Abstract
The aim of the study was to determine the clinical outcome of lung cancer patients with a secondary malignancy according to the time sequence between the lung cancer and the secondary malignancy.
Retrospective review of all lung cancer patients with any secondary cancer treated from June 2004 to July 2012. The survival of patients with a secondary malignancy was compared to those patients without a secondary malignancy. According to the time sequence between the lung cancer and the secondary malignancy, patients were divided into 4 groups. Group I: lung cancer without any other malignancy, Group II: lung cancer with a secondary malignancy at follow-up, Group III: lung cancer with a pre-existing malignancy, Group IV: synchronous malignancies (diagnosis interval between lung cancer and a secondary malignancy of less than 3 months).
Patients with any secondary cancer in their history or at follow up included 157 patients (9.5%). Collectively; the median survival was significantly better for patients with a secondary malignancy, 19.09 months, compared to those without a secondary malignancy, 9.53 months, P < 0.001, HR 0.66 (95% CI 0.55 – 0.79). However, the survival differed significantly according to the time sequence between the lung cancer and the secondary malignancy. The median survival was 47.9 months for group II patients, 12.19 months for group III, 17.51 months for group IV, and 9.53 months for group I; P = 0.001. In Cox proportional hazard analysis, the risk of dying decreased by 68% in group II patients compared to group I patients, HR 0.32 (95% CI 0.21–0.5), P < 0.001. Although the risk of dying for group III and IV decreased by 19% and 16% respectively compared to group I patients, it did not reach statistical significance.
Nowadays, secondary malignancy in lung cancer patients is a frequent finding. Better survival was observed for patient with secondary malignancy following lung cancer.
Keywords: lung cancer, secondary malignancy, survival
1. Introduction
Lung cancer is a deathly disease that affects mostly elderly patient, and it causes more deaths than breast cancer, colon cancer, and pancreatic cancer combined.[1] Technological advancement and screening programs had allowed for detection of lung cancer at earlier stage with potential for curative treatment. However, the clinical outcome of lung cancer is influenced not only by the stage of the disease, but also by the presence of a secondary cancer or comorbidities.[2] The presence of multiple synchronous or metachronous cancers are becoming more and more frequent in today's clinical practice.[3–6] The survival outcome of lung cancer patients with secondary malignancy had conflicting results in the literature.[7–12] In this study, we evaluate the clinical outcome of lung cancer patients with secondary malignancy and the influence of comorbidities.
2. Methods
We performed a retrospective clinical review from June 2004 to July 2012 at Chang-Gung Memorial hospital at Chia-Yi, Taiwan, a tertiary university affiliated hospital. The electronic medical record and cancer center database were cross-matched for the coexistence of lung cancer and any second malignancy. The following clinical data were extracted from the cancer center database: age and date of lung cancer diagnosis, gender, smoking history, lung cancer histology and stage at diagnosis, primary treatment received, and overall survival. The time elapsed between the diagnosis lung cancer and the second malignancy was defined as cancer interval. For lung cancer treatment evaluation, all treatment modalities given within 3 months post diagnosis were considered. Lung cancer staging was performed according to the definitions from the 7th edition of the American Joint Committee on Cancer TNM staging system.[13]
Clinical staging of any patient with lung cancer included physical examination, chest radiography, bronchoscopy, chest computed tomography, spirometry, brain magnetic resonance imaging or brain computed tomography, bone scan and positron emission tomography. Post-treatment follow-ups were carried out at the outpatient clinic every 3 months for 3 years and every 6 months thereafter. Patients with incomplete medical record, creatinine measurement or the absence of pathology report were excluded. Survival time was measured from the day of lung cancer diagnosis till the date of death, or at the end of follow- up (Dec 31, 2014). Patients with loss of follow-up were contacted by telephone by the cancer center case manager. Those patients not reachable by telephone were considered death if were excluded from the National Health Insurance or appeared at the annual cause of death report from the Health Promotion Administration, Ministry of Health and Welfare, Taiwan. This organization releases annual cause of death report of all cancer cases registered to each cancer center for status update, usually with a delay of 12 to 18 months. This study was approved by the institutional review board of Chang Gung Memorial Hospital.
For the evaluation of the effect of comorbidities, Charlson comorbidity index (CCI)[14] and chronic kidney disease (CKD) were used. In the Charlson comorbidity index,[14] the association with mortality is weighted for 19 chronic diseases, and the sum of each point score was used to a total score. CKD was defined as creatinine clearance [CrCl] of < 60 mL/min/1.73 m2 calculated by the Cockcroft–Gault formula[15] in the presence of proteinuria / hematuria or the presence abnormal kidney imaging; otherwise, the mere presence of a creatinine clearance [CrCl] of < 60 mL/min/1.73 m2 will be considered as non-CKD. Cockcroft–Gault formula[15] was selected for the determination renal function status because it is the most commonly used formula in the clinical care of cancer patients. The correlation to measured CrCl in multiple disease settings had been proven.[16] Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)[17] developed a new formulation to estimates renal function. The Kidney Disease Quality Outcomes Quality Initiative (KDOQI)[18] had recommended using the CKD-EPI creatinine equation to predict eGFR in adults. However, its clinical use in routine practice has not been established.
According to the time sequence between lung cancer and the secondary malignancy, patients were divided into 4 groups: Group I: lung cancer without any other malignancy, Group II: lung cancer with a secondary malignancy at follow-up, Group III: lung cancer with a pre-existing malignancy, Group IV: synchronous malignancies (diagnosis interval between lung cancer and a secondary malignancy of <3 months). The Martini and Melamed criteria[19] was used to distinguish synchronous and metachronous metastatic tumors.
3. Statistical analysis
Differences in proportions were compared using Chi-Square or Fisher exact test depending on the cell size. Analysis of variance was used for numerical variables. Continuous variables were categorized using median values as the cut-off point. Age was divided into younger and older than 70 year old, Charlson comorbidity index score divided into less or more than 7, and clinical stage was separated into early stage (stage I-IIIA) versus late stage (stage IIIB and IV) and treatment modality into supportive care, surgical treatment and medical treatment group. The Kaplan–Meier method was used for survival analysis. The log-rank test was used to calculate the difference in survival times. Cox proportional hazard analysis was used to estimate the level of significance with the relative risks and their 95% confidence interval. P value of <0.05 was considered of statistical significance. SPSS 21.0 software (SPSS Inc, Chicago, IL) was used for statistical analysis.
4. Results
During the study period, 1772 patients with the diagnosis of lung cancer were registered in our database. One-hundred and eleven patients were excluded (55 patients had incomplete medical treatment record, 46 patients had no record of creatinine level, and 10 patients were excluded because the diagnosis of lung cancer was not confirmed by our in-house pathologist). After exclusion, 1661 patients were included in this study. Patient without any history of malignancy included 1504 patients (90.5%) and patients with any secondary cancer in their history or at follow up included 157 patients (9.5%).
4.1. Patients with and without secondary malignancy
The age at lung cancer diagnosis, gender, smoking history, creatinine levels, creatinine clearance, Charlson comorbidity index score, CKD proportion and histology type were not significantly different between patients with and without second malignancy. Demographic characteristic is presented in Table 1. The distribution of secondary malignancy's location is presented in Table 2. The proportion of lung cancer patients having a second malignancy with earlier stage (Stage I-IIIA) was 42.7%, compared to 19.2% for patients without second malignancy, P < 0.001. The proportion of patients without second malignancy history receiving surgical treatment was 12.3% compared to 28.7% in of patients with second malignancy, whereas the proportion of patients receiving supportive treatment were 21.4% and 10.2% respectively. The median survival for patient without secondary malignancy was 9.53 months (95% CI 8.73 – 10.32) compared to 19.09 months (95% CI 14.39–23.78), P < 0.001, HR 0.66 (95% CI 0.55 – 0.79). Figure 1 shows the survival curve for patient with and without secondary malignancy.
Table 1.
Demographic characteristics of patients with and without secondary malignancy.
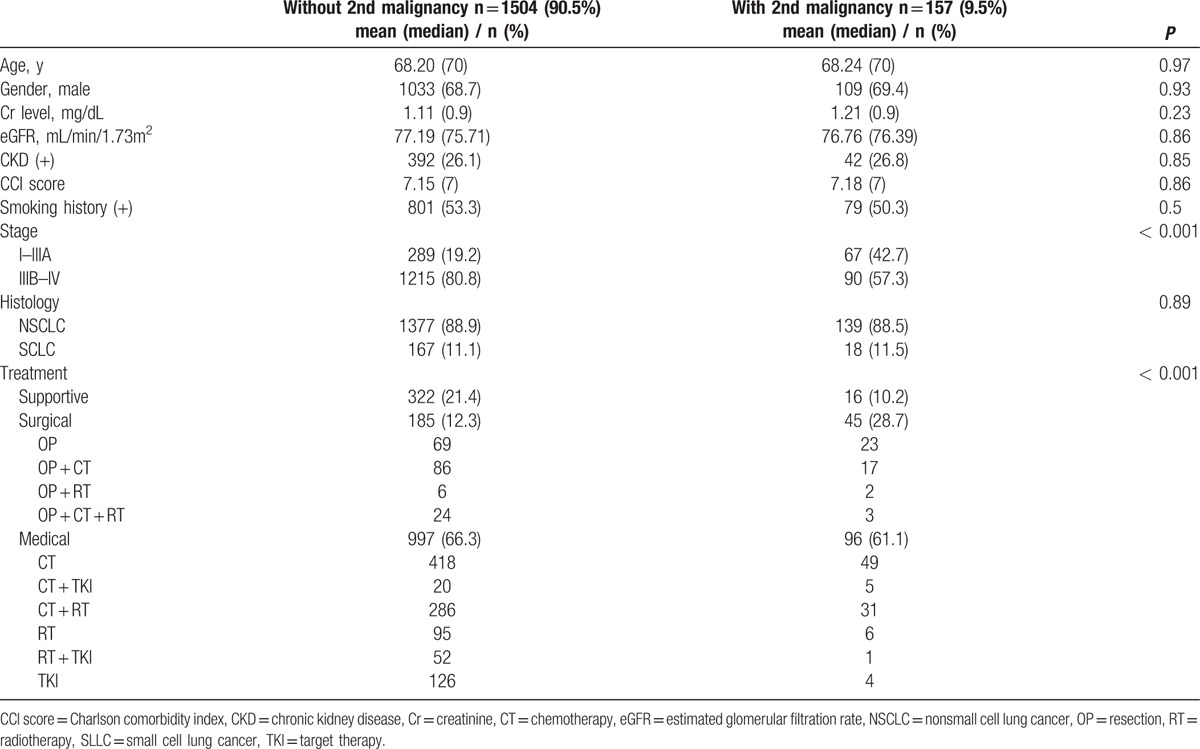
Table 2.
Location of secondary malignancies.
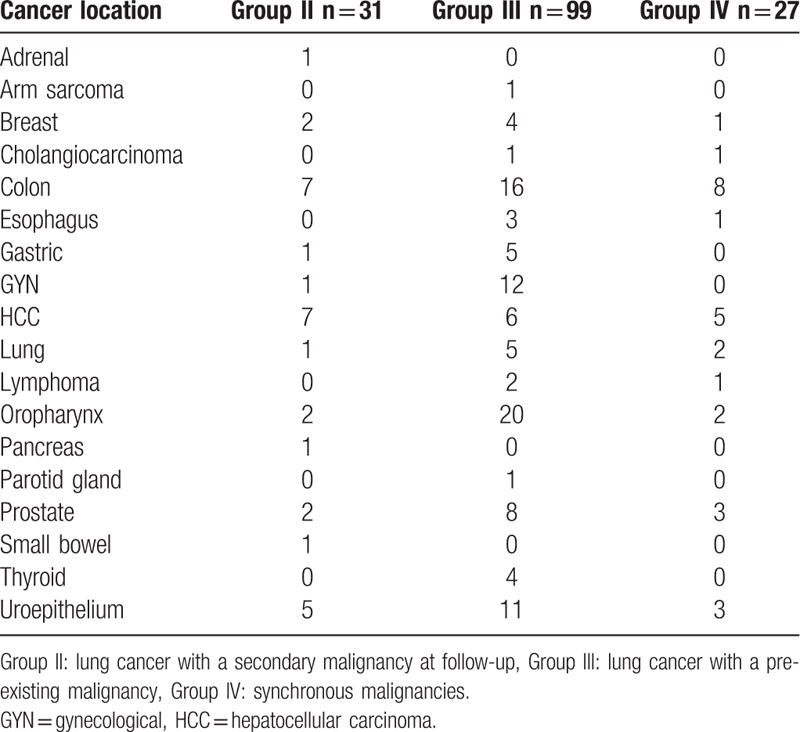
Figure 1.
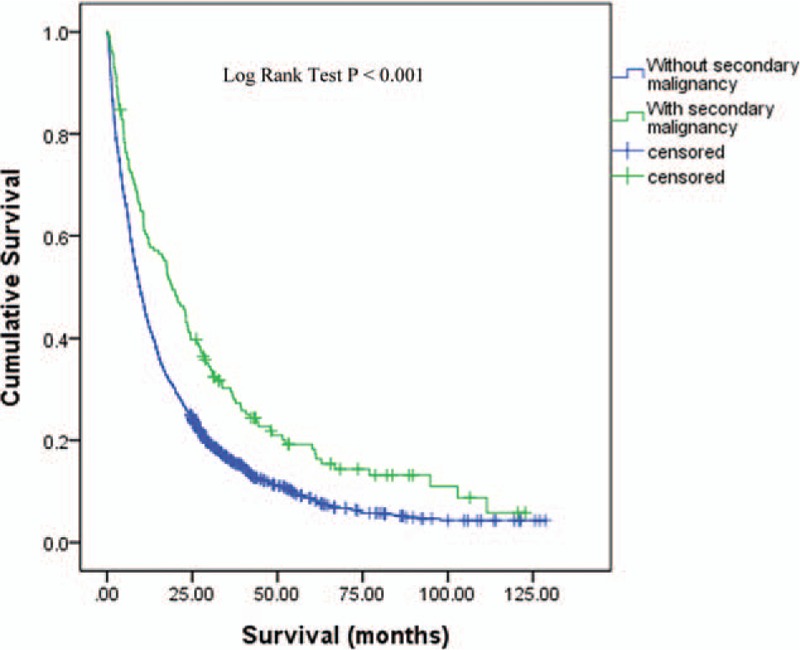
Survival curve for patients with and without secondary malignancy. The median survival for patients without secondary malignancy was 9.53 months compared to 19.09 months, P < 0.001, HR 0.66 (95% CI 0.55–0.79). CI = confidence of interval, HR = hazard ratio. CI = confidence interval, HR = hazard ratio.
4.2. Timing of secondary malignancy diagnosis
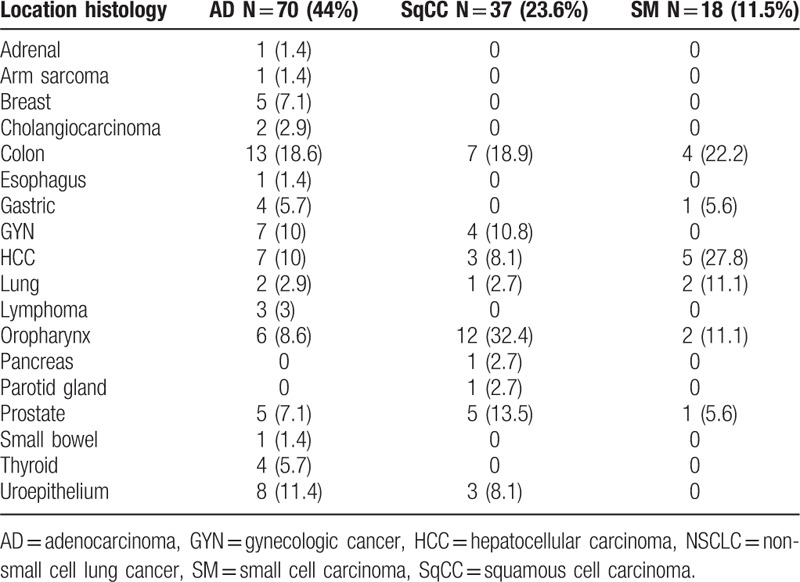
According to the timing of secondary malignancy diagnosis, group II (31 patients, 1.9%) had a median cancer interval of 20.54 months; group III (99 patients,6.0%) had a median cancer interval of 40.28 months; and group IV (27 patients, 1.6%) had a median cancer interval of 0.43 months. The cancer interval between patients with secondary malignancy was significantly different (P < 0.001). The cancer interval between group II and III remained statistically significant after exclusion of patient with synchronous cancer, median 20.54 vs 40.28 months respectively (P = 0.006). The gender, creatinine levels, creatinine clearance, Charlson comorbidity index score, CKD proportion, stage and histology type were not significantly different between patients with different cancer interval (Table 3). The age at lung cancer diagnosis was significantly different among patients with different tumor interval, P = 0.03. Group II patients were significantly younger compared to group III and IV patients, P = 0.04. The proportion of patients with smoking history was highest in group II (77.4%) compared to 42.4% and 48.1% for group III and group IV, respectively, (P = 0.003). Table 4 shows the association between lung cancer histology type (adenocarcinoma, squamous cell carcinoma, and small cell lung cancer) and the location of secondary malignancies. The proportion of tobacco-related secondary malignancy according to lung cancer histology was 65.7% for adenocarcinoma, 81.1% for squamous cell carcinoma, and 94.4% for small cell carcinoma.
Table 3.
Demographic characteristics according to the time sequence of the secondary malignancy. .
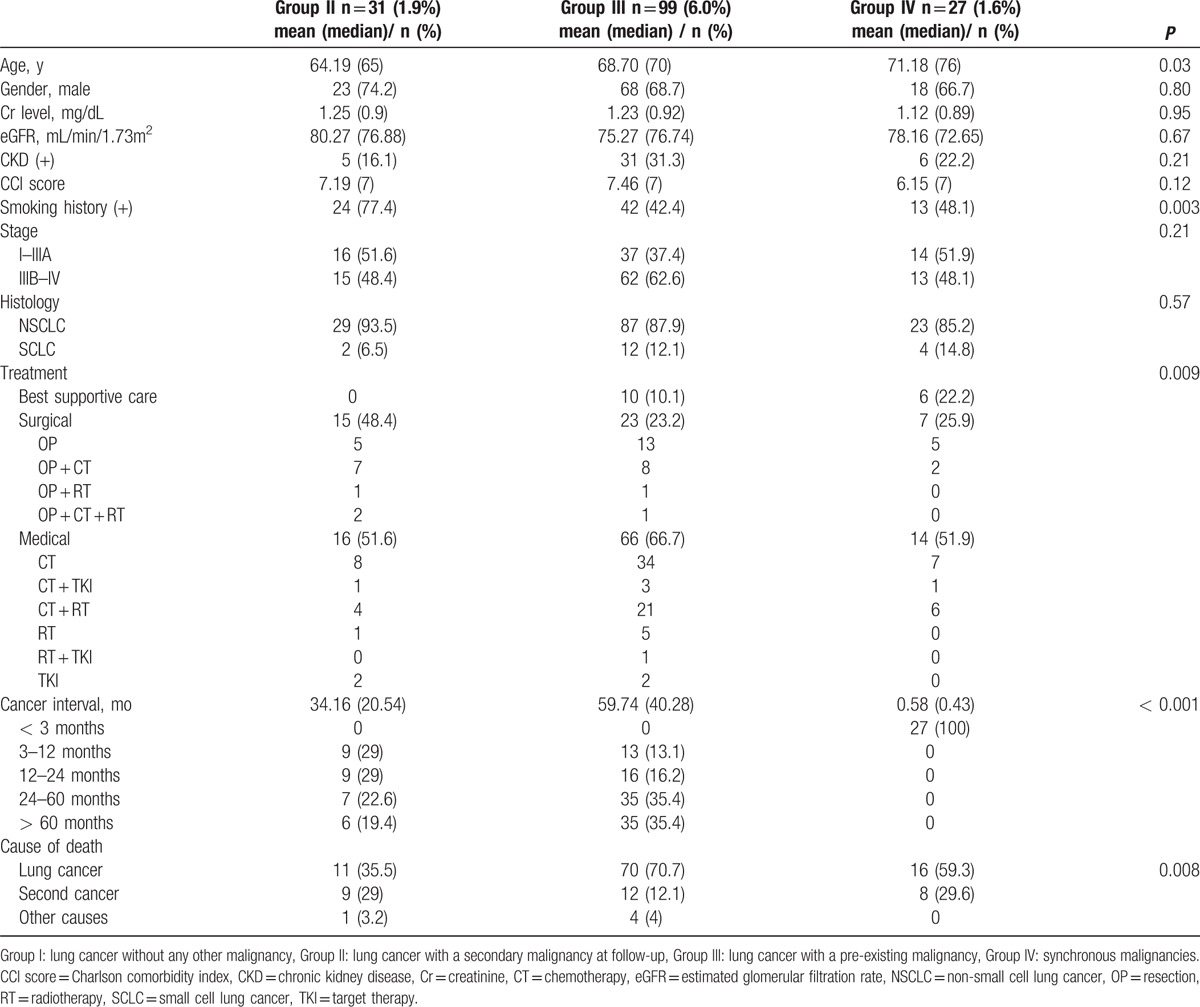
Table 4.
Association between lung cancer histology type and location of secondary malignancies.
4.3. Survival according to the time sequence of the secondary malignancy
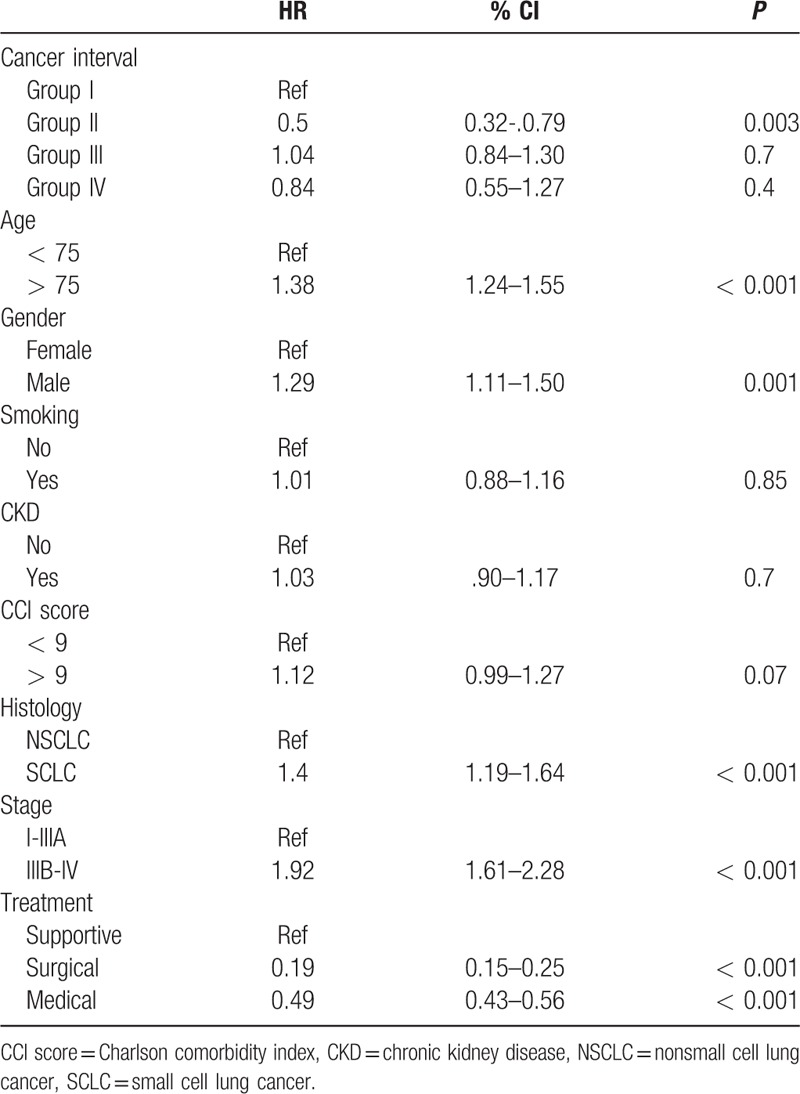
The median survival for patient with secondary malignancy was 47.9 months for group II (95% CI 22.32–73.48), 12.19 months for group III (95% CI 3.23–21.14), 17.51 months for group IV (95% CI 8.59–26.43), and 9.53 months for group I (95% CI 8.73–10.32); P = 0.001. In Cox proportional hazard analysis, the risk of dying for group II patients decreased by 68% compared to group I patients, HR 0.32 (95% CI 0.21–0.5), P < 0.001. Although the risk of dying for group III decreased by 19% (HR 0.81, 95% CI 0.65–1.01, P = 0.06) and 16% (HR 0.84, 95% CI 0.56–1.25, P = 0.39) for group IV, respectively, compared to group I patients, it did not reach statistical significance. Figure 2 shows the survival curve according to the time sequence of the secondary malignancy. The following factors had significant effect on cancer survival in the adjusted model: group II patients, younger than 75 year-old, female gender, nonsmall cell histology, earlier stage, and treatment modality (Table 5).
Figure 2.
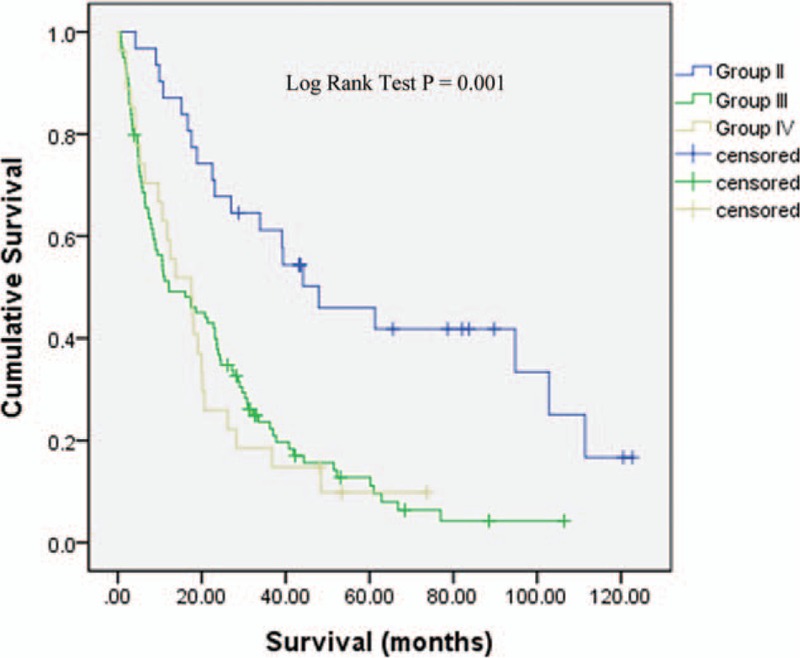
Survival curve according to the time sequence of the secondary malignancy. The median survival for patient with secondary malignancy was 47.9 months for group II, 12.19 months for group III, 17.51 months for group IV, and 9.53 months for group I; P = 0.001.
Table 5.
Cox proportional hazard analysis for factors associated to survival in patients with lung cancer and secondary malignancy.
5. Discussion
Advancement in imaging technology and the development of different cancer screening program have allowed for the detection of cancers at earlier stage. Therapeutic progress, either for curative or supportive modalities, has contributed to a continuous improvement in overall cancer survival. Because of the improving survival, and possibly due to the common etiologic factor and/or individual genetic susceptibility; the presence of multiple synchronous or metachronous cancers are becoming more and more frequent in today's clinical practice.[3–6]
Patients surviving 1 cancer are at risk of developing a new malignancy. This risk has been reported to be 5 to 15 times higher than that in the general population.[20] In this report, 9.5% of lung cancer patient had a second malignancy history, either before or after the diagnosis of lung cancer. This finding is consistent with previous literature report that varied between 0.9% and 26.3%. This wide range may be related to the methodological differences observed in the definition and scope of study.[8 9 12 21–25]
In a previous study in Taiwanese lung cancer patients, Liu et al[23] had found a significant difference between cancer interval in patients with lung cancer as the primary malignant tumor, and patients with lung cancer as secondary malignant tumor (median 10 months vs 46 months, P < 0.001). Our results suggested similar finding with a median cancer interval of 20 months for patients with lung cancer as the primary malignant tumor compared to 40 months, for patients with lung cancer as secondary malignant tumor, P = 0.006. Duchateau and Stokkel [8] had found a cancer interval of 14 months for patients with lung cancer as the primary malignant tumor compared to 83 months for patients with lung cancer as secondary malignant tumor, P < 0.05. In the study by Duchateau and Stokkel, 72.7% of secondary malignancies with lung cancer as primary malignancy had a cancer interval of <1 month.[8] In our report, we considered this short-time frame as synchronous. The development of a secondary malignancy is conditioned by the survival of the primary malignancy. Since lung cancer is a highly lethal disease, patients had to survive long enough to develop a secondary malignancy. Surviving patients are prompt to more intensive follow-up and examinations. Intensive examinations are likely the reason for a shorten cancer interval in patients with lung cancer as primary malignancy. The high prevalence of tobacco-related secondary malignancy indicates a possible common etiology exposure.
In this report, the baseline characteristics in regard to age at lung cancer diagnosis, smoking history, creatinine levels, creatinine clearance, Charlson comorbidity index score, CKD proportion, and histology type were not significantly different between patients with and without second malignancy. Collectively, the overall survival was significantly better for patient with a second malignancy. Patient with a secondary malignancy had greater proportion of patient at earlier stage and surgical resection. The reported literature on the survival of lung cancer patients holding a second malignancy presented with disparate outcome. Several authors had reported similar finding like ours, where lung cancer with a secondary malignancy had improved survival compared to those without secondary malignancy.[7–10] In our cohort, patients with secondary malignancy presents with earlier lung cancer stage and greater proportion of patient receiving surgical treatment. In the study by Hofmann et al,[7] the improved survival by patient with additional secondary malignancy is determined by a shift towards lower tumor stages and fewer SCLCs. However, others had reported better survival for patients without a second malignancy or no difference.[11–12] Although patients with a second malignancy had an improved survival collectively, the time frame order of the lung cancer diagnosis and the secondary malignancy does affect the overall survival. The median survival of patients with secondary malignancy was 47.9 months (95% CI 22.32–73.48) for group II patients, 12.19 months (95% CI 3.23–21.14) for group III patients, and 17.51 months (95% CI 8.59–26.43) for group IV patients; P < 0.001. In Cox proportional hazard analysis, the risk of dying decreased by 68% for group II patients compared to group I patients, HR 0.32 (95% CI 0.21–0.5), P < 0.001. Although the risk of dying for group III and IV decreased by 19% and 16% respectively compared to group I patients, it did not reach statistical significance. According to this, the temporal relationship of lung cancer to other cancer has implication in survival. The improved survival for patient having lung cancer before a secondary malignancy (group II) may be related several factors. Patients in group II were younger with a greater proportion of patients receiving surgical treatment, and no patients receiving supportive care.
The cause of death differs significantly among patients with second malignancy, P = 0.008 (Table 3). Lung cancer-related mortality was registered for 35.5% of patients in group II patients while it was 70.7% and 59.3% for patients in group III and IV, respectively. The proportion of mortality related to the second malignancy was similar in group II patients and group IV patients. In our report, 29% of primary lung cancer patients died from their secondary malignancy. This result is in accordance to the recent report by Donin et al,[26] where they used the Surveillance, Epidemiology, and End Result database and found that 36% of lung cancer patients died from their secondary malignancy. The cause of death among patient with a secondary malignancy demonstrate that the prognosis of lung cancer patients with a secondary malignancy is conditioned by the overall lung cancer survival rather than the secondary malignancy, because NSCLC is the first leading cause of cancer deaths.
The retrospective design of the study, the diversity and no standardized treatment, and the relative small number of patients in a single institution are several of the limitations of this study.
In our limited experience, we had found that lung cancer patients with a secondary malignancy had better survival than those without a secondary malignancy. Lung cancer patients who survive to develop a second malignancy have relative good outcome. Comorbidities are not a major determinant of survival in these patients.
Acknowledgments
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital at Chia-Yi, for their invaluable help.
Footnotes
Abbreviations: AD = adenocarcinoma, CCI = Charlson comorbidity index, CI = confidence interval, CKD = chronic kidney disease, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, CrCl = creatinine clearance, eGFR = estimated glomerular filtration rate, GYN= gynecological cancer, HCC = hepatocellular carcinoma, HR = hazard ratio, KDOQI = Kidney Disease Quality Outcomes Quality Initiative, TNM = tumor, nodes, metastasis.
The authors have no funding and conflicts of interest to disclose.
References
- 1. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2013 Incidence and Mortality Web-based Report. (http://nccd.cdc.gov/uscs/)Atlanta (GA): Department of Health and Human Services, Centers for Diseases Control and Prevention, and National Cancer Institute; 2016. Available at : http://www.cdc.gov/uscs. [Google Scholar]
- 2. Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer 2003; 103:792–802. [DOI] [PubMed] [Google Scholar]
- 3. Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev 2006; 15:2020–2026. [DOI] [PubMed] [Google Scholar]
- 4. Boyle P, Gray N, Henningfield J, et al. Tobacco. Science, Policy and Public Health. Oxford: Oxford University Press; 2004. [Google Scholar]
- 5. Miller YE. Pathogenesis of lung cancer, 100 year report. Am J Respir Cell Mol Biol 2005; 33:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006; 354:333–342. [DOI] [PubMed] [Google Scholar]
- 7. Hofmann HS, Neef H, Schmidt P. Primary lung cancer and extrapulmonary malignancy. Eur J Cardiothorac Surg 2007; 32:653–658. [DOI] [PubMed] [Google Scholar]
- 8. Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 2005; 127:1152–1158. [DOI] [PubMed] [Google Scholar]
- 9. Aguiló R, Macià F, Porta M, et al. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg 2008; 34:1075–1080. [DOI] [PubMed] [Google Scholar]
- 10. Reinmuth N, Stumpf P, Stumpf A, et al. Characteristics of lung cancer after a previous malignancy. Respir Med 2014; 108:910–917. [DOI] [PubMed] [Google Scholar]
- 11. Pagès PB, Mordant P, Grand B, et al. History of multiple previous malignancies should not be a contraindication to the surgical resection of lung cancer. Ann Thorac Surg 2013; 95:1000–1005. [DOI] [PubMed] [Google Scholar]
- 12. Son C, Lee SK, Choi PJ, et al. Characteristics of additional primary malignancies in Korean patients with non-small cell lung cancer. J Thorac Dis 2013; 5:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2:694–705. [DOI] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 15. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 16. Kutluk Cenik B, Sun H, Gerber DE. Impact of renal function on treatment options and outcomes in advanced non-small cell lung cancer. Lung Cancer 2013; 80:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inker LA, Astor BC, Fox CH, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis 2014; 63:713–735. [DOI] [PubMed] [Google Scholar]
- 19. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70:606–612. [PubMed] [Google Scholar]
- 20. Warren S, Gates O. Multiple primary malignant tumor. Am J Cancer 1932; 16:1358–1414. [Google Scholar]
- 21. Surapaneni R, Singh P, Rajagopalan K, et al. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol 2012; 7:1252–1256. [DOI] [PubMed] [Google Scholar]
- 22. Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg 2004; 127:1119–1125. [DOI] [PubMed] [Google Scholar]
- 23. Liu YY, Chen YM, Yen SH, et al. Multiple primary malignancies involving lung cancer-clinical characteristics and prognosis. Lung Cancer 2002; 35:189–194. [DOI] [PubMed] [Google Scholar]
- 24. Teppo L, Salminen E, Pukkala E. Risk of a new primary cancer among patients with lung cancer of different histological types. Eur J Cancer 2001; 37:613–619. [DOI] [PubMed] [Google Scholar]
- 25. Levi F, Randimbison L, Te VC, et al. Second primary cancers in patients with lung carcinoma. Cancer 1999; 86:186–190. [DOI] [PubMed] [Google Scholar]
- 26. Donin N, Filson C, Drakaki A, et al. Risk of primary malignancies among cancer survival in the United States, 1992 through 2008. Cancer 2016; 122:3075–3086.DOI:10.1002/cncr 30164. [DOI] [PMC free article] [PubMed] [Google Scholar]


