positive response receive GES replacement.
GES replacement surgery is similar to the initial GES implantation. The old pacemaker and leads are left in place to avoid contamination and damage to underlying muscle. A new pacemaker pocket is made for insertion of a new GES box. New gastric electrodes are inserted in a different location of the seromuscular layer on the anterior surface between the middle and medial third of the stomach. The stomach is examined endoscopically to make sure the new leads have not penetrated the lumen of the stomach. An EGG is used to record gastric myoelectric activity and for GES programming.
Analysis
Data were analyzed using a paired t test and reported as mean ± standard deviation.
Results
Symptoms scores of the gastroparesis patients who had recurrent symptoms after an initial good response and who subsequently received GES replacement (the study group) were compared with those of gastroparesis patients without recurrent symptoms with initial good response to GES (the control group). In Table 1, we compare the total symptom scores before and after GES therapy for all 5 gastroparesis symptoms using the Likert scale (0–4) for a total scale of 0 to 20. Baseline scores for both groups were recorded prior to initial GES placement, whereas postoperative scores were recorded after the replacement surgery (or after initial GES placement for the control group). Of 15 patients, 12 with replacement GES showed improvement in total symptom scores post–GES replacement. In Table 2, we compare preoperative and postoperative symptom scores for individual symptoms, the mean difference in preoperative and postoperative scores for each group, and the significant difference between the preoperative and postoperative scores. There were statistically significant improvements in vomiting, early satiety, and total symptoms scores for the replacement GES group. In Table 3, we reinforce the physiological similarity between the 2 groups and compare mean serosal EGG values. EGG data include frequency; amplitude; frequency/amplitude ratio; GET at 1, 2, and 4 hours; and total GET.
Table 1.
List of Baseline and Postoperative Total Symptom Scores in Both Groups, 0 to 20.
| Baseline Symptom Scores in Patients Without Recurrent Symptoms | Postoperative Symptom Scores in Patients Without Recurrent Symptoms | Baseline Symptom Scores in Patients With Recurrent Symptoms | Postoperative Symptom Scores in Patients With Recurrent Symptoms |
|---|---|---|---|
| 20 | 13.5 | 18.5 | 15.5 |
| 16 | 18 | 19 | 10 |
| 18.5 | 9 | 17.5 | 17.5 |
| 15 | 14 | 15 | 16 |
| 19 | 8 | 18.5 | 0 |
| 18 | 12.5 | 19 | 15.5 |
| 18 | 8.5 | 18 | 9 |
| 17 | 15 | 16 | 10 |
| 13.5 | 11 | 13.5 | 18.5 |
| 16 | 16 | 16 | 19.5 |
| 16 | 14 | 18.5 | 17 |
| 17.5 | 17 | 18 | 10 |
| 16.5 | 16 | 19 | 16.5 |
| 10 | 8.5 | 17.5 | 15.5 |
| 6 | 8 | 18 | 12.5 |
Table 2.
Comparison of Mean Preoperative and Mean Postoperative Symptom Scores.
| Mean of Preoperative Scores | Mean of Postoperative Scores | Difference in Symptom Scores | P Value of Difference | |
|---|---|---|---|---|
| Replacement (n = 15) | ||||
| Vomiting ± SD | 3.2 ± 1.3 | 2.1 ± 1.3 | 1.2 | .001 |
| Nausea ± SD | 3.9 ± 0.3 | 3.3 ± 1.1 | 0.6 | .088 |
| Early satiety ± SD | 3.3 ± 0.6 | 2.4 ± 1.5 | 0.9 | .041 |
| Bloating ± SD | 3.3 ± 0.6 | 2.5 ± 1.5 | 0.8 | .057 |
| Epigastric pain ± SD | 3.6 ± 0.6 | 3.3 ± 1.4 | 0.3 | .24 |
| Total score ± SD | 17.3 ± 1.6 | 13.6 ± 3.7 | 3.6 | .017 |
| No replacement (n = 15) | ||||
| Vomiting ± SD | 3.0 ± 0.9 | 2.0 ± 1.5 | 1.0 | .019 |
| Nausea ± SD | 3.2 ± 1.2 | 3.0 ± 0.9 | 0.2 | .5 |
| Early satiety ± SD | 3.1 ± 1.0 | 2.4 ± 1.1 | 0.7 | .019 |
| Bloating ± SD | 2.8 ± 1.2 | 2.6 ± 1.1 | 0.2 | .7 |
| Epigastric pain ± SD | 3.1 ± 1.4 | 3.0 ± 1.2 | 0.1 | .88 |
| Total score ± SD | 15.8 ± 3.6 | 12.3 ± 3.5 | 3.5 | .011 |
Abbreviation: SD, standard deviation.
Table 3.
Comparison of Mean Serosal EGG Values.a
| Replacement (n = 15) | No Replacement (n = 15) | P Value | Normal EGG Values | |
|---|---|---|---|---|
| Frequency ± SD | 5.5 ± 3.0 | 5.8 ± 1.5 | .73 | 3.0 ± 0.3 |
| Amplitude ± SD | 0.44 ± 0.6 | 0.6 ± 0.6 | .54 | 0.5 |
| Frequency/Amplitude ratio ± SD | 31.2 ± 31.5 | 32.4 ± 42.3 | .94 | <10 |
| Gastric emptying time (GET), 1, 2, 4 hours (%) | 72, 49, 25 | 76, 48, 22 | .61, .96, .77 | |
| Total GET (%) ± SD | 146 ± 59 | 146 ± 60 | .99 |
Abbreviations: EGG, electrogastrogram; SD, standard deviation.
Table is displayed to reinforce physiological similarity between the 2 groups.
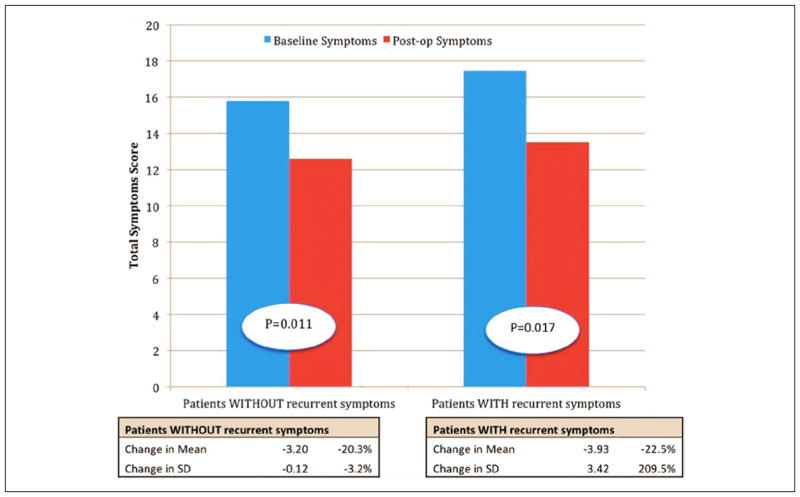
Figure 1 shows the change in mean total symptoms score of the study group compared with that of the control group. Patients without recurrent symptoms (control group) show a 20.3% decrease in mean total symptoms score, whereas patients with recurrent symptoms and GES replacement therapy (study group) show a 22.5% decrease in mean with an absolute reduction of 2.2. This is consistent with what is found in the literature. Musunuru et al30 also found no significant differences in preoperative total symptom scores for GES responders and nonresponders.
Figure 1.
Comparison of the change in mean total symptoms score in patients without (first pair) and with recurrent symptoms (second pair); blue = baseline symptoms, red = postoperative symptoms.
Discussion
GES for gastroparesis is becoming the treatment of choice for gastroparesis refractory to dietary and medical treatment.25,26,28,29 Although GES therapy relieves symptoms in many patients, there is much uncertainty of how to predict response to therapy. Positive predictors of GES response are diabetic versus idiopathic gastroparesis, no analgesic use, normal interstitial cells of cajal (ICC), and absence of mental health factors.30,31 Recent studies suggest that decreased ICC can be predicted by tachygastric rhythm on EGG and may be of use in the future. In our study, 15 patients with GES had an initial positive response to GES and later developed recurrent symptoms. These patients received temporary endoscopic gastric electric stimulation for 1 week and showed symptomatic improvement. With positive response to temporary GES, a second surgery was planned for GES reimplantation. Of the 15 patients, 12 had improved gastroparesis symptoms after GES replacement.
Although there are many unknown factors to GES response, scarring of the gastric wall around the electrodes was correlated with decreased relief with GES therapy in our patients. We believe that temporary endoscopic gastric stimulation is of great value when predicting GES response. All 3 patients who did not respond to GES replacement had a preoperative diagnosis of idiopathic gastroparesis. One patient also suffered from fibromyalgia, which may mimic the symptoms of gastroparesis. The other 2 patients were evaluated for myenteric plexus inflammation with immunocytochemical studies, but the pathology was noncontributory. It is unknown why these patients failed GES replacement therapy.
There are obvious limitations to this study, including the small sample size and the fact that this is a retrospective study. There is no clear explanation of why the original GES did not relieve symptoms. More research is necessary to determine positive and negative predictors of GES therapy.
Conclusion
A trial of temporary endoscopic gastric mucosal electrical stimulation followed by implantation of new leads and stimulator successfully salvages the majority of patients whose primary gastric electrical stimulator is no longer relieving symptoms. Reimplantation of a pacemaker at a new site in the stomach should be considered a viable option for patients who have initially failed GES therapy for gastroparesis.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Thomas L. Abell have COI with Medtronic: as consultant, licensor and investigator.
References
- 1.Yin J, Abell TD, McCallum RW, Chen JDZ. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15:224–231. doi: 10.1111/j.1525-1403.2012.00429.x. [DOI] [PubMed] [Google Scholar]
- 2.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–1816. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 3.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–G191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 5.Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–319. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Runnels JEW, Johnson W, Abell TL. Long-term follow up double masked temporary GES study: the importance of baseline physiologic measures. Neurogastroenterol Motil. 2008;20:A30. [Google Scholar]
- 8.Thompson JJW, Minocha A, Abell TL. Double blinded randomized study of temporary gastric electrical stimulation (GES): preliminary results of the Endostim study (Endoscopic Stimulation Temporarily Implanted Mucosally) Gastroenterology. 2007;132(suppl 2):780. [Google Scholar]
- 9.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–954. doi: 10.1016/j.cgh.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 11.Keld R, Kinsey L, Athwal V, et al. Pathogenesis, investigation and dietary and medical management of gastroparesis. J Hum Nutr Diet. 2011;24:421–430. doi: 10.1111/j.1365-277X.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 12.Aljarallah BM. Management of diabetic gastroparesis. Saudi J Gastroenterol. 2011;17:97–104. doi: 10.4103/1319-3767.77237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddern GJ, Kiroff GK, Leppard PI, Jamieson GG. Domperidone, metoclopramide, and placebo: all give symptomatic improvement in gastroesophageal reflux. J Clin Gastroenterol. 1986;8:135–140. [PubMed] [Google Scholar]
- 14.Erbas T, Varoglu E, Erbas B, Tastekin G, Akalin S. Comparison of metoclopramide and erythromycin in the treatment of diabetic gastroparesis. Diabetes Care. 1993;16:1511–1514. doi: 10.2337/diacare.16.11.1511. [DOI] [PubMed] [Google Scholar]
- 15.Patterson D, Abell T, Rothstein R, Koch K, Barnett J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–1234. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 16.Larson JM, Tavakkoli A, Drane WE, et al. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. Neurogastroenterol Motil. 2010;16:407–413. doi: 10.5056/jnm.2010.16.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin K, Bastani B. Intraperitoneal ondansetron hydrochloride for intractable nausea and vomiting due to diabetic gastroparesis in a patient on peritoneal dialysis. Perit Dial Int. 2002;22:539–540. [PubMed] [Google Scholar]
- 18.Kim SW, Shin IS, Kim JM, et al. Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment. Psychosomatics. 2006;47:440–442. doi: 10.1176/appi.psy.47.5.440. [DOI] [PubMed] [Google Scholar]
- 19.Sawhney MS, Prakash C, Lustman PJ, Clouse RE. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–424. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 20.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009;33:1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortolotti M. Gastric electrical stimulation for gastroparesis: a goal greatly pursued, but not yet attained. World J Gastroenterol. 2011;17:273–282. doi: 10.3748/wjg.v17.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009;21:229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 25.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 26.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–319. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Stanghellini V. Unfulfilled wishes by gastric electrical stimulation. Clin Gastroenterol Hepatol. 2011;9:447–448. doi: 10.1016/j.cgh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 28.McCallum RW, Dusing RW, Sarosiek I, Cocjin J, Forster J, Lin Z. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil. 2010;22:161–167. doi: 10.1111/j.1365-2982.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 29.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 30.Musunuru S, Beverstein G, Could J. Preoperative predictors of significant symptomatic response after 1 year of gastric electrical stimulation for gastroparesis. World J Surg. 2010;34:1853–1858. doi: 10.1007/s00268-010-0586-1. [DOI] [PubMed] [Google Scholar]
- 31.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 2011;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 32.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]