Abstract
Background:
The aim of this article is to assess the influence of comorbidities among elderly patients (at least 70 year old) undergoing surgery for early stage nonsmall cell lung cancer (NSCLC) and to explore the tolerability and efficacy of surgery in relation to stereotactic body radiotherapy (SBRT) in this patient population.
Methods:
A review of the literature on the prevalence of comorbidities among elderly patients with early stage NSCLC, and the impact of comorbidity factors on survival following surgery was conducted. Survival rates and the incidence of complications following SBRT for this patient population were also identified.
Results:
Comorbidities in elderly patients with early stage NSCLC may preclude surgery or lead to poor survival following surgery. However, chronological age alone should not be used as a deciding factor to deny curative treatment in elderly, but fit patients. Stereotactic body radiotherapy is well tolerated by elderly lung cancer patients and may result in survival rates similar to that following surgery.
Conclusion:
SBRT should be the treatment of choice for early stage NSCLC in elderly patients with multiple comorbidities that preclude surgery. The roles of surgery and SBRT for elderly, -fit patients with early stage NSCLC needs to be further defined in future prospective trials.
Keywords: early stage, elderly, lung cancer, SBRT, surgery
1. Introduction
The standard of care for early stage nonsmall cell lung cancer (NSCLC) is lobectomy due to its high cure rate. However, surgery may lead to a higher peri-operative mortality and poorer survival in elderly lung cancer patients who have a high comorbidity score.[1] Many lung cancer patients are former smokers, thus exposing them to the risk of chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), and peripheral vascular disease (PVD), which may preclude them from having surgery.[2 3] In addition, elderly patients may have other comorbidities, such as hypertension, diabetes, and renal disease, which may increase the complication rates following surgery. This may have accounted for a decrease in patient survival.[4 5] Recently, stereotactic body radiotherapy (SBRT), a new technique of radiotherapy delivering a high dose of radiation under image guidance, has been introduced for the curative treatment of early stage NSCLC. Preliminary results following SBRT demonstrated high local control rates and minimal complications even among patients whose medical conditions preclude surgery.[6] Among patients who are candidates for surgery but decline lobectomy, a high survival rate has also been reported following SBRT for early stage NSCLC.[7 8] Thus, the roles of surgery and SBRT needs to be further defined in elderly patients with early stage NSCLC to identify the most optimal treatment approach in this patient population. In this study, we assess the prevalence of comorbidities in elderly patients with NSCLC, and their impact on the efficacy and tolerability of surgical treatment in these patients. In addition, we review selected studies of surgery and SBRT in elderly patients with early stage NSCLC to further assess the efficacy and tolerance of each treatment in this patient population.
2. Materials and methods
A search was conducted based on the PubMed electronic database. The following terms were explored and used for each database search: NSCLC, early stage (stage I), elderly, comorbidity, lobectomy, mortality, survival, and SBRT. A reference list of relevant papers was then searched for additional publications. The following criteria were analyzed in each article: prevalence of comorbidity among elderly lung cancer patients, impact of comorbidity on survival following lobectomy and SBRT, and complications. Articles describing the survival of elderly cancer patients (70 year old or older) following surgery or SBRT were selected. The age cutoff of 70 was chosen because of anthropometric studies reporting increased weight loss and sarcopenia in men and women after the age of 70.[9 10] Sarcopenia has been linked to increased risk of complications and poorer survival after oncologic surgery.[11] The comorbidity index was identified in each article. Only studies reported in English were included. Duplicate articles were excluded.
The University of Arizona Institutional Review Board approved the study as it is a review of the literature and does not require patient consent.
3. Results
A total of 488 articles were screened. However, 82 articles were reviewed. Also, 39 studies were excluded as they did not fit selection criteria. Figure 1 summarizes the Prisma flow diagram. The Charlson comorbidity index was the most common index to analyze complications and mortality. Eighteen studies linked the prevalence of comorbidity and age among lung cancer patients. However, 29 studies and 6 studies reported survival and complications rates among patients who were at least 70 year old who underwent surgery or SBRT for early stage NSCLC, respectively. All studies were retrospective. Thus, selection bias cannot be avoided. Patient selection criteria were identified in each study and reported in the results section.
Figure 1.
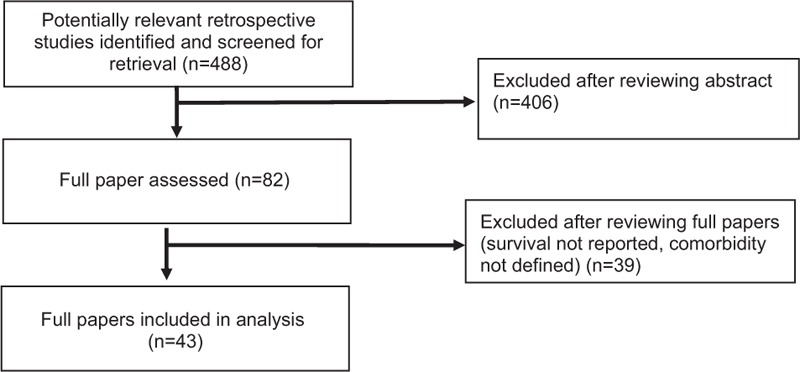
Prisma flow diagram of included studies.
3.1. Prevalence of comorbidity among elderly lung cancer patients
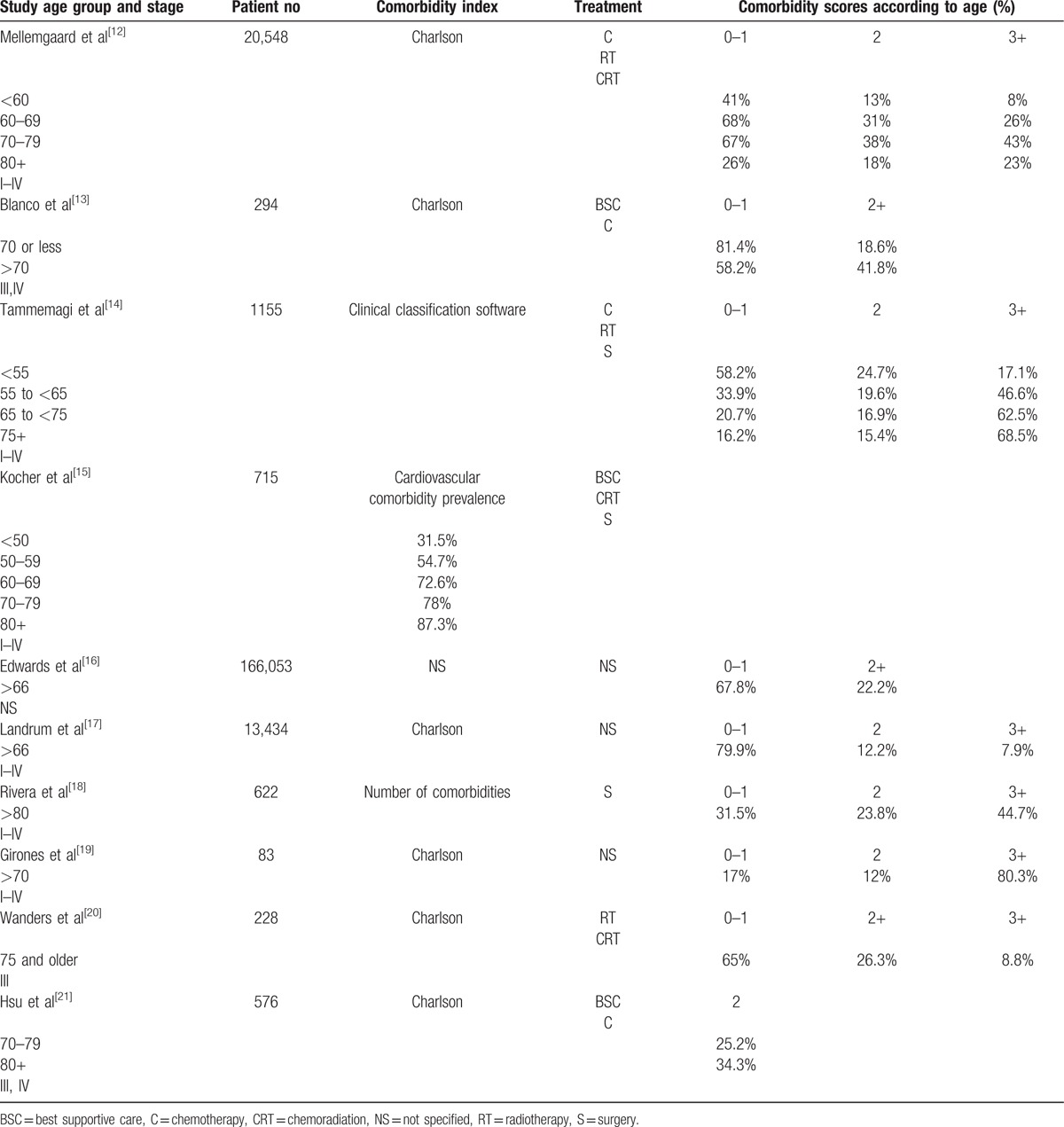
The severity of the comorbidity score increased with age. Among patients who underwent chemotherapy and radiotherapy for lung cancer, Charlson scores of >2 was only 8% for those <60 year old but increased to 26% and 43% for patients between 60- and 69 year old and 70- and 79-year old, respectively.[12] Other studies also showed an increase of prevalence and severity of comorbidity among elderly cancer patients using different indexes.[13–21] The severity of comorbidity scores may be related to the number of comorbidities in the elderly: 44.7% of patients >80 years had >3 comorbidities.[18]
Among studies that used the Charlson comorbidity index, the comorbidity score of grade 2 or higher ranged from 34.3% to 68.5% for patients >80.[12 18 21] Table 1 summarizes the prevalence and severity of comorbidity according to age in patients with NSCLC.
Table 1.
Prevalence and severity of comorbidity according to age in patients with nonsmall cell lung cancer.
3.2. Morbidity and mortality of surgery in elderly patients with early stage nonsmall cell lung cancer
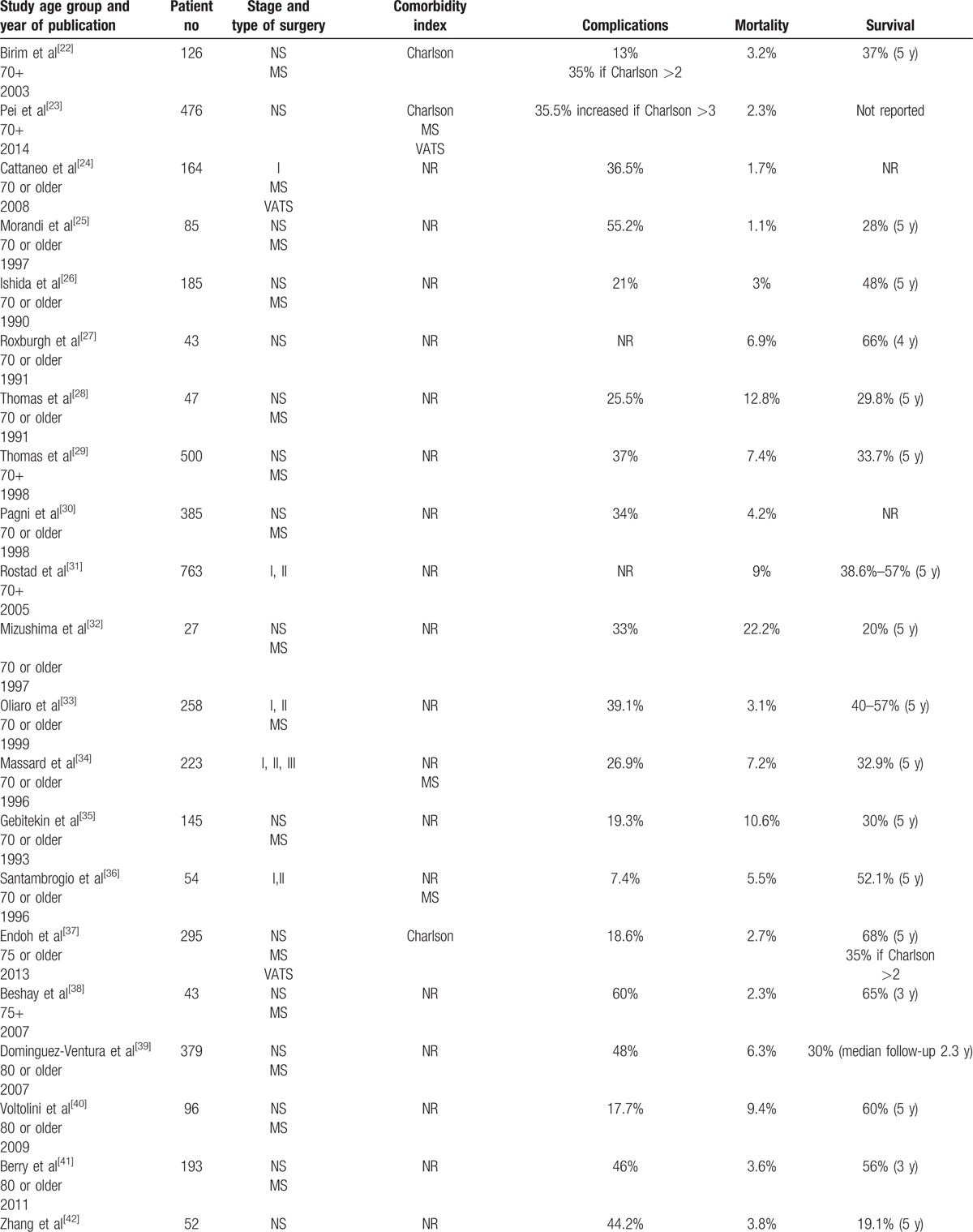
Surgery with either lobectomy or pneumonectomy was performed in elderly lung cancer patients 70, 75, or 80 year old or older.[22–51] Patients selected for surgery were often physically fit and underwent extensive cardio-pulmonary evaluation prior to surgery. Only 5 studies correlated comorbidity index with complications or survival.[22 23 35 41 42] Among studies which included patients who were at least 70 year old, the mortality, and complications rates ranged from 1.1% to 22.2% and 7.4% to 55.2%, respectively.[22–36] Survival of those patients ranged from 20% to 57% at 5 years. In the 2 studies reporting on patients who were at least 75 year old, the mortality, complications, and overall survival rates were 2.3% and 2.7%, 18.6% and 60%, and 65% and 68% at 3 and 5 years, respectively.[37 38] In patients who were at least 80 year old, these rates at 5 years ranged from 1.2% to 12%, 8.4% to 50%, and 19.1% and 65.9% respectively.[39–50] Complications rates increased whereas survival decreased for patients with increased comorbidity scores.[22 23 35 41 42] Table 2 summarizes the morbidity and mortality of elderly lung patients who underwent surgery for early stage NSCLC.
Table 2.
Morbidity and mortality of surgery in elderly patients with early stages nonsmall cell lung cancer.
3.3. Morbidity and mortality of stereotactic body radiotherapy in elderly patients with early stage nonsmall cell lung cancer
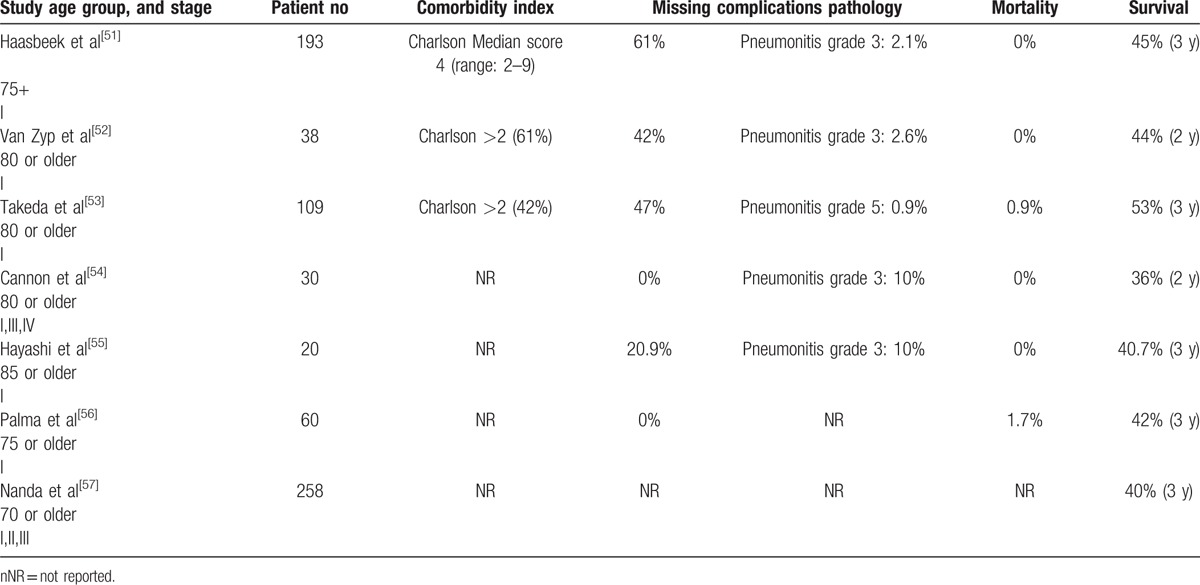
Patients selected for SBRT were often excluded from surgery because of existing comorbidities. Mortality and complication rates of elderly patients with early stage NSCLC who underwent SBRT ranged from 0% to 1.7%, and 0.9% to 10% respectively.[52–57] The 3-year survival ranged from 40.7% to 53%. Three studies reported the Charlson comorbidity scores but did not correlate the index severity with survival or complications. Table 3 summarizes the morbidity and mortality of elderly patients who underwent SBRT for early stage NSCLC.
Table 2 (Continued).
Morbidity and mortality of surgery in elderly patients with early stages nonsmall cell lung cancer.
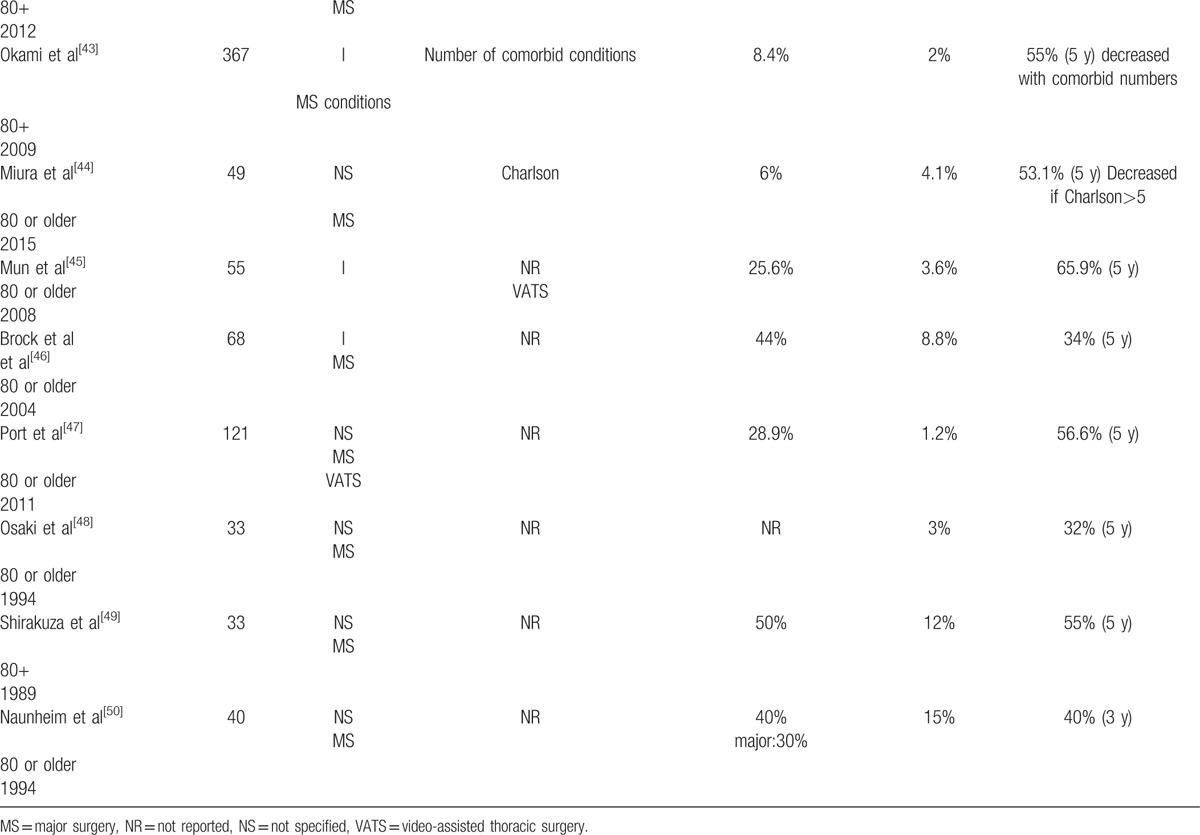
Table 3.
Morbidity and mortality of stereotactic body radiotherapy in elderly patients with early stage nonsmall cell lung cancer.
4. Discussion
Treatment of elderly patients with early stage NSCLC remains a challenge because of the presence of comorbidity factors. The Lung Cancer Study Group identified increasing age as a risk factor for mortality in patients undergoing surgery for NSCLC. Mortality rates were 1.3%, 4.1%, and 7.1% for patients <60 year old, 60 to 69 year old, and 70 year old or older, respectively.[58] Postoperative death occurred in 25% of patients aged 80 or older compared to 5% of those <80.[59] Compared to other cancers such as breast and colorectal cancers, the mortality and complication rates following surgery for NSCLC are higher because of reduced cardio-pulmonary reserve.[59] Even though it is still controversial, the Charlson comorbidity index has been frequently used to assess the influence of comorbidity factors on survival following surgery for NSCLC.[60–62] As an illustration, among patients of all ages undergoing surgery for NSCLC, a 5-year survival of 52%, 48%, and 28% has been reported for patients with Charlson comorbidity grade 0, 1 to 2, and >2 respectively.[60] The poor survival associated with a high Charlson score for NSCLC has been shown in other prospective studies.[61 62] Thus, patients who are selected for surgery usually are in excellent physical condition, with adequate cardiopulmonary function, and had extensive peri-operative evaluation.[34 39 42] In addition, special surgical procedures such as video-assisted thoracoscopic surgery (VATS) may further reduce the incidence of surgical complications, thus allowing surgery in octogenarians.[47] Consequently, 5-year survival rates between 60% and 65.9% has been observed in selected studies for patients 80 year old or older.[40 45] Thus, chronological age should not be a contraindication to curative surgery in all elderly patients with early stage NSCLC. Instead, priority should be given to the evaluation of comorbidities and performance status when evaluating these patients for surgery. Higher complications rates and poorer survival were reported among patients with high comorbidity scores in selected studies that take into consideration the influence of comorbidity on surgical outcomes.[22 23 43 44 50] For example, Endoh et al[37] reported a 5-year survival of 68% for patients who were at least 75 year old. However, the 5-year survival was reduced to 35% among patients with a Charlson score above 2 even though VATS was one of the surgical techniques being reported on this study. The impact of medical comorbidities on the survival of elderly lung cancer patients was also corroborated in other studies.[43 44] Existing evidence suggests that elderly NSCLC patients with severe comorbidity scores may not be the best candidates for surgery. Given the fact that the number of comorbidities increases with age, a significant portion of elderly NSCLC patients with severe comorbidity score would be precluded from surgery.[18 19] Those patients would likely benefit from SBRT as an alternative for cure.[6] Among elderly NSCLC patients who underwent SBRT, 3-year overall survival ranged from 40.7% to 53% despite a high Charlson score.[51 52 55] Excellent local control between 83% to 100% was observed with minimal morbidity.[52–53] Mortality rates were very low (0–1.7%). The most serious complication was grade 3 radiation-related pneumonitis which was reversible and ranged from 2.1% to 10%. Even though the duration of follow-up in SBRT studies (only 3-year survival to date) is relatively short when compared with that in surgical series, current evidence suggests that SBRT should be the treatment of choice for elderly patients with early stage NSCLC in whom medical conditions preclude surgery.
The rate of serious complications is still significant (28.9%) even in physically fit elderly NSCLC patients with minimal comorbidities receiving a less morbid surgical procedure such as VATS.[44] Even though there is still controversy, VATS has been associated with fewer complications and shortened hospital stay when compared with open thoracotomy.[63 64] Surgical complications were mostly cardiopulmonary and ranged from 8.4% to 60% (Table 2 ). These complications may affect patients’ quality of life because of the longer recovery time when compared with younger patients.[65] As the mortality and complications rates of SBRT are significantly less when compared with that following VATS for early stages NSCLC, elderly fit patients may benefit from SBRT if survival comparable to that following surgery can be achieved.[6] A pooled randomized study reported a 3-year survival of 95% and 79% for SBRT and surgery respectively.[7] In this study, grade 3 complications were 10% and 44% for SBRT and surgery, respectively. There was no death in the SBRT arm. One patient in the surgical arm died from complications. Even though the number of patients in this study is small, the study highlights that while both treatment modalities are effective for early stage NSCLC, less complications were observed following SBRT. A matched-pair comparison of SBRT versus surgery for stage I NSCLC also corroborated similar survival rates between the 2 treatments.[66] These studies raise the intriguing questions of whether SBRT may become the treatment of choice for elderly lung cancer patients with early stage NSCLC lung cancer regardless of the presence of comorbidity factors, or physical status. Another argument for SBRT is that it costs less when compared with lobectomy. Among 486 patients at least 66 year old who underwent surgery or SBRT, the estimated Medicare cost over 5 years was 54,968 and 82,641 dollars for SBRT and lobectomy, respectively.[67] As the number of elderly lung cancer patients is rapidly rising in the western world, prospective randomized studies should be conducted in the future to compare the cost-effectiveness of SBRT and surgery for this age group.[68] The effectiveness of SBRT to improve local control and survival while minimizing treatment complications for lung cancer may be related to its ability to precisely target the cancer with an ablative dose of radiation while sparing the normal thoracic organs such as the heart and the lung. Daily pre-treatment tumor localization under image guidance allows the radiation oncologist to reduce the amount of normal lung tissue surrounding the tumor to be included in the PTV (planning target volume to avoid marginal miss), thus minimizing the volume of normal lung tissue to be irradiated to a high radiation dose. In addition, special devices to reduce respiration-induced tumor movement, such as the active breathing control or the abdominal compression device, further decrease excessive lung irradiation which can be substantial if the tumor is located in lower lobe. As a result, there was little change in pulmonary function following SBRT for lung cancer.[69] In another study of 55 medically inoperable patients who underwent SBRT for early stage lung cancer and who had regular pulmonary function test studies (PFTs) every 3 months for the first 2 years and every 6 months after treatment, the mean percentage of forced expiratory volume in the first second (FEV1) and diffusing capacity for carbon monoxide declined by 5.8% and 6.3%, respectively.[70] There were also no significant changes in arterial blood gas and oxygen saturation after 2 years.[70] These results corroborated with the low morbidity observed following SBRT among patients who required chronic oxygen therapy because of lung damage prior to radiotherapy[71] and highlighted the safety of SBRT for elderly lung cancer patients regardless of baseline lung function.[72] Follow-up of a pooled randomized study comparing surgery to SBRT in patients with operable early stage NSCLC suggested that patients’ global heath quality of life may be better with SBRT because of the low morbidity observed but these results should be confirmed in future prospective studies.[73]
We acknowledge the limitations of the current study. In some of the studies reported outside the USA, the absence of pathologic confirmation of cancer in a significant number of patients may raise the question of whether those lung lesions may be benign.[52 53] However, excellent local control with minimal complications has been reported following SBRT for locally more advanced stage (II) NSCLC (>5 cm) in US studies where pathological diagnosis of cancer is practically a legal requirement because of potential lawsuit for misdiagnosis, which highlights the safety of this new radiotherapy technique.[74] Another limitation of SBRT for early stage NSCLC as a treatment approach is the lack of pathologic mediastinal lymph node information which precludes adjuvant systemic treatment which may impair patient survival when compared with surgery. Unless further randomized trials are performed to compare these 2 treatment modalities, these questions will remain unanswered. We recognize the difficulty to conduct randomized trials among elderly cancer patients because of the bias including patient preference, physicians’ reluctance to treat patient in a curative modality because of their age, and the absence of consensus in treating elderly cancer patients. However, such studies will be necessary in the future to guide the treatment of a growing number of elderly cancer patients across the world,[75] which will require collaborations among the nations and the physicians of various specialties.
5. Conclusions
Elderly cancer patients with early stage NSCLC should undergo curative treatment with either surgery or SBRT. Patients with serious comorbidity precluding surgery should have SBRT. The role of surgery and SBRT for the management of elderly fit patients should be tested in future randomized studies.
Acknowledgments
The authors thank Dayleen De Riggs and Kristen Young for their help in the preparation of the manuscript.
Footnotes
Abbreviations: FEV1 = forced expiratory volume in the first second, NSCLC = nonsmall cell lung cancer, PFTs = pulmonary function test studies, PTV = planning target voume, SBRT = stereotactic body radiotherapy, VATS = video-assisted thoracoscopic surgery.
The authors have no funding and conflicts of interest to disclose.
Contributor Information
Collaborators: The International Geriatric Radiotherapy Group
References
- 1. Wang C, Lin Y, Tzao C, et al. Comparison of Charlson comorbity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardio-Thorac Surg 2007; 32:877–881. [DOI] [PubMed] [Google Scholar]
- 2. Kravchenko J, Berry M, Arbeev K, et al. Cardiovascular comorbidities and survival of lung cancer patients: medicare data based analysis. Lung Cancer 2015; 88:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival in patients with early stage non-small cell lung cancer undergoing surgical resection. Chest 2014; 145:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guralnic GM. Assessing the impact of comorbidity in the older population. AEP 1996; 6:376–380. [DOI] [PubMed] [Google Scholar]
- 5. Yancik R, Havlik RJ, Wesley MN, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol 1996; 6:399–412. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen NP, Garland L, Welsh J, et al. Can stereotactic fractionated radiation therapy become the standard of care for early stage non-small cell lung cancer. Cancer Treat Rev 2008; 34:719–727. [DOI] [PubMed] [Google Scholar]
- 7. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015; 16:e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onishi H, Shirato Y, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81:1352–1358. [DOI] [PubMed] [Google Scholar]
- 9. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Health Stat 2012; 11:252. [PubMed] [Google Scholar]
- 10. Beaudart C, Reginster J-Y, Slomian J, et al. Prevalence of sarcopenia: the impact on different diagnostic cut-off limits. J Musculoskelet Neuronal Interact 2014; 14:425–431. [PubMed] [Google Scholar]
- 11. Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology. J Surg Oncol 2015; 112:910. [DOI] [PubMed] [Google Scholar]
- 12. Mellemgaard A, Luchtenborg M, Iachina M, et al. Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J Thorac Oncol 2015; 10:272–279. [DOI] [PubMed] [Google Scholar]
- 13. Blanco JA, Toste IS, Alvarez RF, et al. Age, comorbidity, treatment decision and prognosis in lung cancer. Age Ageing 2008; 37:715–718. [DOI] [PubMed] [Google Scholar]
- 14. Tammemagi CM, Neslund-Dudas C, Simoff M, et al. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidem 2004; 57:597–608. [DOI] [PubMed] [Google Scholar]
- 15. Kocher F, Fiegl M, Mian M, et al. Cardiovascular comorbidities and events in NSCLC: often underestimated but worth considering. Clin Lung Cancer 2015; 16:305–312. [DOI] [PubMed] [Google Scholar]
- 16. Edwards BK, Noone A, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014; 120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Heath Administration versus fee for service Medicare. J Clin Oncol 2012; 30:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivera C, Dahan M, Bernard A, et al. Surgical treatment of lung cancer in the octogenarians: results of a nationwide audit. Eur J Cardio-Thor Surg 2011; 39:981–988. [DOI] [PubMed] [Google Scholar]
- 19. Girones R, Torregrosa D, Gomez-Codina J, et al. Prognosis impact of comorbidity in elderly lung cancer patients: use and comparison of two scores. Lung Cancer 2011; 72:108–113. [DOI] [PubMed] [Google Scholar]
- 20. Wanders R, Steevens J, Botterweck A, et al. Treatment with curative intent of stage III non-small cell lung cancer patients of 75 years: a prospective population study. Eur J Cancer 2011; 47:2691–2697. [DOI] [PubMed] [Google Scholar]
- 21. Hsu C, Chen J, Chen K, et al. Advanced non-small cell in the elderly: the impact of age and comorbidities on treatment modalities and patient prognosis. J Geriatric Oncol 2015; 6:38–45. [DOI] [PubMed] [Google Scholar]
- 22. Birim O, Zuydendorp HM, Maat A, et al. Lung resection for non-small cell lung cancer in patients older than 70: mortality, morbidity, and late survival compared to the general population. Ann Thorac Surg 2003; 76:1796–1801. [DOI] [PubMed] [Google Scholar]
- 23. Pei G, Zhou S, Han Y, et al. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014; 6:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008; 85:231–236. [DOI] [PubMed] [Google Scholar]
- 25. Morandi U, Stephani A, Golinelli M, et al. Results of surgical resection in patients over the age of 70 years with non-small cell lung cancer. Eur J Cardio-Thorac Surg 1997; 11: 432-439-439. [DOI] [PubMed] [Google Scholar]
- 26. Ishida T, Yokoyama, Kaneko S, et al. Long-term results of operation for non-small cell lung cancer in the elderly. Ann Thorac Surg 1990; 50:919–922. [DOI] [PubMed] [Google Scholar]
- 27. Roxburgh JC, Thompson J, Goldstraw P. Hospital mortality and long-term survival after pulmonary resection in the elderly. Ann Thorac Surg 1991; 51:800–803. [DOI] [PubMed] [Google Scholar]
- 28. Thomas P, Sielezneff J, Ragni J, et al. Is lung cancer resection justified in patients over 70 years? Eur J Cardiothor Surg 1993; 7:246–250. [DOI] [PubMed] [Google Scholar]
- 29. Thomas P, Piraux M, Jacques LF, et al. Clinical patterns and trends of outcome of elderly patients with bronchogenic carcinoma. Eur J Cardiothor Surg 1998; 13:266–274. [DOI] [PubMed] [Google Scholar]
- 30. Pagni S, McKelvey A, Riordan C, et al. Pulmonary resection for malignancy in the elderly: is age still a factor? Eur J Cardiothorac Surg 1998; 14:40–45. [DOI] [PubMed] [Google Scholar]
- 31. Rostad H, Naalsund A, Strand T, et al. Results of pulmonary resection in Norway, patients older than 70 years. Eur J Cardiothorac Surg 2005; 27:325–328. [DOI] [PubMed] [Google Scholar]
- 32. Mizushima Y, Noto H, Sugiyama S, et al. Survival and prognosis after pneumectomy for lung cancer in the elderly. Ann Thorac Surg 1997; 64:193–198. [DOI] [PubMed] [Google Scholar]
- 33. Oliaro A, Leo F, Filosso PL, et al. Resection for bronchogenic carcinoma in the elderly. J Cardiovasc Surg (Torino) 1999; 40:715–719. [PubMed] [Google Scholar]
- 34. Massard G, Moog R, Wihlm JM, et al. Bronchogenic cancer in the elderly: operative risk and long-term prognosis. Thorac Cardiovasc Surg 1996; 44:40–45. [DOI] [PubMed] [Google Scholar]
- 35. Gebitekin C, Gupta NK, Martin PG, et al. Long-term results in the elderly following pulmonary resection for non-small cell lung cancer. Eur J Cardiothorac Surg 1993; 7:653–656. [DOI] [PubMed] [Google Scholar]
- 36. Santambrogio L, Nosottti M, Bellaviti N, et al. Prospective study of surgical treatment of lung cancer in the elderly patient. J Gerontol Med Sciences 1996; 51A:M267–M269. [DOI] [PubMed] [Google Scholar]
- 37. Endoh H, Yamamoto R, Satoh Y, et al. Risk analysis of pulmonary resection for elderly patients with lung cancer. Surg Today 2013; 43:514–520. [DOI] [PubMed] [Google Scholar]
- 38. Beshay M, Dorn P, Ris H, et al. Influence of comorbidity on outcome after pulmonary resection in the elderly. Asian Cardiovasc Thorac Ann 2007; 15:297–302. [DOI] [PubMed] [Google Scholar]
- 39. Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006; 82:1175–1179. [DOI] [PubMed] [Google Scholar]
- 40. Voltolini L, Rapiceta T, Ligabue T, et al. Short- and long-term results of lung resection for cancer in octogenarians. Asian Cardiovasc Thorac Ann 2009; 17:147–152. [DOI] [PubMed] [Google Scholar]
- 41. Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardio-Thor Surg 2011; 39:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Xue Z, Chu X, et al. Surgical treatment and prognosis of octogenarians with non-small cell lung cancer. Asian Pac J Trop Med 2012; 5:265–268. [DOI] [PubMed] [Google Scholar]
- 43. Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer. J Thorac Oncol 2009; 4:1247–1253. [DOI] [PubMed] [Google Scholar]
- 44. Miura N, Kohno M, Ito K, et al. Lung cancer surgery in patients aged 80 or older: an analysis of risk factors, morbidity, and mortality. Gen Thorac Cardiovasc Surg 2015; 63:401–405. [DOI] [PubMed] [Google Scholar]
- 45. Mun M, Kohno T. Video-assisted thoracic surgery for clinical stage I octogenarians. Ann Thorac Surg 2008; 85:406–411. [DOI] [PubMed] [Google Scholar]
- 46. Brock MV, Kim MP, Hooker CM, et al. Pulmonary resection in octogenarians with stage I non-small cell lung cancer: a 22 year experience. Ann Thorac Surg 2004; 77:271–277. [DOI] [PubMed] [Google Scholar]
- 47. Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non- small cell lung cancer: ramifications of increased life expectancy and the benefits of minimal invasive surgery. Ann Thorac Surg 2011; 92:1951–1957. [DOI] [PubMed] [Google Scholar]
- 48. Osaki T, Shirakusa T, Koldate M, et al. Surgical treatment of lung cancer in the octogenarian. Ann Surg Thorac 1994; 57:188–192. [DOI] [PubMed] [Google Scholar]
- 49. Shirakusa T, Tsutsui T, Iriki N, et al. Results of resection for bronchogenic carcinoma in patients over the age of 80. Thorax 1989; 44:189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naunheim KS, Kesler KA, D’Orazio SA, et al. Lung cancer surgery in the octogenarian. Eur J Cardiothorac Surg 1994; 8:453–456. [DOI] [PubMed] [Google Scholar]
- 51. Hassbeek CJA, Lagerwaard FJ, Antonisse ME, et al. Stage I nonsmall cell lung cancer in patients in patients >75 years. Cancer 2010; 116:406–414. [DOI] [PubMed] [Google Scholar]
- 52. Van Zyp NC, van der Holt B, van Klaveren R, et al. Stereotactic body radiotherapy using real-time tumor tracking in octogenarians with non-small cell lung cancer. Lung Cancer 2010; 69:296–301. [DOI] [PubMed] [Google Scholar]
- 53. Takeda A, Sanuki N, Eriguchi T, et al. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 86:257–263. [DOI] [PubMed] [Google Scholar]
- 54. Cannon NA, Iyengar P, Choy H, et al. Stereotactic ablative body radiation therapy for tumors in the lung in octogenarians: a retrospective single institution study. BMC Cancer 2014; 14:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hayashi S, Tanaka H, Kajiura Y, et al. Stereotactic body radiotherapy for very elderly patients (age, greater or equal to 85 years) with stage I non-small cell lung cancer. Radiat Oncol 2014; 9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palma D, Visser E, Lagerwaard FJ, et al. Treatment of stage I NSCLC patients in elderly patients: a population-based matched pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011; 101:240–244. [DOI] [PubMed] [Google Scholar]
- 57. Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: a national cancer data base analysis. Cancer 2015; 121:4222–4230. [DOI] [PubMed] [Google Scholar]
- 58. Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983; 86:654–658. [PubMed] [Google Scholar]
- 59. Janssen-Heijnen MLG, Maas HAAM, Houterman S, et al. Comorbidity in older surgical patients: influence on patient care and outcome. Eur J Cancer 2007; 43:2179–2193. [DOI] [PubMed] [Google Scholar]
- 60. Birim O, Kappetein AP, Bogers AJJC. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg 2005; 28:759–762. [DOI] [PubMed] [Google Scholar]
- 61. Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of nonsmall cell lung cancer: a review of National Cancer Institute of Canada clinical trials group trials. J Clin Oncol 2008; 26:54–59. [DOI] [PubMed] [Google Scholar]
- 62. Marcus MW, Chen Y, Duffy SW, et al. Impact of comorbidity on lung cancer mortality-a report from the Liperpool lung project. Oncol Lett 2015; 9:1902–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery vs open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010; 139:976–983. [DOI] [PubMed] [Google Scholar]
- 64. Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interactive Cardiovasc Thorac Surg 2013; 16:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010; 68:115–120. [DOI] [PubMed] [Google Scholar]
- 66. Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011; 101:240–244. [DOI] [PubMed] [Google Scholar]
- 67. Smith BD, Jiang J, Chang JY, et al. Cost effectiveness of stereotactic radiation, sublobar resection, and lobectomy for early non-small cell lung cancers in older adults. J Geriatr Oncol 2015; 6:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009; 10:2758–2765. [DOI] [PubMed] [Google Scholar]
- 69. Guckenberger M, Klement RJ, Kestin LL, et al. Lack of a dose–effect relationship for pulmonary function changes after stereotactic body radiation therapy for early stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 85:1074–1081. [DOI] [PubMed] [Google Scholar]
- 70. Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early stage non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2013; 88:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoshikate T, Nakamura K, Shioyama Y, et al. Stereotactic body radiation therapy for stage I non-small cell lung cancer patients with chronic respiratory insufficiency requiring domiciliary chronic oxygen therapy. Anticancer Res 2012; 32:4041–4044. [PubMed] [Google Scholar]
- 72. Stone B, Mangona VS, Johnson MD, et al. Changes in pulmonary function following image-guided stereotactic lung radiotherapy: neither lower baseline or post SBRT pulmonary function are associated with worse overall survival. J Thorac Oncol 2015; 10:1762–1769. [DOI] [PubMed] [Google Scholar]
- 73. Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non- small cell lung cancer. Radiother Oncol 2015; 117:44–48. [DOI] [PubMed] [Google Scholar]
- 74. Woody NM, Stephans KL, Marwaha G, et al. Stereotactic body radiation therapy for non-small cell lung cancer tumors greater than 5 cm. Int J Radiat Oncol Biol Phys 2015; 92:325–331. [DOI] [PubMed] [Google Scholar]
- 75. Dale DC. Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol 2003; 1:11–17. [PubMed] [Google Scholar]


