Abstract
Background
About one-third of patients undergoing a Roux-en-Y anastomosis develop Roux stasis syndrome, likely because of disordered electrical conduction. GI electrical stimulation has been previously used successfully in the management of postsurgical gastroparesis.
Objective
Endoscopic placement of temporary electrodes and GI electrical stimulation in the management of severe Roux stasis syndrome in a patient with esophagojejunostomy and to determine whether the patient would be a candidate for surgical permanent electrode placement.
Design
Case report.
Setting
Academic medical center.
Patients
This study involved a patient with Roux stasis syndrome.
Intervention
Upper endoscopy was performed, followed by endoscopic placement of two temporary electrodes, one each in the two jejunal limbs. Electrical stimulation was provided by an external stimulation device. The patient was re-evaluated 5 days later.
Main Outcome Measurements
Electrogastrogram (EGG) parameters including frequency, amplitude, and frequency-amplitude ratio and total symptom score and health-related quality of life score.
Results
There was a significant improvement in EGG parameters with electrical stimulation. Also, the patient had a marked improvement in total GI symptom score, from 11 to 4, with a dramatic improvement in the health-related quality of life score from −3 to +3.
Limitations
Single case report.
Conclusion
Endoscopic placement of temporary electrodes is feasible and safe. GI electrical stimulation of the jejunal limb is a potentially effective treatment for Roux stasis syndrome.
High-frequency, low-energy, gastric electrical stimulation (GES) has been shown to be an effective treatment for drug-refractory gastroparesis.1 Temporary GES, via endoscopically placed temporary electrodes, has been shown to produce a rapid and marked improvement in patients with medically refractory gastroparesis and is predictive of clinical outcome with permanent GES.2
Roux-en-Y anastomosis is a common surgical procedure, and about one-third of patients who undergo Roux-en-Y anastomosis have Roux stasis syndrome; they complain of upper abdominal fullness and distension, abdominal pain, nausea or vomiting, and may have significant esophageal reflux of gastric and intestinal contents. This syndrome is related to the stasis of food and secretions in an atonic or abnormally contracting jejunal limb. It has been proposed that the occurrence of Roux stasis syndrome is related to the blockage of electrical conduction caused by cutting of the jejunum.3 The small intestine may lose the stimulation of pacemaker potential initiated from the duodenum because of disruption in continuity of the small intestine.4 Further, this may cause ectopic pacemakers to arise in the Roux limb. These pacemakers drive the contractions of the proximal part of the limb in a reverse or oral direction toward the stomach. This creates stasis in the Roux limb and slows emptying.5,6
GES has been used previously to manage postsurgical gastroparesis, with significant clinical improvements with long-term follow-up.7 We have reported previously using GES, perhaps more appropriately referred to as GI electrical stimulation, in managing severe gastroparesis complicating Roux-en-Y gastric bypass for morbid obesity.8 GI electrical stimulation has not been used previously for management of stasis in the intrathoracic jejunal limbs resulting from disruption of electrical conduction. In this study, we describe and evaluate the role of GI electrical stimulation in a patient with Roux stasis syndrome.
METHODS
A 47-year-old woman with a clinical history consistent with Roux stasis syndrome was evaluated at our institution. This patient had a jejunal interposition, an uncommon procedure, because of the following circumstances. She initially had a total gastrectomy with Roux-en-Y esophagojejunostomy for a diagnosis of adenocarcinoma in the gastric cardia, which we now believe was an extension of a gastroesophageal junction tumor. Because subsequent more proximal esophageal biopsy results were positive, the patient underwent thoracotomy with resection of the distal 15 cm of esophagus followed by the hand-sewn esophagojejunostomy, by using jejunal interposition, which she currently has. This was the reason she had no residual stomach for use as the interposition, as would be the case for standard Ivor-Lewis esophagectomy. The anastomosis is isoperistaltic, and the distance from her anastomosis to the jejunojejunostomy is about 25 to 30 cm. Figure 1 denotes a line diagram of the patient’s gastrojejunostomy.
Figure 1.
Diagram showing the patient’s postsurgical upper intestinal anatomy.
Since surgery, the patient has had uncontrolled reflux symptoms including regurgitation and multiple episodes of aspiration including a recent episode of life-threatening aspiration associated with acute/adult respiratory distress syndrome. She has had severe erosive esophagitis and esophageal strictures requiring dilation over the past 2 years. The patient had multiple upper GI imaging studies demonstrating reflux of jejunal contents into the esophagus, both in upright and prone positions, and the more severe reflux in the prone position likely contributed to her aspiration episodes. There was no evidence of bowel obstruction on any of these studies. She has been refractory to the use of prokinetic agents. Nausea, vomiting, bloating and distension, early satiety, and abdominal pain were each scored on a scale from 0 to 4 based on severity, with 0 being no symptom and 4 being the most severe symptom. The sum of all 5 symptom scores constitutes the total symptom score, 20 being the highest and worst possible score. At the current time, she had a total symptom score of 11 (range 0–20, none to worse), with a quality of life score of −3 (scale −3 to +3, worst to best).
The protocol and endoscopic methods used have been approved by the Institutional Review Board at the University of Mississippi, Jackson, Mississippi, and are in accordance with the guidelines for approval of a humanitarian device exemption. Individual informed consent was obtained for endoscopy and the GI electrical stimulation procedure. During upper endoscopy, performed with the patient under monitored anesthesia care, endoscopic placement of temporary electrodes was undertaken in both jejunal limbs (Video 1, available online at www.giejournal.org). A standard 140-cm-long gastroscope with a 7F accessory channel was used.
The esophagojejunal anastomosis was visualized at 25 cm from the incisors, and there was no evidence of stricture or recurrent tumor. Los Angeles class D erosive esophagitis, likely related to stasis, was noted. Then, a temporary cardiac pacing lead (model 6414-200; Medtronic, Minneapolis, Minn) was inserted through the accessory channel. This temporary lead has an inner bipolar pacing lead and an outer covering sheath. The latter is only 120 cm long, and therefore did not exit the endoscope. The long inner lead exited through the accessory channel and was inserted with a clockwise corkscrew motion into the mucosa of the proximal portion of the afferent (distal) limb (Fig. 2). The lead was inserted through the mucosal and submucosal layers, into the muscularis mucosa. The outer sheath then was removed, leaving the inner lead in place. An endoscopic clipping device (Resolution Clip; Boston-Scientific, Natick, Mass) was passed through the accessory channel; 3 such clips were deployed to hold the lead in place within the jejunal limb (Fig. 3). Placing at least one clip near the distal metallic terminal part of the lead helped to achieve the desirable electrical impedance (400–800 Ω). The above process was repeated for the insertion of a second lead into the mucosa of the proximal portion of the efferent (proximal) jejunal limb. After we obtained mucosal electrogastrogram recordings, the lead from the afferent limb was connected to an external GI electrical stimulation device (Enterra, Medtronic, Minneapolis, Minn) that was then placed in a cardiac telemetry pouch, and the impedance was determined (760 Ω). By using model 7432 Physician Programmer and a model 7457 MemoryMod software cartridge (Medtronic), we programmed the GI electrical stimulation device to previously standardized parameters (frequency 14 Hz, amplitude 5 mA, pulse width 330 μ seconds, cycle on 0.1 second, cycle off 5.0 seconds). These parameters were used as the starting point; modification of the parameters was permitted for optimal response.9 The temporary electrodes remained in place until a clinical evaluation was performed 5 days later. The electrodes then were removed manually by turning them counterclockwise and applying gentle traction.
Figure 2.
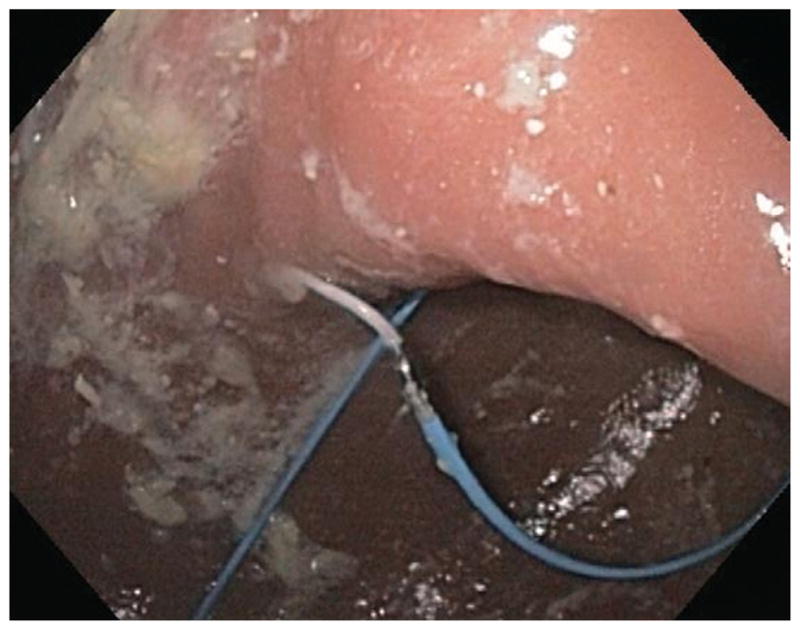
Endoscopic image showing the lead placement in the proximal portion of the afferent (distal) limb.
Figure 3.

Endoscopic image showing 3 endoclips and the secured lead in the jejunum.
RESULTS
With stimulation, the frequency of the afferent (distal) limb changed from 4.1 cycles per minute (cpm) at day 0 to 9.0 cpm at day 5. Frequency of the efferent (proximal) limb changed from 8.2 cpm on day 0 to 7.6 cpm on day 5. This patient had a marked improvement in her total symptom score from 11 to 4, with a dramatic improvement in the health-related quality of life score from −3 to +3. Similar health-related quality of life scores have been validated in previous studies.10 Further, the patient was able to tolerate a soft, low-residue diet very well during the 5-day period. A radionuclide emptying study performed before temporary stimulation demonstrated a normal amount of residual content in the area of what should have been the stomach. This was most probably because of accumulation of the radionuclide material in the thoracic portion of the jejunum and lower esophagus. After temporary stimulation, about 55% of the radionuclide was seen in the area of the stomach, likely denoting improved passage of the contents downstream with electrical stimulation.
DISCUSSION
Disruption of the GI autonomic nervous system because of surgery, such as esophagojejunostomy in this case, can lead to profound effects. Atony or even worse, reverse peristalsis in the jejunal limbs, can lead to the intrathoracic portion of the jejunum becoming a reservoir for fluid and food and lead to electrolyte imbalance, regurgitation, bacterial overgrowth, and pulmonary aspiration.
GES received the Food and Drug Administration’s humanitarian use device approval in 2000 and has been shown to be effective in the management of medication-refractory gastroparesis.11 The use of GES has been shown to result in significant and persistent improvements in symptoms, health-related quality of life, and solid and liquid gastric emptying at long-term follow-up in gastroparesis of diabetic and idiopathic etiology. Further, there is evidence that GES may have a role in the management of postsurgical gastroparesis as well, including patients with various gastrectomy procedures and vagotomy. GES has been used previously for severe refractory delayed emptying of the intrathoracic stomach after Ivor-Lewis esophagogastrectomy.7,8
Electrical pacing is known to suppress ectopic pacemakers in the Roux limb.5 In an animal model, antegrade pacing of the Roux limb abolished the ectopic pacemakers and improved gastric emptying of solids.6 In the patient described here, the application of high-frequency electrical stimulation to the jejunal limbs may have resulted in overriding of the ectopic jejunal pacemakers, resulting in clinical improvement. This concept is supported by the normalization of the electrogastrogram of the afferent (distal) limb with stimulation, with the frequency having increased from 4.1 cpm to 9.0 cpm. The major limitation of this case is that it is limited to a single patient experience. Also, there is a likelihood of spectrum effect/bias in this patient with such severe symptoms; however, the improvement in her electrogastrogram parameters with electrical stimulation as well as the apparent improvement noted on the radionuclide emptying study probably indicates a true improvement secondary to electrical stimulation.
In the current case, the application of GI electrical stimulation in intractable delayed emptying and stasis of the atonic intrathoracic jejunal limbs after esophagojejunostomy seems very promising. As stated earlier, it is also possible that GI electrical stimulation resulted in overriding of ectopic pacemakers in the jejunal limb, thereby abolishing reverse peristalsis. It provides a viable option in the management of this difficult group of patients unresponsive to medical therapy. Implanting permanent electrodes surgically in this patient would have required a thoracotomy or thoracoscopy. Before undertaking such procedures, we sought to assess the response to GI electrical stimulation by temporary endoscopic electrode placement. We have previously demonstrated a good correlation of outcomes between temporary and permanent GES.2
To the best of our knowledge, this is the first reported case of GI electrical stimulation in the management of Roux stasis syndrome, in this case a patient who had undergone total gastrectomy with an intrathoracic Roux-en-Y anastomosis. The patient has been scheduled for thoracotomy with placement of permanent electrodes on the intrathoracic portion of the jejunal limbs. Electrical stimulation of the Roux limb is a potential treatment for patients with Roux stasis syndrome, but further human studies are needed.
Take-home Message.
Endoscopic placement of temporary electrodes is feasible and safe.
GI electrical stimulation of the jejunal limb is a potentially effective treatment for Roux stasis syndrome.
Abbreviation
- GES
gastric electrical stimulation
Footnotes
DISCLOSURE: T. Abell is a licensor, speaker, investigator, and consultant for Medtronic Inc. S-J Tang is a consultant for Olympus America Inc. No other financial relationships relevant to this publication were disclosed.
References
- 1.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 2.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–61. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 3.Zonca S, Rizzo P. Alteration of the Roux Stasis syndrome by an isolated Roux limb: correlation of slow waves and clinical course. Am Surg. 1999;65:666–72. [PubMed] [Google Scholar]
- 4.Schippers E, Willis S, Ruckdeschel G, et al. Small intestinal myoelectrical activity and bacterial flora after Roux-en-Y reconstruction. Br J Surg. 1996;83:1271–5. [PubMed] [Google Scholar]
- 5.Morrison P, Miedema BW, Kohler L, et al. Electrical dysrhythmias in the Roux jejunal limb: cause and treatment. Am J Surg. 1990;160:252–6. doi: 10.1016/s0002-9610(06)80017-6. [DOI] [PubMed] [Google Scholar]
- 6.Miedema BW, Kelly KA. The Roux stasis syndrome: treatment by pacing and prevention by use of an ’uncut’ Roux limb. Arch Surg. 1992;127:295–300. doi: 10.1001/archsurg.1992.01420030057011. [DOI] [PubMed] [Google Scholar]
- 7.Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: long-term outcome. South Med J. 2005;98:693–7. doi: 10.1097/01.smj.0000168660.77709.4d. [DOI] [PubMed] [Google Scholar]
- 8.Salameh JR, Schmieg RE, Jr, Runnels JM, et al. Refractory gastroparesis after Roux-en-Y gastric bypass: surgical treatment with implantable pacemaker. J Gastrointest Surg. 2007;11:1669–72. doi: 10.1007/s11605-007-0331-8. [DOI] [PubMed] [Google Scholar]
- 9.Abidi N, Starkebaum WL, Abell TL. An energy algorithm improves symptoms in some patients with gastroparesis and treated with gastric electrical stimulation. Neurogastroenterol Motil. 2006;18:334–8. doi: 10.1111/j.1365-2982.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 11.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–12. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]



