SYNOPSIS
Intracerebral hemorrhage (ICH) is the deadliest type of stroke and up to half of patients die in-hospital. Blood pressure management, coagulopathy reversal and intracranial pressure control are the mainstays of acute ICH treatment. Prevention of hematoma expansion and minimally invasive hematoma evacuation are promising therapeutic strategies under investigation. The aim of this paper is to provide an updated review on ICH diagnosis and management in the emergency department (ED).
Keywords: Intracerebral hemorrhage, hemorrhagic stroke, neurocritical care, blood pressure, coagulopathy, hematoma expansion
INTRODUCTION AND EPIDEMIOLOGY
Intracerebral hemorrhage (ICH) refers to primary, spontaneous, non-traumatic bleeding occurring in the brain parenchyma. ICH accounts for 10 to 20 % of all cerebrovascular events in the US1 and is the deadliest type of stroke, with 30-day mortality up to 40% and severe disability in the majority of survivors2. Older age, hypertension (HTN), cerebral amyloid angiopathy (CAA) and oral anticoagulant treatment (OAT) are the most important risk factors for ICH1,3,4. Other ICH risk factors are summarized in box 1.
Box 1. Risk factors for ICH.
Hypertension is the most important modifiable risk factor for ICH1. Poor control of blood pressure values is also associated with increased risk of recurrent ICH85.
Cerebral amyloid angiopathy (CAA) accounts for up to 20 % of all spontaneous ICH cases 4. CAA related bleedings typically arise from cortico-subcortical brain regions and frequently affects elderly patients 4.
Alcohol intake: this relationship appears to be dose-dependent 1.
Cholesterol levels and statin use: in contrast to ischemic stroke, hypercholesterolemia has a protective effect against the risk of ICH 1. The association between statins and ICH risk is still unclear 86.
Diabetes: a meta-analysis including almost 70.000 subjects provided evidence in favor of diabetes as a risk factor for ICH87.
Genetics: the gene most strongly associated with ICH is the Apolipoprotein E (APOE) gene and its ε2 and ε4 alleles 1
Ethnicity: ICH incidence is higher in Asian populations 1,2.
Drug abuse: illicit drug consumption, such as cocaine and metamphetamine, is an important risk factor for ICH, especially in young adults 88.
PATHOPHYSIOLOGY
ICH represents an acute manifestation of an underlying progressive small vessel disease. Primary brain damage in the acute phase of ICH is caused by mechanical mass effect of the hematoma, leading to increased intracranial pressure (ICP) and consequent reduced cerebral perfusion and possible herniation5. Intraventricular extension of the hemorrhage (IVH) occurs in up to 40 % of ICH cases and is another important determinant of clinical deterioration and independent predictor of mortality 6.
CLINICAL PRESENTATION AND DIAGNOSIS
The clinical presentation of ICH and ischemic stroke is similar, typically consisting of abrupt onset of a focal neurologic deficit. Decreased level of consciousness, vomiting, headache, seizures and very high blood pressure might suggest the presence of ICH. However, none of these symptoms/signs is specific enough to distinguish hemorrhagic from ischemic stroke and therefore the diagnosis of ICH must always rely on neuroimaging7. A significant proportion of patients with ICH manifest a loss of at least two points on the Glasgow Coma Scale (GCS) during acute evaluation7 and coma can be the presenting symptom of posterior fossa hemorrhages 5.
Clinical assessment
Vital sign measurement and general physical examination should be performed in all patients. The American Heart Association and American Stroke Association (AHA/ASA) recommend routine application of a neurological baseline severity score, and the National Institutes of Health Stroke Scale (NIHSS) score appears to be feasible and useful in ICH patients7. The GCS is a widely known, rapid and reproducible tool for consciousness evaluation. The ICH score is a reliable and validated scale for rapid assessment of ICH severity 8.
Blood tests
In ICH patients complete blood count, electrolytes and creatinine, glucose and coagulation studies should be obtained.
Neuroimaging
A) Noncontrast computerized tomography
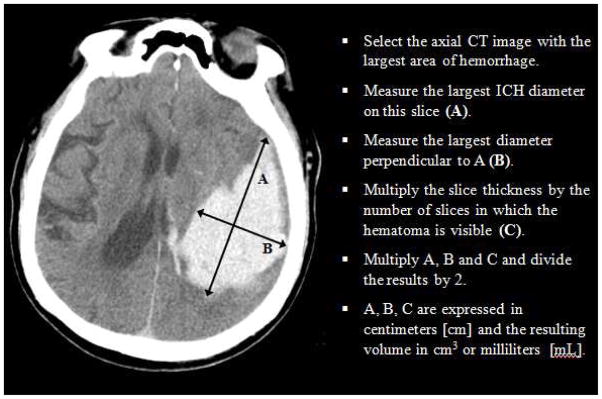
Noncontrast computerized tomography (NCCT) is a fast technique with excellent sensitivity for identifying acute ICH, and given its wide availability is considered the gold standard for the diagnosis of ICH in the ED7,9. Beyond the diagnosis of ICH, NCCT can provide useful elements such as ICH location, intraventricular extension, hydrocephalus, presence and degree of edema, and midline shift or brainstem compression secondary to the mass effect from the hematoma. Furthermore, ICH volume is a strong predictor of ICH outcome10 and can be rapidly estimated in the ED with the ABC/2 technique (figure 1).
Figure 1.
ABC/2 method for ICH volume estimation
B) CT angiography
CT Angiography (CTA) is a useful diagnostic tool in the acute setting of ICH11. It is the most widely available, non-invasive technique for the detection of vascular abnormalities as secondary causes of ICH. The presence of lobar ICH, significant IVH, young age and absence of traditional cerebrovascular risk factors should trigger the suspicion of ICH secondary to vascular malformation or other intracranial pathology7,11. Prompt detection of these lesions is crucial and has a significant impact on patient management. Although CTA is an excellent noninvasive screening tool, digital subtraction angiography remains the gold standard investigation for diagnosis, and frequently endovascular treatment, of cerebral vascular malformations9.
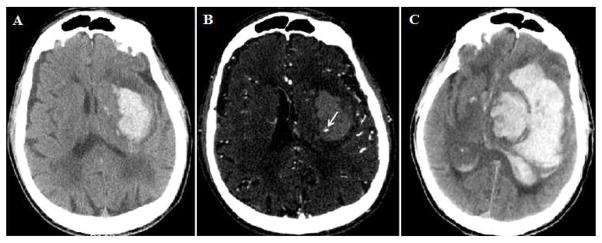
Presence of contrast extravasation within the hematoma on CTA images, also termed spot sign, is an independent predictor of hematoma expansion and poor outcome in patients with supratentorial ICH 12,13 (Figure 2). Furthermore, CTA spot sign is associated with active bleeding during surgical evacuation, and may help guide which patients may benefit from surgery 14. The main drawback of CTA is cost and the additional radiation exposure11. While some are concerned about the risk of contrast induced nephropathy (CIN), there is debate in the literature about whether this entity exists, and there is no evidence that CTA increases the risk of nephropathy in ICH patients15–17.
Figure 2. Spot sign and hematoma expansion.
A) Left deep ICH on NCCT, with baseline volume of 45 mL; B) CTA showing presence of spot sign (arrow); C) Follow-up NCCT at 19 hours demonstrated significant hematoma growth to a volume of 192 mL with severe midline shift and massive intraventricular extension.
C) Magnetic resonance imaging
Magnetic resonance imaging (MRI) sensitivity for the diagnosis of ICH is equivalent to NCCT. MRI can be a useful technique to detect underlying secondary causes of ICH such as neoplastic lesions or hemorrhagic transformation of ischemic stroke9. Finally, in patients with poor kidney function, contrast allergies or other contraindication to CTA, brain vessel imaging can be achieved without contrast through magnetic resonance angiography (MRA). Given the cost, duration of the exam and poor tolerability for some patients, MRI is rarely used in the ED workup of ICH7.
Natural history and clinical evolution
Even though ICH was traditionally viewed as a monophasic disease, growing evidence suggests that ICH is a dynamic disease, characterized by early significant expansion in up to one third of patients18. Early imaging after symptom onset, large baseline hematoma volume, anticoagulant therapy and presence of CTA spot sign are consistently the most powerful predictors of significant hematoma growth13. Other factors associated with clinical deterioration include perihematomal edema, intraventricular extension of the ICH, hydrocephalus, seizures, fever and infections19.
ACUTE MANAGEMENT
Prehospital Care
The main goal of prehospital management of ICH is to provide airway and cardiovascular support to unstable patients, along with careful reconstruction of symptom onset timing, medical history and current medications7. Moreover, early notification reduces the time to NCCT scan in the ED and allows therefore a faster diagnosis of ICH7. Mobile stroke units have been developed to reduce the time from symptom onset to intravenous (IV) thrombolysis administration in patients with ischemic stroke20. This approach seems feasible and potentially useful also for ICH patients, allowing the possibility of early blood pressure management, reversal of coagulopathy and delivery of patients to tertiary care centers with neurosurgical and neurocritical care facilities21.
Airway protection
Patients with ICH are often unable to protect the airway because of reduced consciousness. Endotracheal intubation may be therefore necessary, but this decision should be balanced against the risk of losing the neurologic examination. Rapid sequence intubation is typically the preferred approach in the acute setting. Pretreatment with lidocaine may be preferred as it may blunt a rise in intracranial pressure (ICP) associated with intubation22.
Blood pressure management
The majority of patients suffering ICH present with elevated BP levels in the acute phase. BP elevation is associated with higher risk of hematoma growth and poor outcome5 and is therefore an appealing target for ICH treatment. The most robust data on BP management comes from the INTERACT2 study, a large clinical trial randomizing patients to one of two different BP control strategies (SBP<140mmHg vs. SBP<180mmHg for the first 24 hours). The study failed to meet its primary endpoint, and did not definitively demonstrate improved outcome with intensive BP treatment (Systolic BP target<140 mmHg)23. However, intensive BP lowering appeared safe, and numerous secondary measures of outcome appeared to be superior with the intensive strategy. As a result, some argue that the weight of evidence is now in favor of maintaining SBP<140mmHg in the acute phase. However, this study had numerous limitations, including the disproportionate inclusion of those with small ICH, difficulty achieving target BP quickly, and a heterogeneous range of pharmacological agents were used24,25. The current AHA/ASA guidelines indicate that intensive BP treatment is safe and might be associated with better outcome in patients presenting with systolic BP between 150–220 mmHg. Elevated BP should be treated with short half-life agents such as labetalol or nicardipine to avoid overshoot hypotension. Hydralazine and nitroprusside should be avoided given their possible association with increased ICP24.
Hemostatic treatment
A) Platelet function
The utility and safety of platelet transfusion in ICH patients taking antiplatelet medications remains unclarified and there is not enough evidence to support routine application of a reversal strategy to improve platelet function7. Platelet transfusion is indicated in patients with severe thrombocytopenia with suggested thresholds between 50.000 and 100.000 platelets per microliter26,27.
B) Warfarin-associated coagulopathy
OAT is associated with higher baseline ICH volume, increased risk of hematoma expansion and poor outcome28. Coagulopathy correction is aimed at preventing continued bleeding. Warfarin discontinuation and IV administration of vitamin K are the first therapeutic steps. Vitamin K should be infused slowly (over 10 minutes), at the dose of 10 mg with close monitoring of vital signs given the rare but not negligible risk of anaphylaxis (1/10.000)28. Given its slow onset of action (6 to 24 h), emergent factor repletion is typically provided in addition. Fresh Frozen Plasma (FFP) and Prothrombin Complex Concentrates (PCCs) are commonly used. According to the AHA/ASA guidelines PCCs may be preferred over FFP because of more rapid action7. Two randomized controlled trials of PCC vs. FFP showed that PCCs restore coagulation factors and reverse the International Normalized Ratio (INR) more rapidly than FFP, with no clear difference in thromboembolic risk29–31. While these trials failed to demonstrate improved clinical outcome in ICH patients, some observational studies have suggested improved outcome from more rapid INR reversal 32,33,34. The optimal INR target is still debated and proposed target values range from 1.3 to 1.5 35,36. While it is not clear whether INR values below 1.7 represent clinically relevant coagulation abnormalities, one observational study found that achievement of an INR value below 1.3 within 4h from admission was associated with reduced risk of hematoma expansion37. The European Stroke Organization guidelines do not provide a specific recommendation about the warfarin reversal strategy 38 while the Neurocritical Care Society recommends warfarin reversal with either PCCs or FFP with a target INR<1.5 39. Characteristics of FFP and PCCs and one reasonable reversal strategy for warfarin-associated ICH are shown in table 1 and box 2.
Table 1.
Comparison between FFP and PCCs for warfarin reversal in ICH
| FFP | PCCs |
|---|---|
|
|
3 Factor PCCs: Profilnine SD ® or Bebulin VH ®.
4 Factor PCCs: KCentra ®, Beriplex ®, Octaplex ®, or Confidex ®.
Box 2. Reversal strategy for Warfarin-associated ICH.
Discontinue warfarin treatment
Obtain complete blood count and INR
Administer 10 mg of Vitamin K IV (Infuse over 10 minutes)
-
Administer 4-Factor PCC in weight and INR based dosing:
PCC 20 IU/kg if INR<2.0
PCC 30 IU/kg if INR 2.0–3.0
PCC 50 IU/kg if INR>3.0
If PCCs are not available or desired, administer FFP 10–20 mL/kg
Repeat INR after infusion
C) Heparin-associated coagulopathy
If ICH occurs during IV heparin or low molecular weight heparin treatment, protamine sulfate administration can be used for coagulopathy reversal, at the dose of 1 mg per 100 units of heparin7,28. The maximum dosage should be 50 mg and the infusion must be slow (maximum infusion speed: 5 mg/minute) with vital signs monitoring given the significant risk of hypotension28.
D) Direct Oral Anticoagulants (DOACs)
Alternatives to warfarin are now available, and the most commonly used are the Factor Xa inhibitors Apixaban, Rivaroxaban, and Edoxaban, and the direct thrombin inhibitor Dabigatran40. These agents were traditionally termed NOACs but the International Society of Thrombosis and Hemostasis has recommended the term DOACs; we will use this terminology here 41. Unlike warfarin, there is no specific commercially available laboratory test to assess level of DOAC function; however, some traditional and novel blood tests may assist the clinical provider in estimation of anticoagulant effect. These tests are listed in table 2. Compared to warfarin, DOACs have shorter half-lives and their blood concentration typically follows a peak-trough format42. Therefore, timing of last intake and renal function should always be considered considering whether the patient is still, at the time of presentation, coagulopathic43,44. Current evidence for DOACs reversal is limited and comes from in-vitro and animal models or studies on healthy volunteers45. For dabigatran reversal, a specific antagonist is now available, which addresses coagulopathy but has not yet been shown to reduce expansion 46,47. For reversal of other agents, it may be that no currently available product is effective for this purpose, although more specific reversal agents are under investigation (ClinicalTrials.gov Identifiers NCT02329327 - NCT02220725). Subjects taking DOACs are not deficient in Vitamin K dependent factors, so vitamin K administration is not likely to be of value. Some authorities use activated PCCs for this purpose, to provide excess coagulation factor activity 48,49,50,51. Activated charcoal can be considered if administered within 2 to 3 hours from the last drug intake42,48,49.
Table 2.
| DABIGATRAN | RIVAROXABAN | APIXABAN | EDOXABAN | |
|---|---|---|---|---|
| aPTT |
|
|
|
|
| PT/INR |
|
|
|
|
| Diluted Thrombin time (dTT) |
|
|||
| Ecarin clotting time (ECT) |
|
|||
| Anti Xa activity |
|
|
|
|
| Specific test system | Hemoclot ®: dabigatran calibrated dTT | Rivaroxaban calibrated anti Xa activity | Apixaban calibrated anti Xa activity | Edoxaban calibrated anti Xa activity |
E) Recombinant tissue plasminogen activator (rtPA) – associated coagulopathy
Symptomatic intracerebral hemorrhage (sICH) is the most dangerous complication of rtPA administration in ischemic stroke patients, occurring in about 6 % of patients and leading to increased morbidity and mortality 52. Older age, stroke severity, longer time from onset to treatment, pre-stroke antiplatelet therapy and significant elevation of BP are among the most important predictors of sICH 53. Preventive strategies include strict control of BP (target 180/105 mmHg), and avoidance of any antithrombotic medication in the first 24 h following the infusion of rtPA 52. However, once ICH occurs, it is unclear how best to treat this. Given the short half-life of rtPA, by the time sICH is diagnosed, the agent itself may no longer be present at meaningful levels. As a result, there is great heterogeneity in clinical practice 52,54. One concern is that rtPA can induce a relative hypofibrinogenemia which is present long after rtPA, and if so, this can be treated. As a result, the AHA/ASA guidelines recommend immediate discontinuation of rtPA and administration of cryoprecipitate 55. The optimal dosage of cryoprecipitate is unclear and some suggest empiric treatment with 10 U of cryoprecipitate, followed by further administration until normalization of fibrinogen level 56. Other options can include the anti-fibrinolytic aminocaproic acid (Amicar ®) as a 5 g IV bolus over 15–30 minutes. While other therapeutic approaches including vitamin K, FFP, PCC, platelet transfusion, or rFVIIa have been used, there is no clear biological reason or evidence to support them 52. Finally, decompressive craniotomy or surgical hematoma evacuation may be considered for large hemorrhages with severe mass effect and intracranial hypertension 52,55.
Intracranial pressure management
The more common causes of elevated intracranial pressure (ICP) in ICH patients are mass effect from the hematoma and surrounding edema and IVH with secondary hydrocephalus. The indications for ICP monitoring in ICH are mainly derived from traumatic brain injury studies. Current AHA/ASA guidelines suggest ICP monitoring in patients with coma, significant IVH with hydrocephalus and evidence of transtentorial herniation, with a cerebral perfusion pressure (CPP) target of 50 to 70 mmHg 7. ICP can be measured with parenchymal or ventricular devices. The latter (an external ventricular drain, or EVD) might be preferred in hydrocephalus as it allows cerebrospinal fluid (CSF) drainage. Elevation of the head to 30 degrees, adequate sedation, and avoidance of hyponatremia are mainstays of therapy; hyperosmolar therapy with mannitol or hypertonic saline can be considered in patients at risk of transtentorial herniation7.
Seizures and antiepileptic treatment
Up to 14% of patients with ICH experience seizures in the early course of the disease 57. The main risk factors for development of early seizures are cortical location of the ICH and occurrence of medical complications57,58. However, it is not clear that prophylactic antiepileptic (AED) therapy provides benefit to patients, and some data suggests phenytoin may worsen outcome in this population 59. Therefore, prophylactic administration of AED therapy is not recommended and only subjects with clinical or electroencephalographic (EEG) evidence of seizures should receive antiepileptic drugs7. Continuous EEG monitoring should be considered in patients with impaired mental status that is disproportionate to the degree of brain damage7.
Blood glucose management
Hyperglycemia has been shown to be associated with poor outcome in ICH60 and declining values of glucose appear to be associated with lower risk of hematoma expansion61. Some data suggest that careful glucose control (with sliding scale insulin) may improve neurologic outcome62. However, it is not clear that continuous insulin infusion improves outcomes in this population63,64. The AHA/ASA guidelines suggest to avoid both hyperglycemia and hypoglycemia although a specific blood glucose target level is not provided7.
Temperature management
The presence of fever is a common finding in ICH patients, especially in those with extensive IVH and appears to be independently associated with poor outcome62. Treatment of fever appears therefore reasonable but the optimal temperature management is still unclear7. Therapeutic normothermia failed to improve outcome in one trial 65 although treatment of fever did improve outcome in another62. It appears reasonable to minimize fever, and the role of targeted temperature management in ICH is still under investigation in a randomized clinical trial66.
Surgical treatment
A) IVH management
IVH occurs in nearly half of ICH patients, particularly in those with deep hematomas, and is an independent predictor of poor outcome6. EVD placement is recommended for patients with hydrocephalus, coma and significant IVH, in order to drain blood and CSF and avoid significant elevation of ICP7. Several other approaches have been proposed for IVH treatment 67. A recent meta-analysis showed that EVD placement in conjunction with thrombolytic drugs is associated with reduced mortality and better outcomes68. However, intraventricular fibrinolysis for IVH treatment is still considered investigational7 and further insights will be provided by the ongoing CLEAR III trial69.
B) Surgical hematoma evacuation
Two large randomized controlled trials, the STICH I and STICH II trials, investigated the role of surgical hematoma evacuation, compared to conservative treatment in patients with supratentorial ICH70,71. Neither trial demonstrated a statistically significant benefit of surgery in comparison to best medical management. As a result, the role of surgical therapy remains controversial and surgical evacuation of supratentorial hematomas should be considered only as a life-saving measure in deteriorating patients7. The only case in which there is consensus in favor of surgical intervention is in cerebellar hematomas with clinical or imaging signs of hydrocephalus and/or brainstem compression 7. In these cases, surgical decompression and hematoma evacuation should probably be performed as soon as possible.
C) Decompressive craniotomy with or without hematoma evacuation
Decompressive craniotomy appears a feasible and safe procedure and may be associated with better outcome in a subset of patients with supratentorial ICH72,73. This subset includes those with coma, large hematoma with significant midline shift, or elevated ICP not controlled by optimal medical therapy7.
D) Minimally invasive surgery
While open surgical evacuation has failed to definitively demonstrate benefit, it may be that minimally invasive surgical techniques (MIS) can still offer benefit. The development of less invasive techniques might allow hematoma evacuation with less damage to viable brain tissue and reduce the rate of secondary complications compared to traditional craniotomy67,74. Another potential advantage of MIS over conventional surgery is faster access to the hematoma, with reduction of surgical and anesthesia duration in patients with clinical deterioration and elevated ICP75. Several MIS techniques have been proposed, ranging from endoscopic treatment of IVH to parenchymal hematoma evacuation with or without combined administration of rtPA74,75. The clinical efficacy of all these MIS approaches is still uncertain and clinical trials are ongoing.
ADMISSION TO STROKE UNIT OR NEUROSCIENCE INTENSIVE CARE UNIT
Following diagnosis of ICH, patients should be admitted to a dedicated stroke or neuroscience intensive care unit. Management of ICH in a dedicated stroke unit is associated with reduced mortality and better functional outcome compared to treatment in a general neurology ward76.
PROGNOSIS PREDICTION
Despite progress in primary prevention and acute treatment, ICH is still a disease with high morbidity and mortality. Outcome estimation, palliative care and withdrawal of support are therefore important aspects of care in ICH patients. The ICH score predicts one-month mortality risk while the FUNC score predicts three-month functional independence (Table 3, Table 4)8,77. However, none of these tools should be used as a singular indicator of outcome or guide the decision of medical support withdrawal7. Early limitation of care is an independent predictor of poor prognosis78 and the AHA/ASA guidelines recommend full medical support for all ICH patients at least until the second day of admission, apart from those with preexisting specific directives7.
Table 3.
ICH score for prediction of 30-day mortality
| Component | ICH score |
|---|---|
| GCS | |
| 3–4 | 2 |
| 5–12 | 1 |
| 13–15 | 0 |
| ICH volume (mL) | |
| ≥ 30 | 1 |
| < 30 | 0 |
| IVH presence | |
| Yes | 1 |
| No | 0 |
| Infratentorial ICH | |
| Yes | 1 |
| No | 0 |
| Age (years) | |
| ≥ 80 | 1 |
| <80 | 0 |
| TOTAL SCORE | 0 – 6 |
The mortality rate increases linearly with the ICH score. A total score of 3 is associated with a 30-day fatality rate around 70 %. A total score > 4 confers a 30-day mortality risk close to 100%.
Table 4.
FUNC score for prediction of 90-day functional independence
| Component | FUNC score |
|---|---|
| GCS | |
| ≥ 9 | 2 |
| 3 – 8 | 0 |
| ICH volume (mL) | |
| < 30 | 4 |
| 30 – 60 | 2 |
| > 60 | 0 |
| ICH location | |
| Lobar | 2 |
| Deep | 1 |
| Infratentorial | 0 |
| Age (years) | |
| < 70 | 2 |
| 70 – 79 | 1 |
| ≥ 80 | 0 |
| Pre-ICH cognitive impairment | |
| No | 1 |
| Yes | 0 |
| TOTAL SCORE | 0 – 11 |
The probability of reaching functional independence at 3 months increases steadily with the total score. Total scores > 7 are associated with a functional independence probability over 70%.
FUTURE PERSPECTIVES
Acute treatment of ICH is an active area of research. Hematoma expansion is an appealing target and identification of patients with the higher likelihood to benefit from anti-expansion therapy will be an important challenge. Aggressive BP treatment, hemostatic therapy, neuroprotection, reduction of secondary injury and perihematomal edema development, and MIS are some of the therapeutic strategies under investigation. Some ongoing clinical trials in acute ICH management are listed in table 5.
Table 5.
Some ongoing clinical trials in acute ICH management
| TRIAL | INTERVENTION |
|---|---|
| ATACH II 82 | Intensive BP treatment within 4.5 h from symptom onset with SBP target < 140 mmHg |
| SCORE-IT 83 | Investigate whether CTA spot sign or other imaging markers identify patients with high likelihood to benefit from BP lowering |
|
INCH ClinicalTrials.gov NCT00928915 |
FFP vs PCCs for reversal of coumarin-related coagulopathy |
|
TICH II Tich-2.org |
Administration of intravenous tranexamic acid |
|
STOP-AUST ClinicalTrials.gov NCT01702636 |
Administration of intravenous tranexamic acid to ICH patients with CTA spot sign |
|
STOP-IT ClinicalTrials.gov NCT00810888 |
Administration of recombinant activated factor VII (rFVIIa) to ICH patients with CTA spot sign |
|
SPOTLIGHT ClinicalTrials.gov NCT01359202 |
Administration of recombinant activated factor VII (rFVIIa) to ICH patients with CTA spot sign |
|
iDEF ClinicalTrials.gov NCT02175225 |
Iron chelation with administration of intravenous Deferoxamine |
|
SHRINC ClinicalTrials.gov NCT00827892 |
Enhancing hematoma reabsorption with administration of Pioglitazone |
|
MISTIE III ClinicalTrials.gov NCT01827046 |
MIS plus rtPA for parenchymal hematoma evacuation |
|
CLEAR III
69 ClinicalTrials.gov NCT00784134 |
EVD placement combined with intraventricular administration of rtPA for IVH treatment |
| MISTICH 84 | MIS for evacuation of supratentorial hematomas |
|
SATIH ClinicalTrials.gov NCT00752024 |
Stereotactical hematoma aspiration combined with thrombolysis |
|
SWITCH Clinical Trials.gov NCT02258919 |
Decompressive craniectomy plus best medical therapy |
KEY POINTS.
ICH is a dynamic disease and up to one third of the patients experience early clinical deterioration due to hematoma expansion.
Intensive blood pressure reduction is safe and might improve neurological outcome.
Rapid correction of coagulopathy may minimize the risk of ongoing bleeding.
Surgical evacuation of the hematoma should be considered for patients with clinical deterioration due to cerebellar ICH.
ICH patients should be admitted to a neuroscience intensive care unit or stroke specialty unit.
Footnotes
Conflicts of Interest: none.
DISCLOSURES
Funding sources: NINDS award 5R01NS073344.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14(4):300–306. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MJ, Denis X, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 4.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallevi H, Albright KC, Aronowski J, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70(11):848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemphill JC, Greenberg, Steven M, Anderson C. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 9.Macellari F, Paciaroni M, Agnelli G, Caso V. Neuroimaging in intracerebral hemorrhage. Stroke. 2014;45(3):903–908. doi: 10.1161/STROKEAHA.113.003701. [DOI] [PubMed] [Google Scholar]
- 10.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 11.Khosravani H, Mayer SA, Demchuk A, et al. Emergency noninvasive angiography for acute intracerebral hemorrhage. Am J Neuroradiol. 2013;34(8):1481–1487. doi: 10.3174/ajnr.A3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol. 2012;11(4):307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting Hematoma Expansion After Primary Intracerebral Hemorrhage. JAMA Neurol. 2014;71(2):158. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwers HB, Raffeld MR, van Nieuwenhuizen KM, et al. CT angiography spot sign in intracerebral hemorrhage predicts active bleeding during surgery. Neurology. 2014;83(10):883–889. doi: 10.1212/WNL.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oleinik A, Romero JM, Schwab K, et al. CT angiography for intracerebral hemorrhage does not increase risk of acute nephropathy. Stroke. 2009;40(7):2393–2397. doi: 10.1161/STROKEAHA.108.546127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinert R, Brandler E, Subramanian RA, Miller AC. Does the current definition of contrast-induced acute kidney injury reflect a true clinical entity? Acad Emerg Med. 2012;19(11):1261–1267. doi: 10.1111/acem.12011. [DOI] [PubMed] [Google Scholar]
- 17.Sinert R, Brandler E. Contrast-induced acute kidney injury: Time for a step backward. Acad Emerg Med. 2014;21(6):701–703. doi: 10.1111/acem.12392. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35(3):195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord AS, Gilmore E, Choi HA, Mayer SA. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015;46(3):647–652. doi: 10.1161/STROKEAHA.114.007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebinger M, Kunz A, Wendt M, et al. Effects of Golden Hour Thrombolysis: A Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke (PHANTOMS) Substudy. JAMA Neurol. 2015;72(1):25–30. doi: 10.1001/jamaneurol.2014.3188. [DOI] [PubMed] [Google Scholar]
- 21.Gomes JA, Ahrens CL, Hussain MS, Winners S, Rasmussen PA, Uchino K. Prehospital Reversal of Warfarin-Related Coagulopathy in Intracerebral Hemorrhage in a Mobile Stroke Treatment Unit Result of Initial Pilot Implementation. 2015;46:e118–e120. doi: 10.1161/STROKEAHA.115.008483. [DOI] [PubMed] [Google Scholar]
- 22.Salhi B, Stettner E. In defense of the use of lidocaine in rapid sequence intubation. Ann Emerg Med. 2007;49(1):84–86. doi: 10.1016/j.annemergmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Palesch YY, Martin R, et al. Interpretation and Implementation of Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial ( INTERACT II ) J Vasc Interv Neurol. 2014;7(2):34–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson CS, Qureshi AI. Implications of INTERACT2 and Other Clinical Trials: Blood Pressure Management in Acute Intracerebral Hemorrhage. Stroke. 2014;46(1):291–295. doi: 10.1161/STROKEAHA.114.006321. [DOI] [PubMed] [Google Scholar]
- 26.Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007:172–178. doi: 10.1182/asheducation-2007.1.172. [DOI] [PubMed] [Google Scholar]
- 27.Hunt BJ. Bleeding and Coagulopathies in Critical Care. N Engl J Med. 2014;370(9):847–859. doi: 10.1056/NEJMra1208626. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar MI, Freeman WD. Treatment of Coagulopathy in Intracranial Hemorrhage. Curr Treat Options Neurol. 2010;12(2):113–128. doi: 10.1007/s11940-010-0061-1. [DOI] [PubMed] [Google Scholar]
- 29.Milling TJ, Refaai Ma, Goldstein JN, et al. Thromboembolic Events After Vitamin K Antagonist Reversal With 4-Factor Prothrombin Complex Concentrate: Exploratory Analyses of Two Randomized, Plasma-Controlled Studies. Ann Emerg Med. 2016;67(1):96–105.e5. doi: 10.1016/j.annemergmed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein JN, Refaai Ma, Jr, TJM, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions : a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;6736(14):1–11. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera JA, Gordon E, Zach V, et al. Reversal of coagulopathy using prothrombin complex concentrates is associated with improved outcome compared to fresh frozen plasma in warfarin-associated intracranial hemorrhage. Neurocrit Care. 2014;21(3):397–406. doi: 10.1007/s12028-014-9972-0. [DOI] [PubMed] [Google Scholar]
- 33.Huhtakangas J, Tetri S, Juvela S, et al. Improved survival of patients with warfarin-associated intracerebral haemorrhage: a retrospective longitudinal population-based study. Int J Stroke. 2015;10(6):876–881. doi: 10.1111/j.1747-4949.2012.00926.x. [DOI] [PubMed] [Google Scholar]
- 34.Hickey M, Gatien M, Taljaard M, Aujnarain A, Giulivi A, Perry JJ. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation. 2013;128(4):360–364. doi: 10.1161/CIRCULATIONAHA.113.001875. [DOI] [PubMed] [Google Scholar]
- 35.Marietta M, Pedrazzi P, Girardis M, Torelli G. Intracerebral haemorrhage: An often neglected medical emergency. Intern Emerg Med. 2007;2(1):38–45. doi: 10.1007/s11739-007-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant Reversal, Blood Pressure Levels, and Anticoagulant Resumption in Patients With Anticoagulation-Related Intracerebral Hemorrhage. Jama. 2015;313(8):824. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 38.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 39.Andrews CM, Jauch EC, Hemphill JC, Smith WS, Weingart SD. Emergency Neurological Life Support: Intracerebral Hemorrhage. Neurocrit Care. 2012;17(S1):37–46. doi: 10.1007/s12028-012-9757-2. [DOI] [PubMed] [Google Scholar]
- 40.John Camm a, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 41.Barnes GD, Ageno W, Ansell J, Kaatz S. Recommendation on the nomenclature for oral anticoagulants: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(6):1154–1156. doi: 10.1111/jth.12969. [DOI] [PubMed] [Google Scholar]
- 42.Siegal DM, Garcia Da, Crowther Ma, Dc W. How I treat target-specific oral anticoagulant – associated bleeding. How I Treat How I treat target-specific oral anticoagulant – associated bleeding. 2014;123(D):1152–1158. doi: 10.1182/blood-2013-09-529784. [DOI] [PubMed] [Google Scholar]
- 43.Miller MP, Trujillo TC, Nordenholz KE. Practical considerations in emergency management of bleeding in the setting of target-specific oral anticoagulants. Am J Emerg Med. 2014;32(4):375–382. doi: 10.1016/j.ajem.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 44.Liew A, Eikelboom JW, O’Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol. 2013;29(7 SUPPL):S34–S44. doi: 10.1016/j.cjca.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Siegal DM. Managing target-specific oral anticoagulant associated bleeding including an update on pharmacological reversal agents. J Thromb Thrombolysis. 2015;39(3):395–402. doi: 10.1007/s11239-015-1167-9. [DOI] [PubMed] [Google Scholar]
- 46.Pollack CV, Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6):511–20. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 47.Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386(9994):680–690. doi: 10.1016/S0140-6736(15)60732-2. [DOI] [PubMed] [Google Scholar]
- 48.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(SUPPL 1):141–145. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 49.Steiner T, Böhm M, Dichgans M, et al. Recommendations for the emergency management of complications associated with the new direct oral anticoagulants (DOACs), apixaban, dabigatran and rivaroxaban. Clin Res Cardiol. 2013;102(6):399–412. doi: 10.1007/s00392-013-0560-7. [DOI] [PubMed] [Google Scholar]
- 50.Levine M, Goldstein JN. Emergency Reversal of Anticoagulation: Novel Agents. Curr Neurol Neurosci Rep. 2014;14(8):1–6. doi: 10.1007/s11910-014-0471-7. [DOI] [PubMed] [Google Scholar]
- 51.Baumann Kreuziger LM, Reding MT. Management of bleeding associated with dabigatran and rivaroxaban: A survey of current practices. Thromb Res. 2013;132(2):161–163. doi: 10.1016/j.thromres.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaghi S, Eisenberger a, Willey JZ. Symptomatic Intracerebral Hemorrhage in Acute Ischemic Stroke After Thrombolysis With Intravenous Recombinant Tissue Plasminogen Activator: A Review of Natural History and Treatment. JAMA Neurol. 2014;71(9):1181–1185. doi: 10.1001/jamaneurol.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43(6):1524–1531. doi: 10.1161/STROKEAHA.111.644815. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein JN, Marrero M, Masrur S, et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol. 2010;67(8):965–969. doi: 10.1001/archneurol.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 56.Yaghi S, Boehme AK, Dibu J, et al. Treatment and Outcome of Thrombolysis-Related Hemorrhage. JAMA Neurol. 2015;72(12):1451. doi: 10.1001/jamaneurol.2015.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Herdt V, Dumont F, Hénon H, et al. Early seizures in intracerebral hemorrhage: Incidence, associated. Neurology. 2011;77(20):1794–1800. doi: 10.1212/WNL.0b013e31823648a6. [DOI] [PubMed] [Google Scholar]
- 58.Pezzini A, Grassi M, Del Zotto E, et al. Complications of acute stroke and the occurrence of early seizures. Cerebrovasc Dis. 2013;35(5):444–450. doi: 10.1159/000348704. [DOI] [PubMed] [Google Scholar]
- 59.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40(12):3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 60.Stead LG, Jain A, Bellolio MF, et al. Emergency Department hyperglycemia as a predictor of early mortality and worse functional outcome after intracerebral hemorrhage. Neurocrit Care. 2010;13(1):67–74. doi: 10.1007/s12028-010-9355-0. [DOI] [PubMed] [Google Scholar]
- 61.Qureshi AI, Palesch YY, Martin R, et al. Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocrit Care. 2011;15(3):428–435. doi: 10.1007/s12028-011-9541-8. [DOI] [PubMed] [Google Scholar]
- 62.Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomised controlled trial. Lancet. 2011;378(9804):1699–1706. doi: 10.1016/S0140-6736(11)61485-2. [DOI] [PubMed] [Google Scholar]
- 63.Finfer S, Chittock DR, Su SY-S, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 64.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6(5):397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 65.Lord AS, Karinja S, Lantigua H, et al. Therapeutic temperature modulation for fever after intracerebral hemorrhage. Neurocrit Care. 2014;21(2):200–206. doi: 10.1007/s12028-013-9948-5. [DOI] [PubMed] [Google Scholar]
- 66.Rincon F, Friedman DP, Bell R, Mayer SA, Bray PF. Targeted temperature management after intracerebral hemorrhage (TTM-ICH): methodology of a prospective randomized clinical trial. Int J Stroke. 2014;9(5):646–651. doi: 10.1111/ijs.12220. [DOI] [PubMed] [Google Scholar]
- 67.Dey M, Stadnik A, Awad IA. Spontaneous intracerebral and intraventricular hemorrhage: advances in minimally invasive surgery and thrombolytic evacuation, and lessons learned in recent trials. Neurosurgery. 2014;74(Suppl 1):S142–S150. doi: 10.1227/NEU.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan NR, Tsivgoulis G, Lee SL, et al. Fibrinolysis for intraventricular hemorrhage: an updated meta-analysis and systematic review of the literature. Stroke. 2014;45(9):2662–2669. doi: 10.1161/STROKEAHA.114.005990. [DOI] [PubMed] [Google Scholar]
- 69.Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) Int J Stroke. 2014;9(4):536–542. doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial A. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 71.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013;382(9890):397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fung C, Murek M, Z’Graggen WJ, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 2012;43(12):3207–3211. doi: 10.1161/STROKEAHA.112.666537. [DOI] [PubMed] [Google Scholar]
- 73.Hayes SB, Benveniste RJ, Morcos JJ, Aziz-Sultan MA, Elhammady MS. Retrospective comparison of craniotomy and decompressive craniectomy for surgical evacuation of nontraumatic, supratentorial intracerebral hemorrhage. Neurosurg Focus. 2013;34(5):E3. doi: 10.3171/2013.2.FOCUS12422. [DOI] [PubMed] [Google Scholar]
- 74.Barnes B, Hanley DF, Carhuapoma JR. Minimally invasive surgery for intracerebral haemorrhage. Curr Opin Crit Care. 2014;20(2):148–152. doi: 10.1097/MCC.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beynon C, Schiebel P, Bösel J, Unterberg AW, Orakcioglu B. Minimally invasive endoscopic surgery for treatment of spontaneous intracerebral haematomas. Neurosurg Rev. 2015;38(3):421–8. doi: 10.1007/s10143-015-0606-6. [DOI] [PubMed] [Google Scholar]
- 76.Langhorne P, Fearon P, Ronning OM, et al. Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke. 2013;44(11):3044–3049. doi: 10.1161/STROKEAHA.113.001564. [DOI] [PubMed] [Google Scholar]
- 77.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008;39(8):2304–2309. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 78.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68(20):1651–1657. doi: 10.1212/01. [DOI] [PubMed] [Google Scholar]
- 79.Diener HC, Foerch C, Riess H, Röther J, Schroth G, Weber R. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol. 2013;12(7):677–688. doi: 10.1016/S1474-4422(13)70101-7. [DOI] [PubMed] [Google Scholar]
- 80.Veltkamp R, Horstmann S. Treatment of Intracerebral Hemorrhage Associated with New Oral Anticoagulant Use. Clin Lab Med. 2014;34(3):587–594. doi: 10.1016/j.cll.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Cuker A, Husseinzadeh H. Laboratory measurement of the anticoagulant activity of edoxaban: a systematic review. J Thromb Thrombolysis. 2015:288–294. doi: 10.1007/s11239-015-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qureshi A, Palesch Y. Expansion of recruitment time window in antihypertensive treatment of acute cerebral hemorrhage (ATACH) II trial. J Vasc Interv Neurol. 2012;5(supp):6–9. [PMC free article] [PubMed] [Google Scholar]
- 83.Goldstein JN, Brouwers HB, Romero J, et al. SCORE-IT: the Spot Sign score in restricting ICH growth—an Atach-II ancillary study. J Vasc Interv Neurol. 2012;5(supp):20–25. [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng J, Li H, Guo R, et al. Minimally invasive surgery treatment for the patients with spontaneous supratentorial intracerebral hemorrhage (MISTICH): protocol of a multicenter randomized controlled trial. BMC Neurol. 2014;14:206. doi: 10.1186/s12883-014-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biffi A, Anderson CD, Battey TWK, et al. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. Jama. 2015;314(9):904. doi: 10.1001/jama.2015.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauer A, Greenberg SM, Gurol ME. Statins in Intracerebral Hemorrhage. Curr Atheroscler Rep. 2015;17(8):46. doi: 10.1007/s11883-015-0526-5. [DOI] [PubMed] [Google Scholar]
- 87.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin-Schild S, Albright KC, Hallevi H, et al. Intracerebral hemorrhage in cocaine users. Stroke. 2010;41(4):680–684. doi: 10.1161/STROKEAHA.109.573147. [DOI] [PMC free article] [PubMed] [Google Scholar]