Abstract
Background
Gastric electric stimulation (GES) at a high-frequency, low-energy setting is an option for treating refractory gastroparesis. The currently available commercial stimulator, the Enterra neurostimulator (Medtronic Inc, Minneapolis, MN), however, requires surgical implantation and is powered by a nonrechargeable battery.
Objective
To develop and test a miniature wireless GES device for endoscopic implantation in an experimental model.
Design
In-vivo gastric signals were recorded and measured in a nonsurvival swine model (n = 2; 110-lb animals).
Intervention
An endoscopically placed, wireless GES device was inserted into the stomach through an overtube; the two GES electrodes were endoscopically attached to the gastric mucosa and secured with endoclips to permit stimulation.
Main Outcome Measurements
Stable electrogastrogram measures were observed during GES stimulation.
Results
Electrogastrogram recordings demonstrated that gastric slow waves became more regular and of constant amplitudes when stomach tissues were stimulated, in comparison with no stimulation. The frequency-to-amplitude ratio also changed significantly with stimulation.
Limitation
Nonsurvival pig studies.
Conclusion
Gastric electric stimulation is feasible by our endoscopically implanted, wireless GES device.
INTRODUCTION
Gastroparesis (GP) is associated with disordered gastric emptying of a solid meal and with abnormal gastric myoelectric activity that hampers gastric slow wave frequency and amplitude.1,2 Gastric electric stimulation (GES), effective in the treatment of GP,3–5 is now most commonly provided through the Enterra neurostimulator (Medtronic Inc, Minneapolis, MN), which the U.S. Food and Drug Administration has approved for humanitarian use. This stimulator produces at its lowest setting an electric pulse train with a frequency of 14 Hz and a current output of 5 mA, effectively decreases the symptoms of GP, and has a variable effect on gastric emptying.1,6–9
The Enterra neurostimulator measures 60 mm × 55 mm × 10 mm, necessitating surgical implantation. Its power source, a nonrechargeable battery of limited life, requires subsequent replacement surgical procedures. To circumvent these shortcomings, we have developed a prototype miniature gastric electric stimulator, which is wirelessly rechargeable and small enough for endoscopic implantation, and have tested it in an experimental model.
MATERIALS AND METHODS
Animals
This nonsurvival study protocol, approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, was conducted at its Animal Endosuite by using two 5-month-old male pigs, approximately 110 lb each, with a cardiac rate of approximately 110 cycles per minute and a respiratory rate of 12 cycles per minute.
Wireless GES device
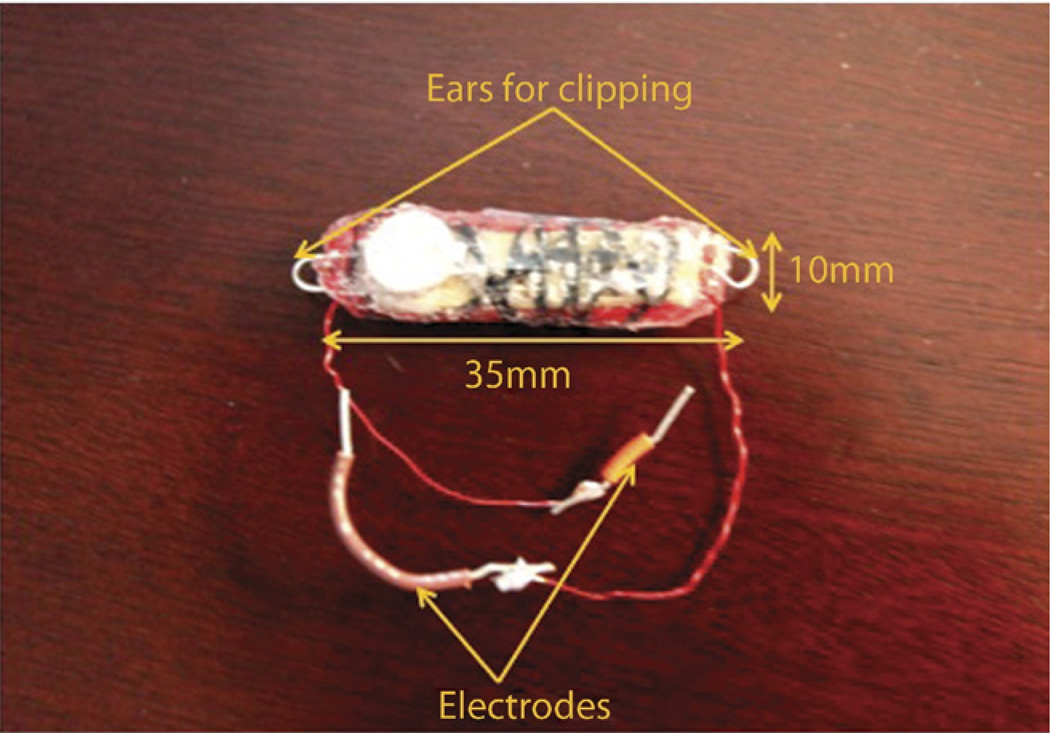
Our prototype wirelessly rechargeable stimulator measures 35 mm × 10 mm × 8 mm (Fig. 1) and weighs 5.2 g. It can be alternated from stimulation to charging mode through a magnetic reed switch (KSK-1A80-1015), operated by an external magnet. Apart from two ear-shaped, stainless steel structures (“ears”) with which the device is secured during endoscopic attachment, the entire stimulator is coated with a biocompatible polymer polydimethylsiloxane for packaging to avoid electric shortcircuit.10
Figure 1.
Endoscopically implantable gastric electric stimulator (GES).
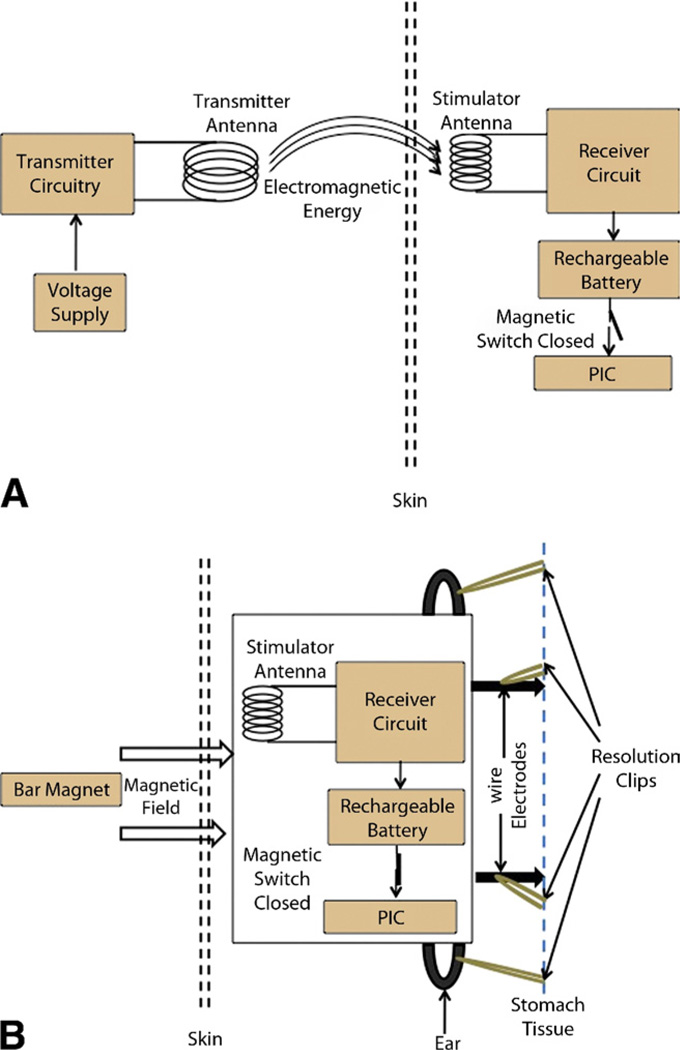
The stimulator’s external transmitter (11.5 cm × 11.5 cm) transmits 3 W of electromagnetic power at a radio frequency of 1.3 MHz to the stimulator to permit charging of the 3.0-V, 11-mAh, rechargeable, lithium-ion battery (MS920S-FL27E, Seiko Instruments, Torrance, CA, USA, Inc.) (Fig. 2A).10,11 The coin cell battery must be recharged after 19 hours of use, with approximately 30 minutes required for each charging cycle, and can sustain 100 recharging cycles (about 3 months of operation). Hence, this battery would need to be reloaded after 100 recharging cycles, entailing replacement of the device.
Figure 2.
Block diagram illustration of the wireless-rechargeable GES device in A, charging mode and B, stimulation mode.
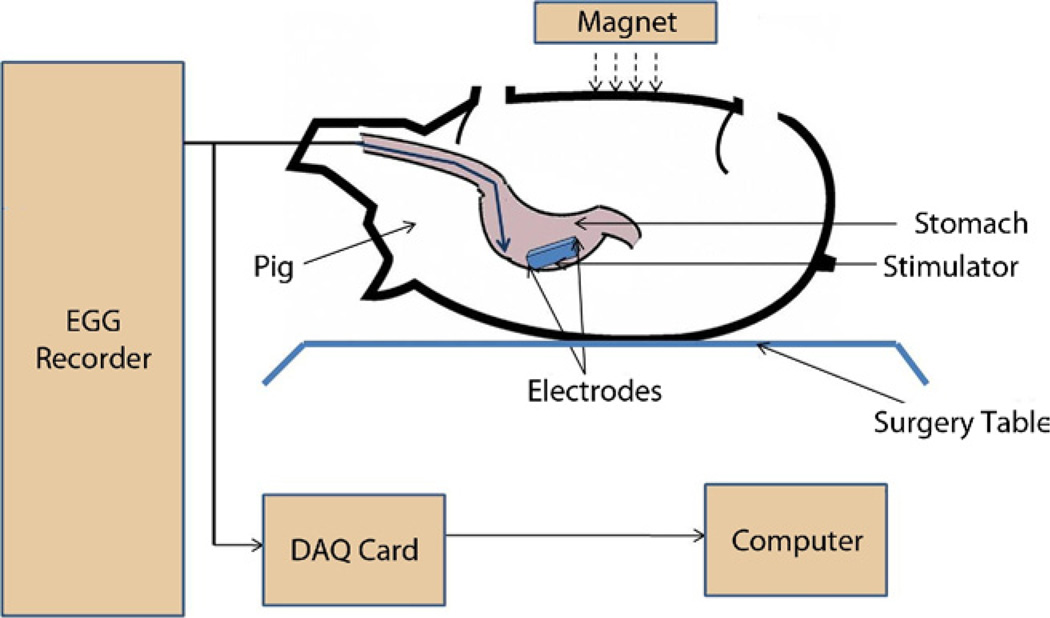
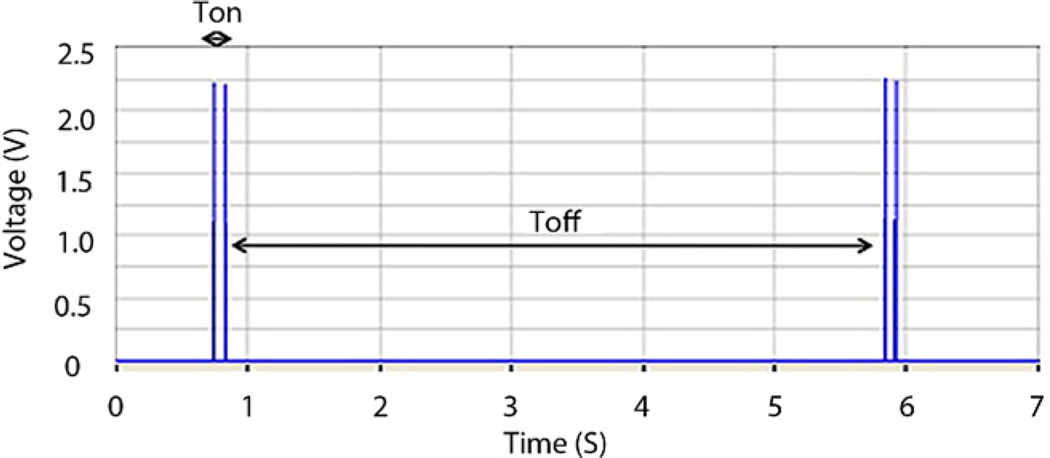
Stimulator pulses are generated by a preprogrammed peripheral interface controller, which becomes activated once the magnet is brought into proximity to the patient. With this activation, the reed switch closes, permitting a connection between the battery and the peripheral interface controller to be established (Fig. 2B). The stimulator then produces pulses at a frequency of 14 Hz with a “pulse ON” time of 330 µs and a duty cycle of 0.46%. The “cycle ON” (TON) and “cycle OFF” (TOFF) times are 0.1 s and 5 s, respectively. Figure 3 shows the stimulation pulses delivered to tissues. Figure 4 shows the experimental setup for testing the effects of the GES device.10
Figure 3.
Stimulation output pulse train btained with 500Ω load attached.
Figure 4.
Animal experimental setup.
Endoscopic placement and attachment of device
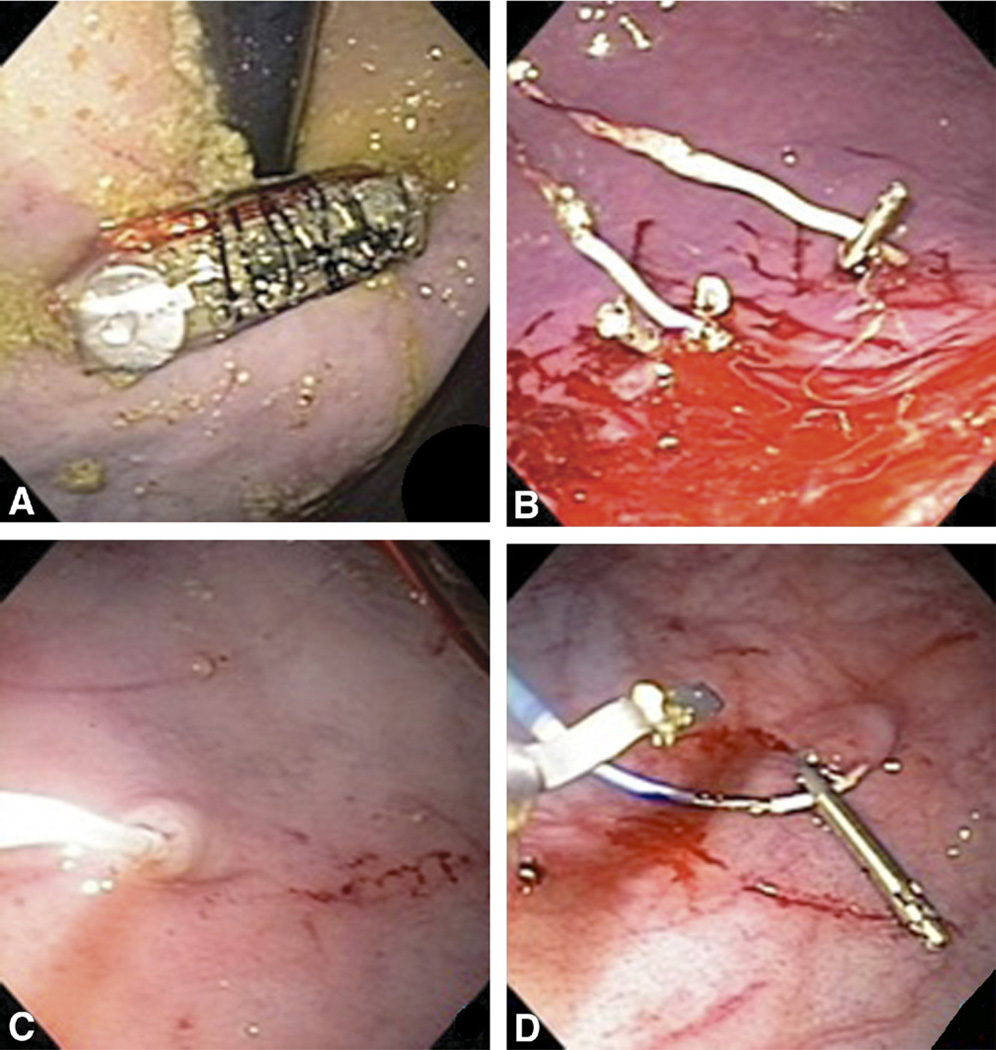
We used a diagnostic gastroscope (Olympus GIF-Q160, Olympus America, Center Valley, PA) for endoscopic visualization and device attachment. An overtube (US Endoscopy, Mentor, OH) was gently pushed into the esophagus over the endoscope for subsequent endoscopic esophageal reintubation (Fig. 5A). The overtube has an internal diameter of 16.7 mm, an outer diameter of 19.5 mm, and a length of 25 cm. After endoscopic examination of the stomach, the miniature stimulator was easily deployed through the overtube into the esophagus and pushed by endoscope into the stomach.
Figure 5.
A, Device insertion by endoscopic overtube. B, Pinning of wire electrodes into the gastric mucosa. C, Temporary transvenous cardiac pacing lead screwed into the mucosa for EGG measurement. D, Resolution clip securing of the temporary leads.
The wire electrodes (Fig. 1) were pinned into the gastric mucosa at the distal body with an endoscopic rat tooth forceps (Olympus America) and secured by endoclip (Resolution clip, Boston Scientific, Natick, MA) (Fig. 5B) (Video 1, available online at www.giejournal.org).12,13 Another pair of endoclips further secured the device with one clip arm through the electrode’s built-in “ear” loop (not shown). The resolution clips can stay attached to the gastric mucosa for as long as 4 weeks.
Attachment of electrogastrogram recording leads
A temporary transvenous cardiac pacing lead (model 6414-200 cm, Medtronic Inc., Minneapolis, MN), inserted through the endoscopic channel, was then screwed into the mucosa by applying torque on a lead wire situated within an outer sheath (Fig. 5C). After the sheath was removed, the lead was secured by 3 endoclips to the underlying mucosa (Fig. 5D), with 1 endoclip placed on the lead’s metallic end to achieve the desired electric impedance.
Signal measurement and analysis
Once the cardiac pacing lead had been attached to the mucosa, the outside end was connected to the electrogastrogram (EGG) recorder (Sandhill Scientific, Highlands Ranch, Colorado) for myoelectric signal recording of the stomach. These myoelectric signals were then quantitatively analyzed by averaging mean frequencies and amplitudes as well as the frequency-to-amplitude ratios (FARs) of the gastric slow waves. The EGG recordings were obtained in three steps, as follows. The first reading was recorded for 30 minutes with the stimulator turned OFF (Baseline-1). The second reading was recorded with the stimulator turned ON for 30 minutes (Stimulation). The third reading was recorded with the stimulator turned OFF again (Baseline-2). Between each reading, an interval of 4 minutes without EGG measurement was allowed to elapse to ensure separation between the readings. Slow wave frequency was analyzed qualitatively as regular or irregular rhythm, amplitudes were analyzed as equal or unequal, during recordings with and without stimulation. The FAR, depicting the stability of slow wave signals, is shown in Table 1. Table 2 (Appendix, available online at www. giejournal.org), displays the pilot findings on EGG parameters in healthy patients, patients with GP, and GP patients after gastric stimulation.
TABLE 1.
Electrogastrogram signal summary
| Devices | Impedance (Ω) |
O/P volt (V) |
O/P current (mA) |
Rhythm | Freq range (bpm) |
Mean freq (bpm) |
Amp of wave |
Amp range (mV) |
Mean amp (mV) |
FAR (bpm/mV) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline-1 | 1751 | – | – | IRR | 2.00–4.00 | 3.00 | UA | 0.0625–0.525 | 0.1831 | 16.382 |
| Mucosal stimulation |
1751 | 1.9 | 1.097 | RR | 3.00–4.00 | 3.33 | EA | 0.1100–0.1500 | 0.1283 | 25.955 |
| Baseline-2 | 1751 | – | – | IRR | 3.00–5.00 | 3.67 | UA | 0.0500–0.1375 | 0.0858 | 42.657 |
RR, Regular rhythm; IRR, irregular rhythm; Amp, amplitude; Freq, frequency; EA, equal amplitude; UA, unequal amplitude; FAR, frequency-to-amplitude ratio; O/P, output.
Mucosal DC impedance was measured to be 1751 Ω with the Enterra controller. A data acquisition system (DAQ USB 6210, National Instruments, Austin, TX), which interfaced between the stimulator electrode wires and the computer, was used to measure voltage outputs. The electric current delivered to the tissues was computed from voltage readings, and DC impedance was measured. A 20-minute recording was taken for each EGG measurement.
RESULTS
Endoscopic attachment of the miniature stimulator was successfully performed. The measured output voltage of the pulses was 1.9 V, indicating an electric current of 1.097 mA delivered to the tissues; however, the gastrostimulator when tested during a bench-top experiment delivered 3.2 mA, with the highest impedance (800Ω) obtained in human models.1 The Baseline-1 EGG signals showed irregular rhythm, with unequal amplitudes as rhythm and amplitude changed during stimulation. The FAR seen during Baseline-1 was lower than the FAR observed during stimulation. Rhythmic activity worsened during Baseline-2, with irregular rhythm and unequal amplitudes observed. The FAR during stimulation was 25.955 bpm/mV but increased to 42.657 bpm/mV when the stimulation was turned OFF and the second baseline reading was recorded.
The operation of the reed switch in the GES, which lay beneath muscle, fat tissues, and the stomach wall, was effected by a magnet. Stimulation was turned ON by holding the magnet at a distance of 3 cm from the skin, for a total distance of approximately 7 cm from magnet to stimulator.
DISCUSSION
We successfully developed and tested a prototype miniature, wirelessly rechargeable, gastric electric stimulator for endoscopic implantation in an experimental model. The miniature stimulator was easily attached endoscopically (Fig. 5B) and was well secured by the use of endoclips, wire electrodes pinned into the mucosa, and “ears” clipped into place. Stimulator electrodes were placed in the distal gastric body, along the greater curvature or posterior wall. The cardiac (EGG) lead was placed in the proximal aspect of the gastric body, close to the cardia. We have used endogastric electrodes positioned close to the signal origin to facilitate precision and accuracy in EGG recording. We were able to modulate the myoelectric activities of the porcine stomach with this endoscopically implanted stimulator.
This miniature stimulator was designed for the human stomach, where mucosal impedance varies between 200 Ω and 800 Ω.1 The delivered current measured in the pig model, at 1.9 mA, was lower than the anticipated value of 5 mA value for humans. This is also verified by the measured impedance of 1751 Ω in the pig stomach, which is higher than that expected for the human stomach.
Future applications in patients will not include the wires used for the measurements reported here. A significantly longer delay between measurements is suggested for obtaining more accurate EGG recordings, independently of prior stimulation effects. Because of the physical condition of the pig, and to permit maximum possible data capture, we limited the time interval between recordings to 4 minutes, but for human intervention the delays between the baseline and the stimulation readings need to be increased. In future studies, we will obtain more data but will allow longer intervals between experimental observations.
In the Baseline-2 recording, a significant increase in FAR (by 16.702 bpm/mV) was noted, as compared with the FAR during stimulation. Slow wave amplitude was impaired during this Baseline-2 recording, and at times unequal peaks were observed. However, slow wave rhythmic activity had also been irregular at the Baseline-1 reading, before initial stimulation, perhaps in response to the insertion procedure and/or minor scratches in the mucosa occurring during insertion.
Our results predict the feasibility of an endoscopically implantable, miniature wireless system that can be operated and recharged in patients. Further studies are needed to analyze the potential long-term impact and damages, if any. Future systems will deliver different pulses to stomach tissues according to the severity of the gastric anomaly.
CONCLUSION
Our endoscopically implantable, rechargeable miniature gastrostimulator may eventually eliminate the replacement and surgical drawbacks of the neurostimulator currently in use. This being the first endoscopic implantable gastric stimulator, further development is feasible by investigations of the different endoscopic attachment procedures of the stimulator. The gastrostimulator may further provide a nonsurgical means of assessing any difference in the effects of permanent or long-term temporary gastric stimulation, as compared with permanent stimulation via serosal electrodes.
Take-home Message.
This breakthrough research has permitted a significant reduction in gastrostimulator size and has made this prototype device completely wireless and wirelessly rechargeable. Moreover, it is endoscopically implantable and therefore has the potential to eliminate the surgical procedure that many patients with gastroparesis now undergo each year.
Abbreviations
- GES
gastric electric stimulation
- EGG
electrogastrogram
- GP
gastroparesis
- FAR
frequency-to-amplitude ratio
APPENDIX TABLE 2
Typical EGG parameters in healthy patients and patients with gastroparesis
| Patients | Frequency (bpm) |
Amplitude (mV) |
FAR (bpm/mV) |
|---|---|---|---|
| Patients | |||
| Healthy Patient | 3.0 | 0.5 | 6.0 |
| GP Patient | |||
| Normal frequency |
3.0 | 0.2 | 15 |
| High Frequency |
7.0 | 0.2 | 35 |
| GP Patient after GES |
|||
| Normal frequency |
4.5 | 0.4 | 11.25 |
| High Frequency |
5.0 | 0.4 | 12.5 |
GP, gastroparesis, GES, gastric electrical stimulation.
Footnotes
DISCLOSURE: The authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 2.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Ross RA, McCallum RW, et al. Two-channel gastric pacing with a novel implantable gastric pacemaker accelerates glucagon-induced delayed gastric emptying in dogs. Am J Surg. 2008;195:122–129. doi: 10.1016/j.amjsurg.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster J, Sarosiek I, Lin Z, et al. Further experience with gastric stimulation to treat drug refractory gastroparesis. Am J Surg. 2003;186:690–695. doi: 10.1016/j.amjsurg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol. 2006;9:351–360. doi: 10.1007/s11938-006-0017-4. [DOI] [PubMed] [Google Scholar]
- 6.Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437–442. doi: 10.1016/j.jpedsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:257–258. doi: 10.1111/j.1572-0241.2003.08681.x. [DOI] [PubMed] [Google Scholar]
- 8.Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- 9.O’Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009;33:1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deb S, Tang SJ, Abell TL, et al. Miniature wireless gastro-stimulators for gastric dysmotility. Med Eng Phys. In press. [Google Scholar]
- 11.Ativanichayaphon T, Tang S-J, Hsu L-C, et al. An implantable batteryless wireless impedance sensor for gastroesophageal reflux diagnosis. IEEE International Microwave Symposium; May 23–28, 2010; Anaheim, CA. [Google Scholar]
- 12.Daram SR, Tang SJ, Abell TL. Temporary gastric electrical stimulation for gastroparesis: endoscopic placement of electrodes (ENDOstim) Surg Endosc. 2011;25:3444–3445. doi: 10.1007/s00464-011-1710-5. [DOI] [PubMed] [Google Scholar]
- 13.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496.e3–503.e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]