Abstract
Brain-derived neurotrophic factor (BDNF) is a growth factor that is dynamically expressed in the brain across postnatal development, regulating neuronal differentiation and synaptic plasticity. The neurotrophic hypothesis of psychiatric mood disorders postulates that in the adult brain, decreased BDNF levels leads to altered neural plasticity, contributing to disease. Although BDNF has been established as a key factor regulating the critical period plasticity in the developing visual system, it has recently been shown to also play a role in fear circuitry maturation, which has implications for the emergence of fear-related mood disorders. This review provides a detailed overview of developmental changes in expression of BDNF isoforms, as well as their receptors across postnatal life. In addition, recent developmental studies utilizing a genetic BDNF single nucleotide polymorphism (Val66Met) knock-in mouse highlight the impact of BDNF on fear learning during a sensitive period spanning the transition into adolescent time frame. We hypothesize that BDNF in the developing brain regulates fear circuit plasticity during a sensitive period in early adolescence, and alterations in BDNF expression (genetic or environmental) have a persistent impact on fear behavior and fear-related disorders.
Keywords: anxiety, adolescence, BDNF prodomain, polymorphism, fear conditioning, extinction learning
INTRODUCTION
Advances in psychiatry and neuroscience have enhanced our understanding of the neural circuitry implicated in anxiety- and stress-related disorders, with a primary focus on characterizing inappropriate and exaggerated fear responses in the developed brain. Current clinical treatments have been implemented based on their efficacy in a physiologically mature neural framework. Although existing behavioral and psychopharmacological therapies offer significant benefit to adult patients suffering from anxiety disorders, such as PTSD, a comparative lack of knowledge about the development of fear neural circuitry may limit successful treatment outcomes in children and adolescents.[1] In addition, the peak emergence of anxiety- and stress-related disorders occurs in pediatric and adolescent populations,[2] which highlights the importance of identifying not only the circuit-based but also molecular mechanisms for fear regulation from a developmental perspective.
One of the prevailing molecular frameworks thought to underlie mood disorders, including anxiety disorders, has been the neurotrophic hypothesis. This hypothesis postulates that growth factors in the adult brain, which provide continuing trophic support, are decreased by environmental and physiological stressors leading to altered neural plasticity in key regions implicated in anxiety and fear responses, including the hippocampus, prefrontal cortex, and amygdala. It is important to point out that chronic stress has opposite effects on brain-derived neurotrophic factor (BDNF) in the amygdala compared to hippocampus, with an increase in expression observed in the amygdala and a decrease in hippocampus. Ultimately, changes in activity of inhibitory and excitatory inputs in both structures result in enhanced net activation of the hypothalamic-pituitary-adrenal axis, which controls stress response. Excellent reviews of the studies establishing this neurotrophic hypothesis have been published.[3–5] In contrast, less is known about how these trophic factors impact the developing fear circuitry, especially in a time frame that corresponds to the peak onset and emergence of these disorders during the transition from childhood to adolescence. This review will provide a detailed overview of developmental expression changes of one such trophic factor, BDNF as well as its receptors. In addition, although BDNF has been established as a key factor in the developing brain for regulating the critical period related to visual system plasticity,[5, 6] its role in the developing fear circuitry has only recently been examined. This review will detail recent developmental studies utilizing a human genetic BDNF single nucleotide polymorphism (SNP; Val66Met) knock-in mouse that highlights the functional consequence of altered BDNF availability on fear learning during a sensitive period spanning the transition into adolescence. Clinical implications for neuropsychiatric disease and future directions will also be discussed.
NEUROTROPHINS
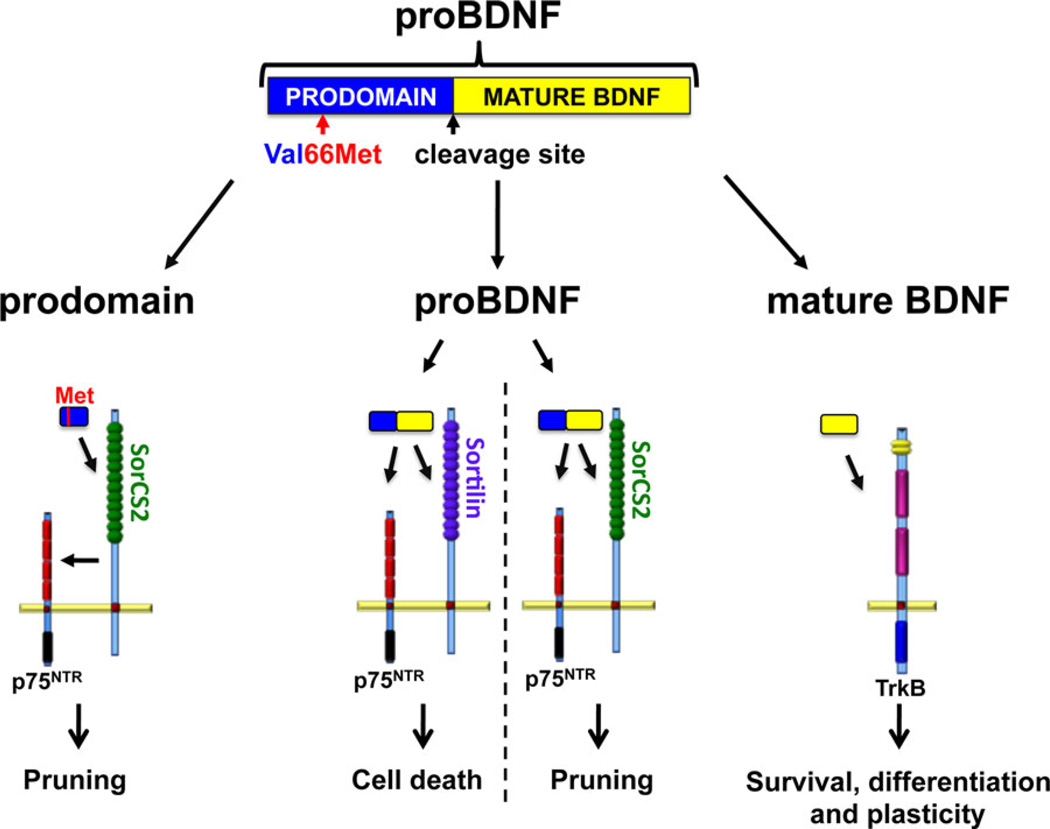
Neurotrophins, comprising of NGF, BDNF, NT-3, NT-4, are a unique family of polypeptide growth factors that influence the proliferation, differentiation, survival, and death of neuronal and non-neuronal cells. The effects of neurotrophins depend upon their levels of availability, their binding affinity to transmembrane receptors, and the downstream signaling cascades that are engaged following receptor activation. Although the biological roles for neurotrophins were initially characterized during early prenatal development of the peripheral nervous system, it is now clear that they have multiple roles in the adult central nervous system, such as regulating neuronal connections, plasticity, and neurotransmitter release.[7] The predominant neurotrophin in the brain is BDNF, which is initially synthesized as a precursor or a proneurotrophin that is subsequently cleaved to release the mature, active proteins[8, 9] (Fig. 1). Mature BDNF binds preferentially to the tropomyosin-related kinase B (TrkB) receptor, activating the downstream signaling molecules mitogen-activated protein kinase (MAPK), phospholipase C gamma, and phosphatidylinositol-3 kinase. These signaling cascades result in neuronal survival, differentiation, and enhancement of neuronal mechanisms regulating learning and memory[10–14] (Fig. 1).
Figure 1.
BDNF isoforms and their receptors. BDNF is initially synthesized as the precursor protein proBDNF, which is cleaved to release the prodomain and mature BDNF. All three BDNF isoforms display biological functions. In BDNF isoforms containing the Val66Met SNP, the prodomain binds to SorCS2, which in turn interacts with the p75NTR to induce growth cone pruning. proBDNF binds specifically to p75NTR, which leads to apoptosis and pruning of dendritic spines through interaction with sortilin and SorCS2, respectively. Mature BDNF binds preferentially to TrkB receptors, activating downstream signaling cascades resulting in neuronal survival, differentiation, and synaptic plasticity.
ProBDNF is also a biologically active signaling molecule,[15–17] however, its effects on neuronal survival and functioning are to a large degree opposite to those of mature BDNF.[18] Unlike mature BDNF, proBDNF binds specifically to the p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor super family.[7] p75NTR activation leads to decreased neuronal complexity and increased apoptosis[19–21] (for review, Roux and Barker[22]; Fig. 1). In addition, proBDNF negatively regulates neuronal mechanisms implicated in learning and memory,[17, 19, 23, 24] again indicating an opposite role in synaptic plasticity compared to mature BDNF.
EXPRESSION PATTERNS FOR BDNF ISOFORMS AND RECEPTORS ACROSS DEVELOPMENT
Brain-Derived Neurotrophic Factor
Detection of BDNF and its isoforms has proven challenging in the developing brain due to relatively low abundance of the growth factor. ELISA-based immunoassays have been the main methodology utilized to assess BDNF protein levels, however, the main disadvantage of this technique is that it does not differentiate between the pro- and mature isoforms of the polypeptide. Nevertheless, ELISA assays have provided valuable information on relative total BDNF expression throughout postnatal life in rodents. The consistent pattern observed in multiple studies is that endogenous levels of BDNF in the brain rise dramatically in early postnatal life and peak in the third to fourth postnatal week, a time period that corresponds with the transition into adolescence. Developmental expression of BDNF in the rat brain has shown that at postnatal day (P)0, very low levels are detected in the hippocampus, septum, olfactory bulb, and hypothalamus but none in cerebral cortex, striatum, and cerebellum.[25] At P10, BDNF is weakly detected in all brain regions tested except in the cerebellum whereas in the hypothalamus levels are almost five times higher than P0. At P20, levels are again increased and BDNF expression exhibits a pattern unique to each brain region. In the hippocampus, BDNF expression gradually rises between P20 and P120, at which point it reaches a plateau. Interestingly, in the cortex, BDNF levels peak at P20, after which they gradually decline to about 50%. In the striatum, the highest level of BDNF expression is attained at P30 followed by a decrease. In the olfactory bulb and hypothalamus, levels also peak between P20 and P30. Confirming results from rat brains, in the mouse brain BDNF levels increase between P1 and P21 in the cortex, hippocampus, striatum, septum, cerebellum, and olfactory bulb. The largest increase is detected in the hippocampus where levels rise from ~25 ng per gram (ng/g) wetweight to about 300 ng/g.[26] This developmental pattern is also supported by polymerase chain reaction analyses of BDNF mRNA in mouse hippocampus indicating a peak during week 3 of postnatal life.[27, 28] In all, these rodent studies suggest that in multiple brain regions, BDNF levels increase significantly during the first month of postnatal development, which may be followed by a slight decline into adulthood (Fig. 2A).
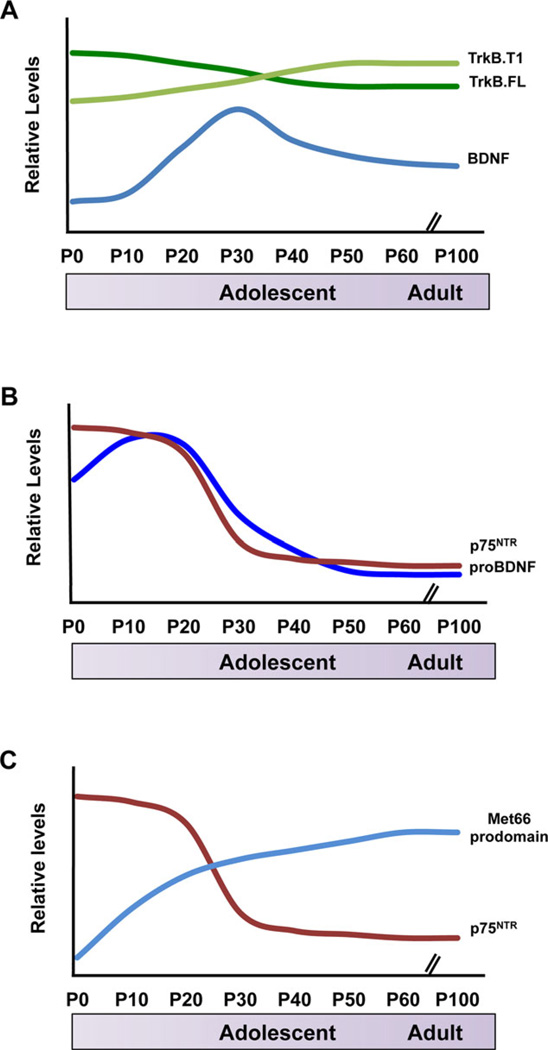
Figure 2.
Schematic summary of expression levels of BDNF isoforms and their receptors in rodent hippocampus relative to developmental stage. (A) BDNF levels peak in early adolescence, followed by a plateau in adulthood. Full-length TrkB is the predominant receptor isoform in early development, however, truncated TrkB levels increase in late adolescence.[25, 26, 45–47] (B) Levels of both, proBDNF and its receptor p75NTR are highest during the first weeks of postnatal development, after which expression rapidly decreases to low levels in adulthood.[15, 17] (C) Met66 prodomain levels rapidly increase within the first month of life, which coincides with high levels of p75NTR expression.[9, 15]
ProBDNF
ProBDNF and mature BDNF utilize distinct receptors to mediate divergent neuronal functions[18, 29] suggesting that the two isoforms may also exhibit a differential expression pattern in the developing brain. To address this question, Hempstead and colleagues have generated a knock-in mouse in which wild-type(WT) BDNF is expressed with a C-terminal hemagglutinin (HA) epitope tag (Bdnf-HA) to enhance BDNF protein detection.[15] Hippocampal lysates from BDNF-HA/+ mice have been subjected to immuno-analysis using HA-specific antibodies, allowing for the pro- and mature BDNF polypeptides to be resolved based on size. ProBDNF is easily detectable at P4 and levels significantly increase by the second postnatal week, however, by 10 weeks of age, levels are below those of P4, indicating a hippocampal proBDNF peak in early postnatal life. In contrast to proBDNF, the mature isoform is weakly detected on P4 and is expressed throughout postnatal development.[15] A follow-up study utilizing the same BDNF-HA/+ mouse model, further elucidates the early postnatal proBDNF expression in hippocampus by showing low expression at P0 but levels are rapidly increased at P15 followed by a gradual reduction at P21, P42, and into adulthood. On the other hand, mature BDNF levels are highest at P21 and although they decrease at later ages, this isoform is still readily detectable in adulthood.[17] Thus, unlike mature BDNF, proBDNF developmental effects may be most relevant very early in postnatal life when axonal projections are being established and synapses are forming (Fig. 2B). The transient postnatal peak in pro-BDNF has been hypothesized to correspond to later development of BDNF-processing enzymes[15] as well as higher levels of p75NTR in the hippocampus at this age,[15, 23] particularly in CA1 pyramidal cell apical dendrites, postsynaptic to the Schaffer collateral axon terminals.[23] ProBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus, which occurs at the highest rate in early postnatal development.[17] Thus, the developmental timing of proBDNF peak expression may be closely related to its function in establishing efficient circuitry connections in the maturing brain. Conversely, immunoanalysis of BDNF from WT murine hippocampi has suggested that the mature form is predominant at all ages.[30] Methodological differences may account for these diverging results.
BDNF Prodomain
The BDNF prodomain is highly conserved across species highlighting its importance in CNS function. Detection of the prodomain in murine hippocampus is first observed at P5. At 1 month of age, levels have significantly increased by nearly 4.5-fold and they remain high into adulthood.[9] Developmentally, WT prodomain levels peak after the time of highest proBDNF expression, when cleavage of proBDNF becomes more efficient.[15] The prodomain is secreted from cultured neurons and alters neuronal processes involved in learning and memory.[9, 31] A common SNP has been identified in the human BDNF gene, which results in an amino acid substitution from valine (Val) to methionine (Met) at position 66 in the BDNF prodomain.[32] This SNP is expressed in about 30% of the Caucasian population[33] and is associated with structural and functional alterations of the hippocampus.[32, 34–36] Neuropsychiatric disorders such as major depressive disorder as well as elevated expression of anxiety-related traits including harm avoidance, fear of uncertainty, and anticipatory worry are more prevalent in Met allele carriers than noncarriers.[37, 38] Additionally, patients with PTSD carrying the Met allele exhibit poorer response to exposure-based cognitive behavior therapy.[39] At a cellular level, the presence of the Met allele results in altered availability of the variant BDNFMet protein.[32, 40–43] Interestingly, this SNP introduces a structural shift in the BDNF prodomain conferring biologically relevant function. The Met66 prodomain negatively regulates development of hippocampal neurons by binding to SorCS2, a VPS10 family member, which subsequently interacts with p75NTR (Fig. 1). Met66 prodomain levels are developmentally regulated and they follow the general expression pattern of the Val66 (WT) prodomain, however, in comparison, Met66 hippocampal content is lower at all ages. There is an increase in Met66 expression between P5 and 4 weeks of age in murine hippocampus and another increase in levels into adulthood.[9] Met66 prodomain signaling might have a narrow window of biological relevance in early adolescence, when both, prodomain and p75NTR, levels are relatively high[9, 15] (Fig. 2C). Developmental expression of SorCS2 remains to be elucidated. Additionally, in carriers of the Val66Met mutation, even though prodomain levels are lower than Val66, the Met66 peptide is produced and it has biological relevance that is not observed with the WT prodomain.[9]
TrkB Receptor
In rodents, full-length TrkB (TrkB.FL) mRNA levels remain relatively even from birth throughout development and into adulthood.[27, 44, 45] Conversely, hippocampal mRNA levels of the truncated TrkB isoform (Trk.T1), lacking a catalytic domain, gradually increase between P1 and P21 followed by a reduction.[44] Additionally, regional and species specificity in the expression patterns of mRNA for both receptor isoforms has been reported. A peak at about P10–P15 has previously been reported in rat forebrain for TrkB.T1 mRNA.[45] In rat hippocampus, TrkB.FL mRNA expression slightly but significantly decreases from P1 through P7 to P14, and remains relatively stable into adulthood. TrkB.T1 mRNA expression strongly increases during the first two postnatal weeks, and again at 2 months of age, which is then followed by a decrease.[46]
Full-length TrkB protein levels vary from the mRNA expression pattern. In the rat hippocampus, TrkB.FL protein is highly expressed during the first few days of postnatal life and expression levels are maintained until P15–P21. This plateau is followed by a drop into adulthood.[46, 47] Protein expression in the rat forebrain follows a similar pattern.[45] The truncated TrkB protein, on the other hand, increases between P2 and P50 and may be the predominant form in adult rat hippocampus, hypothalamus, and forebrain[45–47] (Fig. 2A). In addition, onset of protein expression for the two isoforms varies with TrkB.T1 being first detected at P1, whereas low levels of full-length TrkB are observed at late embryonic stages.[48]
p75NTR Receptor
Additional support for the early-life biological relevance of proBDNF and the BDNF prodomain comes from the expression pattern of their neurotrophic coreceptor, p75NTR. Analyses in mice have shown that hippocampal p75NTR levels are highest in the first postnatal week, with a significant reduction by 4 weeks and minimal expression by 6 weeks of age, recapitulating the proBDNF expression pattern[15, 17] (Fig. 2B). Furthermore, p75NTR mRNA levels in rat primary visual cortex parallel developmental p75NTR protein levels observed in mouse hippocampus.[49]
IMPACT OF ALTERED BDNF SIGNALING ON FEAR CIRCUITRY IN THE ADULT
BDNF signaling through the TrkB neurotrophin receptor has been established as a major regulator of adult fear circuitry function as well as expression of fear behavior.[50] Neural circuitry regulating expression of learned fear is a complex network of fronto-limbic brain structures with the amygdala being the main control center. During auditory cued fear conditioning, cortical and thalamic projections converge on the lateral nucleus of the amygdala (LA),[51–53] which, along with the basal nucleus of the amygdala (BA) integrate appropriate sensory information and relay it to the central nucleus (CE).[54] During extinction learning, the prelimbic cortex (PL), which is located dorsally within the vmPFC, is necessary for the expression of learned fear behaviors,[55] whereas the more ventrally located infralimbic cortex (IL) is associated with suppression of conditioned fear responses.[56–60] Infusion of BDNF protein into the IL but not the PL facilitates fear extinction even without extinction training, whereas sequestering endogenous BDNF diminishes extinction learning.[61, 62] Furthermore, extinction training increases BDNF expression in the ventral hippocampus, which is associated with increased IL activity, facilitating extinction learning.[62] Additionally, lentiviral expression of the truncated TrkB receptor in the basolateral amygdala impairs consolidation of fear extinction in rats.[63] BDNF haploin-suffiecient (BDNF+/−) mice display impairment of contextual fear learning.[64] In accordance, full-length TrkB overexpression leads to enhanced contextual fear learning and hippocampal long term potentiation (LTP).[65] Thus, modulation of BDNF signaling alters functioning of the fear circuitry leading to divergent behavioral outcomes in adult rodents.
CHANGES IN BRAIN MATURATION AND FEAR-RELATED PHENOTYPES ACROSS DEVELOPMENT
The prefrontal and subcortical brain regions, which regulate fear learning and expression, undergo pronounced developmental changes from childhood into adulthood. However, structural and functional development of the human brain is a nonlinear process,[23, 29, 66] giving rise to a developmental mismatch model, suggesting that during adolescence subcortical regions are further ahead in their maturation trajectories compared to prefrontal ones.[67] Consistent with this model, magnetic resonance imaging (MRI) scans of human cortical gray matter for subjects between the ages of 4 and 21 years show that high-order association cortices mature later in development compared to sensorimotor regions, whose function they integrate.[68] In the prefrontal cortex, maximum synaptic density peaks at around 4 years of age,[69] followed by a substantial reduction in gray matter between adolescence and adulthood thought to be due to increased myelination in peripheral regions of the cortex that regulate cognitive processes.[70] There is an about 17% decrease in gray matter volume in the human prefrontal cortex between the ages of 10 and 26.[71] This earlier maturation of limbic regions compared to prefrontal regions in humans provides a basis for nonlinear changes in behavioral phenotypes across development.[72] Mirroring changes in the circuitry maturation, fear expression also exhibits qualitative changes across development in both humans and rodents.[73] In WT mice, it has been shown that there is a sensitive period for both fear extinction as well as contextual fear retrieval that spans the same developmental stage. Specifically, in both rats and mice, fear extinction learning and retention are attenuated during adolescence.[74–76] Relative to pre- and postadolescent animals, adolescents exhibit diminished fear extinction learning that is paralleled by an absence of extinction learning-induced plasticity within the IL.[76] This blunted fear extinction during adolescence is associated with a lack of activity in the prefrontal cortex, specifically IL, as assessed by phospho-MAPK immunohistochemistry[74] or c-Fos immunohistochemistry[76] compared with younger and older ages. Electrophysiological recordings in the prefrontal cortex across development reveal that fear conditioning-induced potentiation as well as extinction-induced enhancement of IL synaptic plasticity in adult mice is lacking in adolescent mice.[76] These studies suggest that the development of cued fear extinction progresses in a nonlinear manner, with adolescents showing diminished extinction learning relative to preadolescents and adults. In contrast to cued fear extinction learning, adolescent mice appear to be insensitive to contextual fear conditioning. Unlike juvenile and adult mice, adolescent mice returned to the context in which they experienced an aversive event do not display a fear response. This suppression of contextual fear during adolescence is due to a failure of contextual fear retrieval as opposed to acquisition because the same animals tested in early adulthood display a fear response to the context.[77] Thus, during early adolescence, WT mice undergo a disjunction in fear expression in which they are particularly sensitive to cued fear associations and insensitive to contextual fear associations.
Human studies of fear learning across development have been hindered by methodological limitations and the design of appropriate aversive testing conditions for children and adolescents. These settings include but are not limited to the use of white noise, unpleasant images, and the combination of the two as unconditioned stimuli, which correspond to the mild electric shock in the rodent paradigm.[78, 79] Similarly to rodents, humans also exhibit selective attenuation in fear extinction in adolescent development compared to children and adults.[76] Furthermore, a functional connectivity MRI study between the medial prefrontal cortex and the amygdala shows that from childhood to adolescence there is a change in activity correlation between these two regions involved in fear expression regulation, which is then stabilized into adulthood. These findings suggest dynamic maturational interaction for the medial prefrontal cortex and amygdala during adolescence.[80]
THE EFFECT OF BDNF ON CRITICAL AND SENSITIVE PERIOD PLASTICITY
The impact of postnatal BDNF signaling on circuitry maturation has been most clearly delineated in the context of plasticity in the mouse visual cortex. Monocular deprivation during a defined postnatal time window leads to persistent loss of visual acuity. In mice, this critical period has been confined to P19–P32.[81] Timed intracortical delivery of BDNF protein alters the formation of ocular dominance columns, as well as cortical plasticity after monocular deprivation.[82] In addition, in transgenic mice overexpressing BDNF, the critical period for ocular dominance plasticity is shifted to an earlier time frame and leads to accelerated maturation of visual acuity. Constitutive postnatal overexpression of BDNF also enhanced the maturation of GABAergic innervation in the visual cortex, in particular the parvalbumin-positive neurons, suggesting that BDNF produced and secreted from excitatory cortical neurons impacts the maturation of the inhibitory circuit during this period that spans the transition into adolescence.[6]
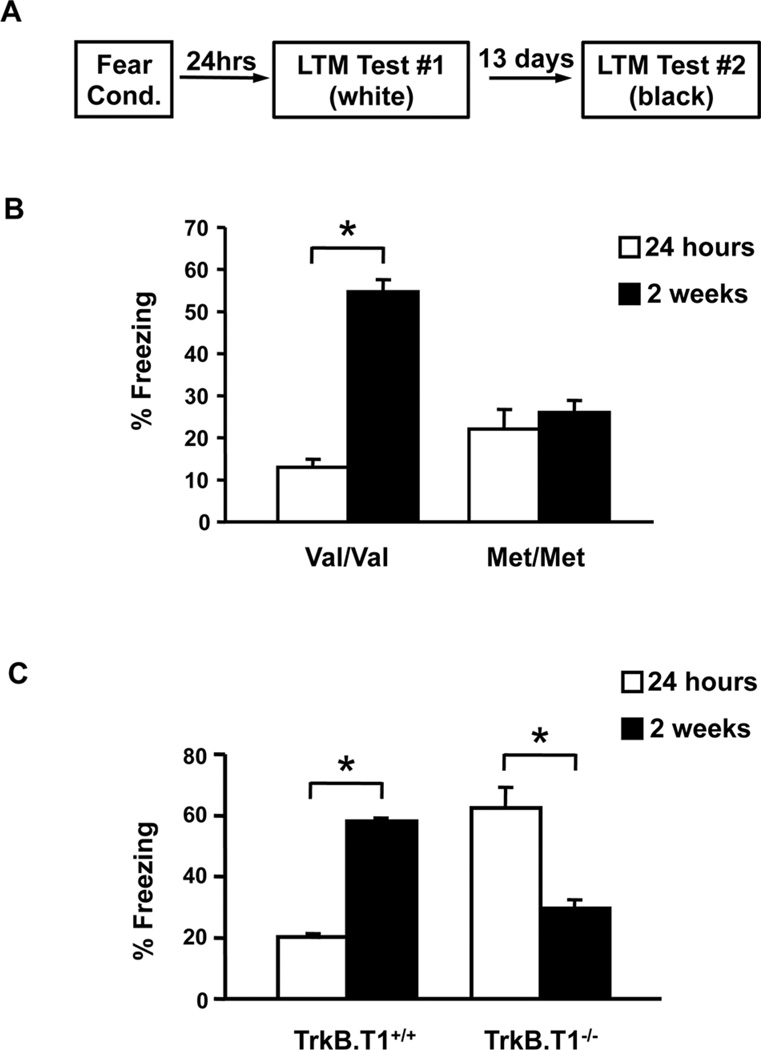
In contrast, less is known about the impact of BDNF signaling on fear-related circuits in this time frame that spans the third to fourth postnatal week. Recently, we have performed a series of studies assessing the impact of diminished BDNF signaling during this early adolescent sensitive period. We have utilized a knock-in mouse line, which carries the human Val66Met SNP leading to decreased BDNF bioavailability.[32, 40] Adult mice expressing the Met allele recapitulate the phenotypic hallmarks of humans with this polymorphism, especially with regard to altered anxiety- and fear-related behaviors.[40, 83] First, we have sought to determine the impact of the BDNF SNP on the contextual fear retrieval. In WT mice, contextual fear expression is temporarily suppressed during a distinct period in adolescence, but re-emerges at later, postadolescent ages.[77] We have previously shown that adult BDNFMet/Met mice have a deficit in acquisition of contextual fear associations, possibly related to high BDNF dependence of hippocampal plasticity.[40] Interestingly, BDNFMet/Met mice, in early adolescence (P29), do not exhibit alterations in contextual fear suppression as compared to age-matched WT littermates. However, when previously conditioned BDNFMet/Met mice are tested in adulthood, they fail to display the delayed expression of contextual fear compared to WT mice[44] (Fig. 3A and B), suggesting that diminished BDNF bioavailability may permanently alter hippocampal function in Met allele carriers, leading to persistent functioning that is indistinguishable from that in early adolescence.
Figure 3.
BDNF modulates contextual fear learning during adolescence. (A) Schematic representation of behavioral paradigm. Average freezing behavior to context at 24 hrs or 2 weeks following fear acquisition in (B) BDNF Val66Met and (C) truncated TrkB receptor-deficient mice compared to littermate controls.[44] All results are presented as means ± SEM. *P < .001.
This behavioral outcome of diminished BDNF availability would also predict that increasing BDNF-TrkB signaling results in early emergence of contextual fear in rodents. To better address this question, we have used a mouse line with a genetic deletion of one of BDNF’s receptors, the truncated TrkB receptor (TrkB.T1−/−), which lacks the intracellular tyrosine kinase domain.[45, 84] TrkB.T1 receptors primarily play a role as BDNF scavengers or dominant negative receptors sequestering BDNF that would otherwise lead to activation of full-length TrkB receptors and downstream signaling cascades.[85–87] When tested at P29, TrkB.T1−/− mice display an opposite behavioral pattern, exhibiting significantly heightened contextual fear expression in early adolescence compared to WT littermates (Fig. 3A and C). Contextual fear in P29 TrkB.T1−/− mice is indistinguishable from adult contextual fear levels for control mice (Fig. 3C), suggesting that complete loss of TrkB.T1 may have led to an enhanced functional maturation of hippocampal-amygdala-prefrontal circuitry via increased signaling through full-length TrkB.
EFFECT OF THE BDNF MET ALLELE ON THE DEVELOPMENT OF CUED FEAR EXTINCTION LEARNING
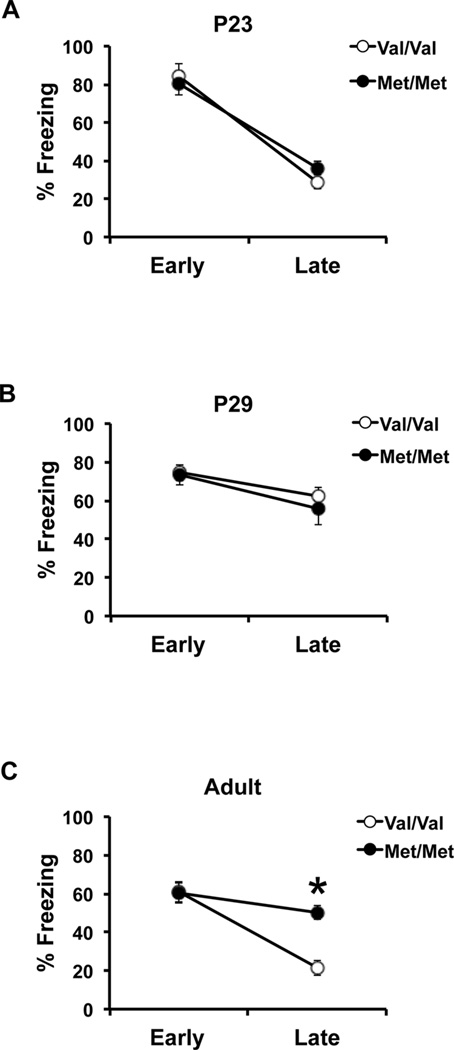
Our previous studies implicate BDNF in modulating contextual fear learning during adolescence,[44] however, it is still unclear what the developmental impact of the BDNF Val66Met SNP is on cue fear extinction. In order to address this question, we have analyzed preadolescent (P23) and early adolescent (P29) extinction learning in the BDNFMet/Met mice and compared it to fear extinction in adult BDNFMet/Met mice. All animal care is in accordance with Weill Cornell Medical College, Institutional Animal Care and Use Committee, National Institutes of Health Care and Use of Laboratory Animals. Cued fear conditioning apparatus and procedure have also previously been described.[88] Briefly, mice are fear conditioned with three tone-shock pairings. Extinction training consists of five tone presentations in a novel context repeated daily for 4 days after fear acquisition. At P23, preadolescent BDNFMet/Met mice exhibit fear extinction levels similar to those of WT littermates (two-way analysis of variance (ANOVA) with post hoc Bonferroni test; trial × genotype interaction: [F (1, 12) = 1.15, P > .05]; effect of trial: [F (1, 12) = 85.88, P < .0001]; Fig. 4A). Both groups show a significant decrease in freezing behavior to tone following repeated presentations. In early adolescence, there is no effect of the Met allele on extinction learning and BDNFMet/Met mice as well as controls display blunted extinction learning (two-way ANOVA with post hoc Bonferroni test; trial × genotype interaction: [F (1, 26)=0.26, P > .05]; Fig. 4B). This behavioral phenotype has previously been associated with altered synaptic plasticity in prefrontal cortical regions at this developmental stage.[76] However, adult BDNFMet/Met mice continue to display high freezing compared to age-matched controls, which exhibit enhanced extinction learning comparable to preadolescent mice (two-way ANOVA with post hoc Bonferroni test; trial × genotype interaction: [F (1, 30) = 9.70, P < .01]; effect of genotype: [F (1, 30) = 28.36, P < .0001]; Fig. 4C). These results suggest that, similar to the context fear expression, the impairment in cued fear extinction emerges in a time frame corresponding to the transition from adolescence to adulthood. In all, these findings also highlight that the impact of the Met allele in both contextual fear expression and cued fear extinction emerges as BDNF levels and BDNF-TrkB signaling are declining after peaking in early adolescence. The developmental onset of fear behavior deficits in BDNFMet/Met mice also parallels the late adolescent emergence of elevated anxiety-like behaviors in these mice.[89]
Figure 4.
Adolescent mice homozygous for the BDNF Val66Met polymorphism exhibit reduced extinction learning, which persists into adulthood. Average freezing behavior during early (extinction day 1) and late (extinction day 4) extinction trials of BDNF WT (Val/Val) and knock-in (Met/Met) mice during (A) early development, (B) adolescence, and (C) adulthood. All results are presented as means ± SEM obtained from 3–10 mice per group. *P < .001. P, postnatal day.
CONCLUSION AND FUTURE DIRECTIONS
Adolescence is a developmental stage, behaviorally and physiologically conserved across species, characterized by the transition of mammals from dependence to independence.[90] During this period, learning the optimal fear response to various environmental cues is a crucial adaptation for survival. During this time period the fear circuitry has been shown to be particularly plastic and is greatly affected by genetic factors. In this review, we have discussed the developmental trajectories of neurotrophin expression, including BDNF, proBDNF, and the prodomain as well as their respective receptors, highlighting the effects that changes in availability of these factors during adolescence may have on the maturation of fear circuitry and ultimately anxiety-related behavior. We have then focused on a particular human BDNF SNP that confers decreased BDNF bioavailability, and utilizing a knock-in mouse model identified alterations in the developmental trajectories of fear learning. With regard to the implications for the human BDNF Val66Met SNP, the absence of delayed contextual fear expression observed in BDNFMet/Met mice may be suggestive of an adaptive role in protecting BDNF Met allele carriers from re-experiencing previously conditioned aversive fears, despite their increased tendency for higher anxiety-like behavior in adulthood.[40, 91] Future studies are needed to investigate this apparent discrepancy between high anxiety-like behavior during adulthood and a lack of expression of previously conditioned contextual fear memories in BDNFMet/Met mice. In addition, understanding this phenomenon may shed light on the effects of the BDNF Met allele on the long-term behavioral consequences of early-life stress. In all, these analyses of the BDNF neurotrophin system across postnatal development demonstrate the complexity of the interactions between molecular function, development, and behavioral subdomains and that a polymorphism may confer both risk and resilience for anxiety, depending on the timing and nature of fear exposures. Ultimately, exploratory analyses in polymorphic mice can generate and refine hypotheses for association testing in human population samples.
In addition, our studies with the BDNFMet/Met mice support the idea that alterations in neurotrophin signaling during a sensitive period in early adolescent development may underlie risk for psychiatric disorders later on in life. Thus, elucidating the precise developmental changes in neurotrophin signaling impacting fear and anxiety circuitry maturation would facilitate the appropriate timing of intervention for optimal behavioral outcomes. We have identified a discrete window of sensitivity during early adolescence, which might present an opportunity for therapeutic intervention to normalize fear-related behaviors by correcting circuit-level deficiencies associated with the human BDNF Met-allele.
Acknowledgments
Contract grant sponsor: Sackler Institute (F.S.L.); Contract grant sponsor: NewYork-Presbyterian Youth Anxiety Center (F.S.L.); Contract grant sponsor: National Institutes of Health (F.S.L.); Contract grant numbers: MH079513, NS052819.
Footnotes
Conflict of interest. The authors declare no conflicts of interest.
REFERENCES
- 1.Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44(10):1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367(1601):2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Boyle LM. A neuroplasticity hypothesis of chronic stress in the basolateral amygdala. Yale J Biol Med. 2013;86(2):117–125. [PMC free article] [PubMed] [Google Scholar]
- 6.Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 7.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anastasia A, Deinhardt K, Chao MV, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. 2013;4:1–12. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucchelli C, Vantaggiato C, Ciamei A, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34(5):807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblum K, Futter M, Voss K, et al. The role of extracellular regulated kinases I/II in late-phase long-term potentiation. J Neurosci. 2002;22(13):5432–5441. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110(2):167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 14.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10(12):850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Siao CJ, Nagappan G, et al. Neuronal release of proBDNF. Nat Neurosci. 2009;12(2):113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng HK, Teng KK, Lee R, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25(22):5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Harte-Hargrove LC, Siao CJ, et al. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7(3):796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deinhardt K, Chao MV. Shaping neurons: long and short range effects of mature and proBDNF signalling upon neuronal structure. Neuropharmacology. 2014;76(Pt C):603–609. doi: 10.1016/j.neuropharm.2013.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: recent advances and challenges. Dev Neurobiol. 2010;70(5):350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Je HS, Yang F, Ji Y, et al. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc Natl Acad Sci USA. 2012;109(39):15924–15929. doi: 10.1073/pnas.1207767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Lim Y, Li F, et al. ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One. 2012;7(4):e35883. doi: 10.1371/journal.pone.0035883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67(3):203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 23.Woo NH, Teng HK, Siao CJ, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 24.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 25.Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69(1):34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 26.Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72(5):1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 27.Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett. 2001;307(1):21–24. doi: 10.1016/s0304-3940(01)01905-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Yang M, Song L, et al. Developmental and degenerative modulation of brain-derived neurotrophic factor transcript variants in the mouse hippocampus. Int J Dev Neurosci. 2014;38:68–73. doi: 10.1016/j.ijdevneu.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Galvin C, Lee FS, Ninan I. Alteration of the centromedial amygdala glutamatergic synapses by the BDNF Val66Met polymorphism. Neuropsychopharmacology. 2015;40(9):2269–2277. doi: 10.1038/npp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauskolb S, Zagrebelsky M, Dreznjak A, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30(5):1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizui T, Ishikawa Y, Kumanogoh H, et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci USA. 2015;112(23):E3067–E3074. doi: 10.1073/pnas.1422336112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 33.Petryshen TL, Sabeti PC, Aldinger KA, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15(8):810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dincheva I, Glatt CE, Lee FS. Impact of the BDNF Val66Met polymorphism on cognition: implications for behavioral genetics. Neuroscientist. 2012;18(5):439–451. doi: 10.1177/1073858411431646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhagen M, van der Meij A, van Deurzen PA, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 38.Montag C, Basten U, Stelzel C, et al. The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010;179(1):86–90. doi: 10.1016/j.psychres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Felmingham KL, Dobson-Stone C, Schofield PR, et al. The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry. 2013;73(11):1059–1063. doi: 10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZY, Patel PD, Sant G, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24(18):4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ZY, Ieraci A, Teng H, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25(26):6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou H, Kim SK, Zaitsev E, et al. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45(2):245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 44.Dincheva I, Pattwell SS, Tessarollo L, et al. BDNF modulates contextual fear learning during adolescence. Dev Neurosci. 2014;36(3–4):269–276. doi: 10.1159/000358824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fryer RH, Kaplan DR, Feinstein SC, et al. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374(1):21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132(3):613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29(3):436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escandon E, Soppet D, Rosenthal A, et al. Regulation of neurotrophin receptor expression during embryonic and postnatal development. J Neurosci. 1994;14(4):2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bracken BK, Turrigiano GG. Experience-dependent regulation of TrkB isoforms in rodent visual cortex. Dev Neurobiol. 2009;69(5):267–278. doi: 10.1002/dneu.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 51.Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7(2):97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15(5):1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 53.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 55.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29(26):8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hefner K, Whittle N, Juhasz J, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16(8):486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berretta S, Pantazopoulos H, Caldera M, et al. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132(4):943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Likhtik E, Popa D, Apergis-Schoute J, et al. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328(5983):1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal—prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39(9):2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9(7):870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24(36):7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koponen E, Voikar V, Riekki R, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8(10):1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- 67.Boskovic Z, Alfonsi F, Rumballe BA, et al. The role of p75NTR in cholinergic basal forebrain structure and function. J Neurosci. 2014;34(39):13033–13038. doi: 10.1523/JNEUROSCI.2364-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Sowell ER, Thompson PM, Holmes CJ, et al. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 71.Frankland PW, Josselyn SA, Bradwejn J, et al. Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. J Neurosci. 1997;17(5):1838–1847. doi: 10.1523/JNEUROSCI.17-05-01838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeo JF, Tang FR, Leong SK. Expression of glutamate receptor subunit 1 and nitric oxide synthase in the hypoglossal nucleus and dorsal vagal nucleus in the rat after neurectomy. Int J Neurosci. 1997;90(1–2):9–20. doi: 10.3109/00207459709000622. [DOI] [PubMed] [Google Scholar]
- 73.Stamford JA, Isaac D, Hicks CA, et al. Ascorbic acid is neuroprotective against global ischaemia in striatum but not hippocampus: histological and voltammetric data. Brain Res. 1999;835(2):229–240. doi: 10.1016/s0006-8993(99)01587-5. [DOI] [PubMed] [Google Scholar]
- 74.Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex. 2011;21(3):530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- 75.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35(10):2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pattwell SS, Duhoux S, Hartley CA, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci USA. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattwell SS, Bath KG, Casey BJ, et al. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci USA. 2011;108(3):1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casey BJ, Pattwell SS, Glatt CE, Lee FS. Treating the developing brain: implications from human imaging and mouse genetics. Annu Rev Med. 2013;64:427–439. doi: 10.1146/annurev-med-052611-130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shechner T, Britton JC, Ronkin EG, et al. Fear conditioning and extinction in anxious and nonanxious youth and adults: examining a novel developmentally appropriate fear-conditioning task. Depress Anxiety. 2015;32(4):277–288. doi: 10.1002/da.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267(5204):1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 83.Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carim-Todd L, Bath KG, Fulgenzi G, et al. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29(3):678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biffo S, Offenhauser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121(8):2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 87.Eide FF, Vining ER, Eide BL, et al. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16(10):3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pattwell SS, Bath KG, Perez-Castro R, et al. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32(7):2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bath KG, Chuang J, Spencer-Segal JL, et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72(6):499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 91.Spencer JL, Waters EM, Milner TA, et al. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA. 2010;107(9):4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]