SUMMARY
During implantation, uterine luminal epithelial (LE) cells first interact with the blastocyst trophectoderm. Within 30 hr after the initiation of attachment, LE cells surrounding the blastocyst in the implantation chamber (crypt) disappear, allowing trophoblast cells to make direct physical contact with the underneath stroma for successful implantation. The mechanism for the extraction of LE cells was thought to be mediated by apoptosis. Here, we show that LE cells in direct contact with the blastocyst are endocytosed by trophoblast cells by adopting the nonapoptotic cell-in-cell invasion process (entosis) in the absence of caspase 3 activation. Our in vivo observations were reinforced by the results of co-culture experiments with primary uterine epithelial cells with trophoblast stem cells or blastocysts showing internalization of epithelial cells by trophoblasts. We have identified entosis as a mechanism to remove LE cells by trophoblast cells in implantation, conferring a role for entosis in an important physiological process.
Graphical abstract
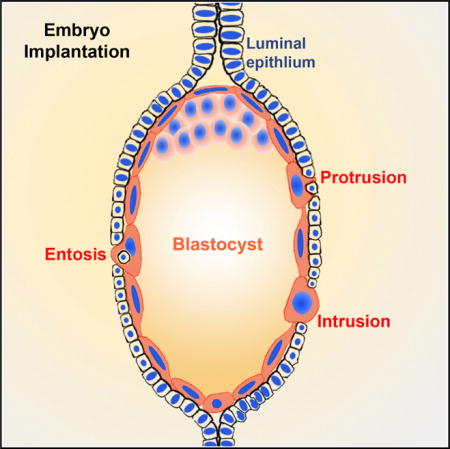
INTRODUCTION
A reciprocal interaction between a blastocyst and the receptive uterus is critical to implantation. In mice, blastocysts are positioned within the implantation chambers (crypt) formed by the evagination of the luminal epithelium (LE) at a regular space at the antimesometrial pole of the uterus on day 4 of pregnancy (day 1 = vaginal plug) (Cha et al., 2014). The implantation process involves several stages—blastocyst apposition, adhesion, and attachment with the LE, ultimately allowing the trophectoderm to erode the LE barrier to make direct contact with the underlying stroma. The attachment phase (initiation of implantation) is coincident with localized endometrial vascular permeability exclusively at the site of blastocyst that occurs in the evening of day 4 (Das et al., 1994). The process is more prominent on day 5, and by day 6, blastocysts are in direct contact with uterine stromal cells. Defects during the early implantation events result in either pregnancy failure or late-stage pregnancy defects (Cha et al., 2012; Wang and Dey, 2006).
Upon attachment of the blastocyst with the LE, abstraction of the LE for the passage of the trophectoderm into the stroma is one of the first steps in the process of implantation. Genetic studies provide evidence that a cause for implantation failure is the blockade of the trophectoderm transit through the LE barrier (Daikoku et al., 2011; Sun et al., 2012). Although trophectoderm-LE interactions have been studied for decades, the mechanism by which LE cells are devoured still remains unclear. The relative importance of trophoblast and uterine participation in the removal of uterine LE cells has been debated for many years. Based mainly on electron microscopy (EM) studies, some investigators hypothesized that degeneration of LE cells is intrinsic to the uterus in which embryos play a minor role (Finn and Hinchliffe, 1964; Krehbiel, 1937), whereas others suggested that trophoblast cells trigger LE apoptosis (Parr et al., 1987). Nonetheless, most investigators believed that LE cells enclosing the blastocyst have characteristics of apoptosis, including cellular shrinkage and nuclear fragmentation following implantation on day 5 (Parr et al., 1987; Welsh and Enders, 1993). The general accord was that the apoptotic LE cells are phagocytized by trophoblast cells (El-Shershaby and Hinchliffe, 1975; Parr et al., 1987). However, these notions were primarily based on observations of cell integrity and structure, but no molecular markers of apoptosis were used in these studies. Therefore, no direct evidence for apoptosis of LE cells during their initial encounter with the trophectoderm was presented to exclude the possibility that disappearance of LE cells is dependent on a different mechanism and the possibility that embryonic trophoblast cells play a critical role in the abstraction of LE cells during implantation under normal pregnancy conditions.
In recent years, a new cell-in-cell invasion phenomenon, also called entosis, has been described (Overholtzer et al., 2007). Both entosis and phagocytosis involve engulfment of one cell by another cell. However, in phagocytosis, only dead or dying cells are engulfed by live cells, whereas in entosis live cells internalize live cells. Entosis has mainly been studied in vitro using cancer cell lines (Overholtzer et al., 2007; Purvanov et al., 2014). In vivo, entosis disrupts normal cytokinesis, resulting in aneuploidy in human breast cancers (Krajcovic et al., 2011). Our results provide evidence that entosis has a role in a normal physiological process.
Here we report that during blastocyst implantation in mice, trophoblast cells actively engulf proximate LE cells, resulting in elimination of the epithelial barrier for direct contact with the stroma and facilitating anchorage of the embryo within the stromal bed. This observation challenges the dogma that uterine epithelial cells undergo apoptosis attributed by maternal responses with minimal role played by embryonic cells in eliminating the LE cells. Our results derived from in vivo and in vitro experiments rather suggest that trophoblast cells engulf the LE cells by entosis. Using reliable apoptosis and epithelial cell markers at different time points during implantation in mice, we found no evidence of apoptosis in the LE cells adjacent to trophoblast cells during the clearance of the LE cells; rather, evidence for entosis was noted in a series of experiments. Our speculation of entosis operating in the removal of LE cells by trophoblast cells was further reinforced by in vitro experiments of co-cultured primary uterine epithelial cells with trophoblast stem cells (TSCs) both labeled with different fluorescent cell trackers, or co-cultured epithelial cells with zona-free Rosa-Tomato reporter blastocysts. The results provide evidence that trophoblast cells are able to engulf uterine epithelial cells in culture. Taken together, these results support that entosis plays a key role in a normal physiological process, such as embryo implantation, where trophoblast cells engage this mechanism to efficiently remove the epithelial barrier for implantation.
RESULTS
LE Cells around the Blastocyst Are Breached on Day 5 Evening
Blastocysts are positioned in the implantation chamber (crypt) in the afternoon of day 4 (Cha et al., 2014). Prior to and upon initiation of blastocyst attachment, changes in gene expression are observed in both LE and stromal cells encircling the blastocyst, suggesting blastocyst-uterine interactions (Das et al., 1994; Lim et al., 1997). We found that LE cells were tightly held together surrounding the blastocyst in the implantation chamber on the morning of day 5 of pregnancy. In contrast, in the evening of day 5 (18:00–20:00 hr), multiple gaps within the LE lining the lateral sides of the crypt were noted with the remaining epithelial cells showing an uneven apical alignment, indicative of decreased apicobasal cell polarity (Figure 1A). By day 6 morning, the LE separating the embryo from the stroma completely disappeared, indicating that breaching of the epithelial barrier occurred in the evening of day 5.
Figure 1. Uterine LE Surrounding the Blastocyst Is Breached on Day 5 Evening.

(A) H&E-stained sections of implantation sites on days 5 (D5) and 6 (D6). Arrows point to the gaps on the LE. Em, embryo.
(B) Immunofluorescence of E-cad and β-catenin in sections of implantation sites on days 5 and 6. Signals for E-cad show LE is removed in the evening of day 5. An area with breached epithelium delineated by a rectangle is presented at a larger magnification. Arrows point to the breaches in the LE. Em, embryo; M, mesometrial pole; AM, antimesometrial pole.
To further examine the epithelial integrity, we demarcated the LE by immunolocalization of E-cadherin (E-cad) and β-catenin, which are components of the adherens junction. At 18:00 hr on day 5, the E-cad signal was distinct in the LE surrounding the blastocyst (Figure 1B). By 20:00 hr, interrupted areas appeared in the LE, and most of the LE lining disappeared 4 hr later. These results suggest that the extraction of the LE begins in the evening between 18:00 and 20:00 hr on day 5 of pregnancy. Signals of β-catenin were observed at the apical and basolateral sides of the LE around the blastocyst on day 5 at 18:00 hr and in decidualizing stromal cells beginning on day 5 at 20:00 hr (Figure 1B). With decidualization process in progress, the expression zone of β-catenin became larger and stronger in intensity, since decidualizing cells (primary decidual zone) around the embryo show epithelial-like characteristics during this time (Paria et al., 1999). In contrast, the signal intensity of β-catenin in the LE around the implantation chamber became discontinuous, suggesting breaching of the LE. All of these results suggest that the apicobasal polarity of LE cells decreases around the implantation chamber heralding the clearance of LE cells, which are in direct contact with the implanting blastocyst.
Removal of LE Cells in Direct Contact with the Trophoblast Cells Is Independent of Caspase 3 Activation
Previous studies have suggested that maternal responses to implantation prompt LE cells in the implantation chamber to undergo apoptosis and that cell corpses are then endocytosed by trophoblast cells (Parr et al., 1987; Welsh and Enders, 1993). The activation of Caspase3 (cleaved form) is a well-established pro-apoptotic cell marker (Salvesen, 2002). Therefore, we performed immunofluorescence of cleaved Caspase3 on sections of implantation sites around the time of LE breaching (18:00–20:00 hr on day 5). We found that cytokeratin 8 (CK8)-labeled LE cells in direct contact with the embryos were negative for cleaved Caspase3 signals at all-time points examined (Figure 2A). In contrast, LE cells at the bottom of the implantation chamber not in direct contact with the blastocyst began to show cleaved Caspase3 signals around midnight of day 5 (24:00 hr) and became prominent in almost all the epithelial cells in that area on day 6 morning (Figure 2A). Scattered signals were also observed in the stroma around the implantation chamber, the significance of which is not clear. These findings provide evidence that epithelial cells in direct contact with the embryo (the entry points of trophoblast cells into the stroma) are removed before the appearance of pro-apoptotic signals. It may be argued that LE cells in direct contact with the embryo are removed by senescence or autophagy-related cell death. However, we did not find evidence for senescence-associated β-galactosidase (β-gal) and LC3 (autophagy marker) staining on the sections of implantation sites in the morning or evening of day 5 (Figures S1 and S2). These results suggest that a different mechanism operates in removing LE cells in direct contact with the blastocyst trophoblasts. Cells undergoing apoptosis ultimately show DNA fragmentation, which can be detected by the TUNEL assay (Negoescu et al., 1996). We performed this assay in sections of the implantation site around the time of breaching of the LE. No TUNEL-positive cells were evident at the time of LE breaching, but sporadic TUNEL-positive signals were observed in dead LE cells within the trophoblasts and in some stromal cells at midnight of day 5, and almost in all LE cells in the bottom of the implantation chamber away from the implanting embryo on day 6 morning (area demarcated by dotted lines in Figure 2B). These observations suggest that the mechanism of cell death in two different subsets of epithelial cells situated at two distinct sites is different.
Figure 2. Extraction of LE Cells Surrounding the Embryo Is Independent of Caspase 3 Activation.
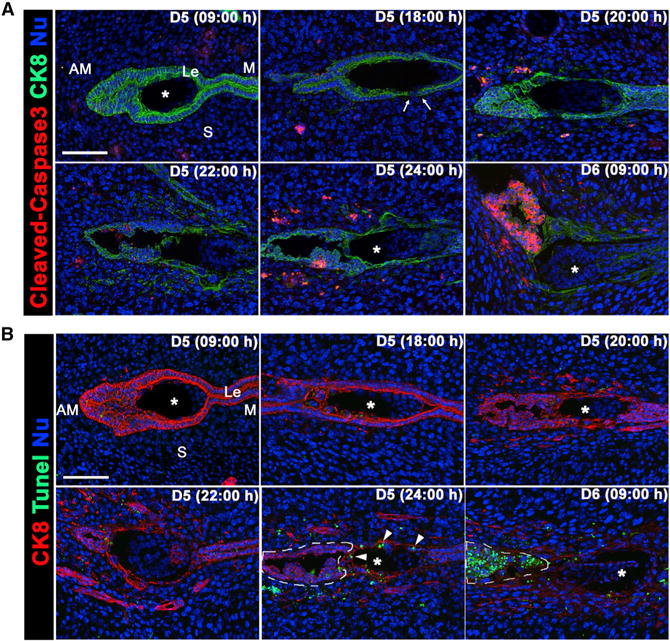
(A) Immunofluorescence of cleaved caspase 3 and CK8 in sections of implantation sites on days 5 and 6. Signals for activated caspase 3 are absent from the LE in direct contact with the embryo. The area of LE breaching is indicated by arrows. Asterisks depict the position of the embryo. Le, luminal epithelium; S, stroma; M, mesometrial pole; AM, antimesometrial pole.
(B) TUNEL and CK8 staining in sections of implantation sites on days 5 and 6. TUNEL-positive cells were absent in the LE prior to and at the time of its breaching (D5, 20:00 hr). Arrowheads point to the TUNEL-positive cells engulfed by trophoblast cells. Very few TUNEL-positive cells were observed in the anti-mesometrial extension of LE not in contact with the blastocyst as outlined by dotted lines on day 5 (24:00 hr); these cells became TUNEL positive on day 6 morning. Scale bars represent 100 μm.
Trophoblast Cells Engulf LE Cells in the Evening of Day 5
To further explore the mechanism of disappearance of the LE barrier, we demarcated the LE and trophoblast cells by immunofluorescence of CK8 and junctional protein ZO-1. The results showed that few trophoblast cells on the lateral side of the embryo formed dome-shaped bulges protruding into stromal bed (dotted circles in Figure 3A), and within these bulged trophoblast cells, a second nucleus was identified (red arrows in Figure 3A). Immunohistochemistry of E-cad on paraffin sections of implantation sites on day 5 at 20:00 hr confirmed this observation. The E-cad-positive LE became discontinuous and gaps appeared in the LE lining at this time point. The trophoblasts occupied the site of the missing epithelial cells, and interestingly, cells with pyknotic nuclei were observed within the trophoblast cells (red arrows in Figure 3B), suggesting that LE cells are internalized within the trophoblast cells (blue arrows in Figure 3B). A glycosylated lysosomal-associated membrane protein 1 (LAMP1) is concentrated in the lysosomal membrane (Howe et al., 1988). We found that the engulfed LE cells are encircled by LAMP1, suggesting that the engulfed cell is within a lysosome (white arrow head in Figure 3C).
Figure 3. Trophoblast Cells Engulf LE Cells in the Evening of Day 5.
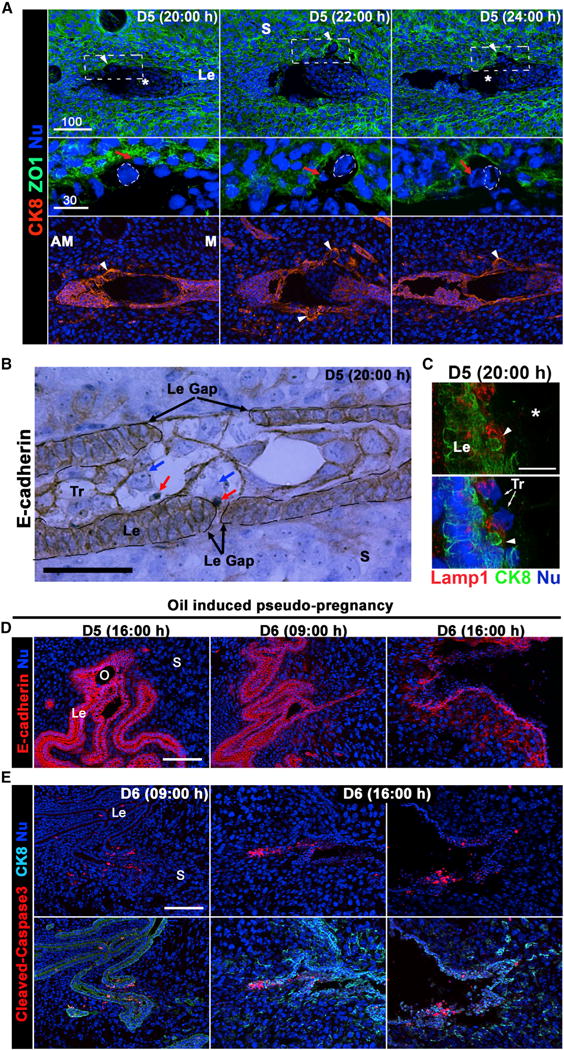
(A) Immunofluorescence of CK8 and ZO1 in sections of implantation sites on days 5 and 6. Arrowheads point to the protruding trophoblast cells. Areas containing protruding trophoblast cells outlined by dotted lines are presented at higher magnification. Red arrows show epithelial cells engulfed by the protruding trophoblast cells, which are outlined by dotted circles. Asterisks indicate the position of the embryo. Le, luminal epithelium; S, stroma; M, mesometrial pole; AM, antimesometrial pole. Scale bar represents 100 μm.
(B) Immunohistochemistry of E-cad in a section of day 5 implantation site at 20:00 hr. The section is counterstained with hematoxylin. E-cad signals depict the borders of the trophectoderm and LE. At the LE (Le) gaps, trophoblast cells are in direct contact with the basal membrane of LE cells. Blue arrows point to the nuclei of trophoblast cells, while red arrows point to cells engulfed by trophoblast cells. Scale bar represents 50 μm.
(C) Immunofluorescence of LAMP1 and CK8 in sections of a day 5 implantation site at 20:00h. The detached LE cell as indicated by an arrowhead is within a LAMP1-positive endosome, a marker of endosome. Trophoblast (Tr) nuclei are indicated by arrows. Scale bar represents 50 μm.
(D) LE cells undergo apoptosis on day 6 of pseudopregnancy after an induction of decidual reaction by intraluminal oil infusion on day 4. Immunofluorescence of E-cad in oil-infused pseudopregnant uterine sections on days 5 and 6. LE cells start to disintegrate on day 6 of pseudopregnancy. O, oil drop; Le, luminal epithelia; S, stroma.
(E) Immunofluorescence of cleaved-caspase3 and CK8 in sections of pseudopregnant uteri of day 6 following intraluminal oil infusion. caspase 3 is activated in LE (Le) on day 6 (16:00 hr). Two representative sections from two different mice are shown. Scale bars represent 100 μm.
Previous studies have shown that neutrophils and monocytes are present close to the implantation chamber at the beginning of implantation (McMaster et al., 1993). Therefore, it is possible that these cells around the implantation site also play a role in clearing the epithelial cells surrounding the embryo. Thus, we performed immunofluorescence of F4/80 (marker of macrophage) and GR-1 (neutrophil marker) on the sections of implantation sites on day 5 (20:00 hr) of pregnancy (Figure S3). While monocytes were present close to implantation sites in the morning of day 5, they were absent with the formation of primary decidual (PDZ) in the evening. Similarly, neutrophils were detected sporadically around the implantation chamber, suggesting a limited role of macrophages or neutrophils in the removal of LE cells. Collectively, these results suggest that the LE cells on lateral sides of the implantation chamber are removed in the evening of day 5 of pregnancy due to engulfment of them by trophoblast cells.
On-Time Elimination of LE Cells Requires the Presence of Implanting Blastocyst
With these data in hand, we asked whether cell death in the LE around the implantation chamber is intrinsic to a maternal response to implantation or an active process executed by the intrusive trophoblast cells; earlier studies have implicated that epithelial cell death is an intrinsic maternal response of the uterus in which the conceptus plays a minor role (Finn and Lawn, 1968; Welsh and Enders, 1993). It was thought that the trophoblast cells phagocytize dead epithelial cells, but not actively participate in breaching the LE barrier. To study whether the blastocyst plays an active role in removing the LE cells, we induced implantation-like responses by intraluminal infusion of oil in the receptive uterus of pseudopregnant mice to examine LE cell death in the absence of blastocysts. Sesame oil (10 μl) was injected into the lumen of one uterine horn on day 4 of pseudopregnancy. Uterine tissues were collected on days 5 and 6 of pregnancy as indicated and were subject to CK8 immunofluorescence. In the evening of day 5 (16:00 hr), small oil droplets formed within the lumen, creating chambers similar to those of implantation chambers (Figure 3D). Surprisingly, the LE layer remained intact until the morning (09:00 hr) of day 6, but was only breached in the evening of day 6 (16:00 hr). This observation is consistent with immunofluorescence of activated caspase 3, which showed sparse distribution on day 6 morning and became more widespread in the evening of day 6 (Figure 3E). These results provide evidence that the demise of LE cells in the “pseudo-chamber” is delayed in the absence of an embryo, and the mechanism of LE cell death is different within the pseudopregnant versus pregnant uterus; i.e., the pseudopregnant LE cells after oil infusion undergo apoptosis by the activation of caspase 3 as opposed to entosis during normal implantation.
Trophoblast Cells Are Capable of Internalizing Epithelial Cells by Entosis
Given that the LE barrier is interrupted at locations adjacent to blastocysts much earlier in implantation in a caspase 3-independent manner, we think that the embryo plays an active role in this process. Similar to earlier evidence that MCF7 cells can engulf live cells by entosis (Overholtzer et al., 2007), we also observed internalization of LE cells within trophoblast cells, supporting our hypothesis that trophoblast cells remove the surrounding LE cell barrier by entosis during implantation, and this on-time elimination of the LE barrier is critical to normal implantation. To test this hypothesis, WT TSCs labeled by red fluorescence tracker dye were incubated in vitro with primary uterine epithelial cells labeled with green tracker fluorescent dye. We observed that epithelial cells were engulfed by TSCs after 6 hr of incubation (Figure 4A; Movie S1). As shown, a TSC nucleus was squeezed aside and became crescent shape with the engulfed epithelial cell occupying most of the cytosolic space of the TSC. Staining of β-catenin demarcates the membrane of the engulfed epithelial cells, demonstrating that engulfed epithelial cells are surrounded by TSCs (Figure 4B). These results suggested that TSCs are capable of engulfing epithelial cells in vitro. To make the in vitro condition closer to a physiological setting, we repeated the experiments by replacing TSCs with blastocysts, which were collected by flushing uteri from Rosa-Tomato reporter mice on day 4 of pregnancy. Blastocysts were cultured overnight to make them zona free. After 6 hr of co-incubation with primary uterine epithelial cells, some trophoblast cells on the surface of the blastocyst became enlarged and formed dome-shaped bulges (Figure 4C), similar to what we observed in vivo (Figure 3A). Furthermore, blastocyst trophoblast cells were noted to have internalized epithelial cells (Figure 4C; Movie S2). Collectively, these data suggest that both TSCs and blastocyst trophoblast cells are capable of engulfing uterine epithelial cells by the process of entosis in vitro.
Figure 4. TSCs and Blastocysts Endocytose Primary Uterine Epithelial Cells In Vitro.
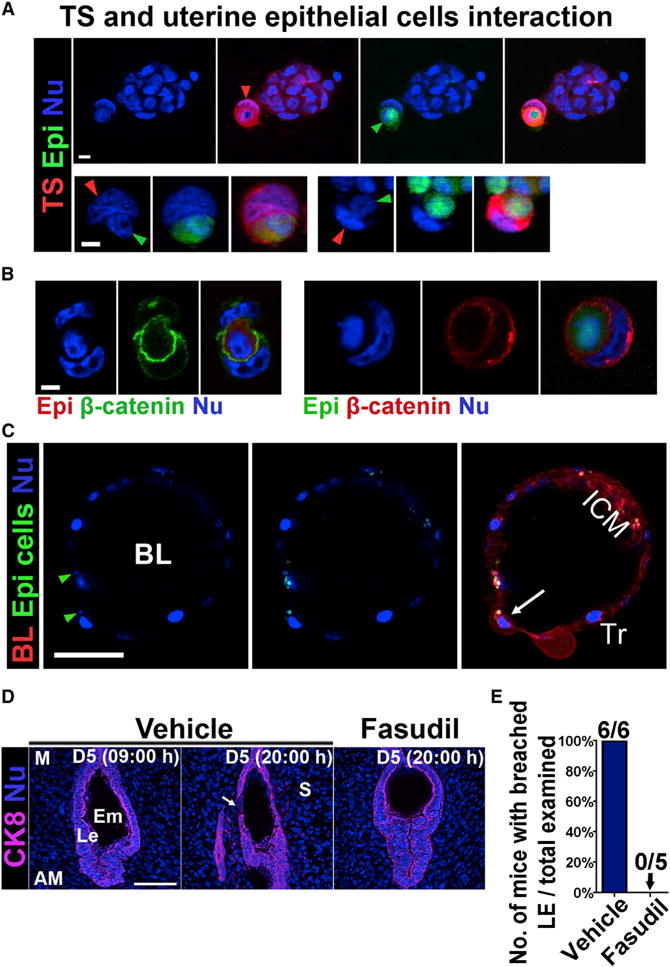
(A) TSCs engulf primary epithelial cells. Red arrowhead points toward the nucleus of one TSC (red), whose nucleus is squeezed to a crescent shape by an epithelial cell (green). The green arrowhead points toward the engulfed epithelial cell. The bottom panels show two other engulfed epithelial cells (green) by TSCs (red). Scale bar represents 10 μm.
(B) Immunofluorescence of β-catenin in engulfed epithelial cells. Epithelial cells were stained with either red or green tracker as indicated. The boundary of the engulfed epithelial cells and the TSCs is highlighted by β-catenin staining. Scale bar represents 10 μm.
(C) Trophoblast cells in a Rosa-tomato reporter blastocyst showing engulfment of primary epithelial cells (green). Green arrowheads point to the nuclei of engulfed epithelial cells. A white arrow points to an enlarged trophoblast cell which had engulfed an epithelial cell. BL, blastocyst; ICM, inner cell mass. Tr, trophectoderm. Scale bar represents 50 μm.
(D) A ROCK inhibitor Fasudil impedes the removal of LE cells at 20:00 hr of day 5. The LE barrier begins to disappear in control (vehicle) females, as indicated by the white arrow, whereas the LE encasing the blastocyst remained intact in Fasudil-treated females at 20:00 hr of day 5. Le, luminal epithelium; S, stroma; Em, embryo; M, mesometrial pole; AM, antimesometrial pole. Scale bar represents 100 μm.
(E) Percentage of mice with breached luminal epithelia versus total mice examined at 20:00 hr on day 5. All five mice treated with Fasudil had intact LE surrounding embryos, whereas all six control (vehicle-treated) mice had implantation sites (IS) with breached LE.
Inhibition of ROCK Impedes the Process of Entosis during Implantation
There is evidence that small GTPase ROCK is required for entosis (Overholtzer et al., 2007). Therefore, we examined whether the elimination of the LE barrier is inhibited by blocking the ROCK activity. WT pregnant females were treated with a cell permeable ROCK inhibitor (Fasudil) or vehicle (control) in the morning (09:00 hr) of day 5 of pregnancy. At 20:00 hr on day 5, the LE barrier began to disappear in control females (n = 6), whereas the LE encasing the blastocyst remained intact in all Fasudil-treated females (n = 5; Figures 4D and 4E). These results suggest that the disintegration of the epithelial cells around the embryo during implantation was disrupted by inhibiting ROCK activity.
DISCUSSION
One of the early prerequisites for successful implantation is the removal of the LE barrier encasing the blastocyst. Earlier studies in mice and rats indicated that the demise of LE cells around the implantation chamber is due to apoptosis resulting from an intrinsic uterine response followed by trophoblast cell-led phagocytosis (Finn and Hinchliffe, 1964; Finn and Lawn, 1968). In contrast, our present results show that the removal of the LE for the passage of the embryonic trophoblast cells into the stromal bed involves nonapoptotic cell death by entosis, a process by which one live cell engulfs another live cell. We argue that the intrusive nature of trophoblast cells is consistent with this process. This is reinforced by our observation of the absence of activated caspase 3, a reliable marker of apoptosis, in the LE cells at the time of their removal, suggesting that this event is not mediated by apoptosis. Furthermore, the initial removal of LE cells from the lateral sides of the implantation chamber enclosing the blastocyst does not appear to be due to cell death by senescence, autophagy, or their removal by phagocytosis by macrophages or neutrophils because these activities are not impressive at this time. Rather, entosis is due to active participation of the implanting blastocyst since this process is not observed by induction of implantation-like responses by oil infusion into the uterine lumen of pseudopregnant mice. Interestingly, senescence-associated β-gal staining is primarily seen in day 2 uteri when apoptosis is also prominent.
Previous reports showed that apoptotic LE cells are phagocytized by trophoblast cells (El-Shershaby and Hinchliffe, 1975; Parr et al., 1987). As mentioned before, the difference between phagocytosis and entosis is that a dead cell is phagocytized, whereas in entosis a live cell is internalized by another live cell. We argue that LE cells internalized by trophoblast cells adopting entosis is due to the following reasons: first, the window of clearance of LE cells is narrow (18:00–22:00 hr on day 5 of pregnancy); second, there is no Caspase3 activation in LE cells before or during the LE cell clearance. Finally, TUNEL-positive cells were only observed after the removal of LE cells, and positive staining was only present in engulfed LE cells at 24:00 hr on day 5, suggesting that LE cells undergo cell death and nuclear disintegration after internalization by entosis. Cell death after engulfment is associated with the digestion of LE cells in lysosomes of trophoblast cells. These in vivo observations are supported by in vitro results that trophoblast cells engulf primary uterine epithelial cells.
We have shown previously that LE cells begin to lose their apicobasal polarity pending blastocyst implantation (Cha et al., 2014; Daikoku et al., 2011). On day 5, the intrusive trophoblast cells take advantage of the less polarized loosened LE cells and adopt a cell-in-cell invasive behavior to engulf and internalize bordering LE cells to make contact with stromal cells. Transition of LE cells from high to low polar state seems critical since the LE retains high polarity in mice with uterine inactivation of Msx, Klf5, or overexpression of Wnt5a with blastocysts remaining encased within the intact epithelium past day 6 of pregnancy, resulting in implantation failure (Cha et al., 2014; Daikoku et al., 2011; Sun et al., 2012). Therefore, the initial phase of the implantation process allowing entosis requires a low polar state of the epithelium, and thus, successful operation of this process requires cooperation between the uterus and embryo.
Our argument of the participation of entosis by trophoblast cells is consistent with our in vivo observations of swallowing of LE cells located at the lateral side of the crypt in the absence of caspase 3 activation or TUNEL-positive cells. These observations were further strengthened by our co-culture experiments with epithelial cells and TSCs or epithelial cells with zona-free Rosa-Tomato reporter blastocysts expressing red fluorescence. Given that ROCK activity is involved in entosis (Overholtzer et al., 2007), our observation of the persistent presence of the LE surrounding the blastocyst past evening of day 5 of pregnancy by intraperitoneal administration of a ROCK inhibitor is a testament to the entosis process in implantation. There is a report of participation of entosis in aneuploidy in breast cancer (Krajcovic et al., 2011). However, our study implicates entosis as an important process in embryo implantation in a normal physiological setting; whether this process plays a role in other physiological processes warrants further investigation. The mechanism of intercalation seen in the clearance of mesothelial cells by ovarian cancer cell clusters could also be at play in addition to entosis, both requiring ROCK activity (Iwanicki et al., 2011).
Interestingly, epithelial cells at the anti-mesometrial side below the extension of implantation chamber not in contact with the embryo undergo apoptosis on day 6 of pregnancy, as marked by caspase 3 and TUNEL-positive cells. This suggests that epithelial cells at this site undergo demise by apoptosis that occurs much later and perhaps attributes to the intrinsic uterine responses as previously thought (Joswig et al., 2003). The LE cell death in oil-induced uterine lumens of pseudopregnant females in the absence of embryos also involves apoptosis that occurs much later, suggesting that LE cell death in close proximity to trophoblast cells occurs at a much more rapid pace and does involve entosis.
It would be interesting to see whether the process of entosis is operative during implantation in other species, including humans. This process in humans could possibly be examined by utilizing in vitro fertilization (IVF)-derived blastocysts in culture. Another intriguing aspect of the present findings could be to explore whether the processes of entosis and/or intercalation are active in other gynecological cancers including human choriocarcinoma, a cancer of trophoblastic origin.
EXPERIMENTAL PROCEDURES
Animals
All mice used in this investigation were housed in barrier facilities in the Cincinnati Children’s Hospital Medical Center’s Animal Care Facility according to NIH and institutional guidelines for the use of laboratory animals. Experimental procedures to analyze implantation sites are provided in the Supplemental Information.
TSC Culture
Cells were maintained in a proliferative state in media (TSC media) containing 70% embryonic mouse fibroblast-cell-conditioned medium, 30% TSC medium, 25 ng/ml FGF4, and 1 μg/ml heparin, incubated in a humidified tissue culture incubator at 37°C saturated with 5% CO2 in air (Sun et al., 2010).
Isolation of Uterine Primary Epithelial Cells
Epithelial cells were isolated by enzymatic digestion of uterine tissues on day 4 of pseudopregnancy as previously described (Chung and Das, 2011; Daikoku et al., 2011). Sheets of epithelial cells were grown on matrigel-coated cell culture dishes with complete media, consisting of DMEM/F12 supplemented with charcoal-stripped 10% fetal bovine serum (FBS).
Cell Tracker Assays
Cell tracker assays were performed as previously described (Overholtzer et al., 2007) and are detailed in the Supplemental Information.
Tunnel Assay
Tunnel assays were performed using a DeadEnd Fluorometric TUNEL kit from Promega following manufacturer’s instructions.
Supplementary Material
Highlights.
Blastocyst trophoblasts engulf uterine epithelial cells by entosis (cell-eat-cell)
Entosis allows prompt entry of trophoblasts to the underlying stroma
This non-apoptotic process of entosis occurs without caspase 3 activation
Entosis is thus a central concept in embryo implantation
Acknowledgments
We thank Serenity Curtis for editing this manuscript. This work was supported in part by NIH grants (HD068524, DA006668, and PO1CA7783) and grants from the March of Dimes to S.K.D. We thank the Cincinnati Children’s Hospital Confocal Imaging Core for support and services.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two movies and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.03.035.
AUTHOR CONTRIBUTIONS
Y.L., X.S., and S.K.D. designed experiments. Y.L. and X.S. performed all animal, biochemical, histological, molecular, and cell biological experiments. Y.L., X.S., and S.K.D. analyzed data and wrote the manuscript.
References
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Bartos A, Park C, Sun X, Li Y, Cha SW, Ajima R, Ho HY, Yamaguchi TP, Dey SK. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep. 2014;8:382–392. doi: 10.1016/j.celrep.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Das SK. Mouse primary uterine cell coculture system revisited: ovarian hormones mimic the aspects of in vivo uterine cell proliferation. Endocrinology. 2011;152:3246–3258. doi: 10.1210/en.2011-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, Dey SK. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21:1014–1025. doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- El-Shershaby AM, Hinchliffe JR. Epithelial autolysis during implantation of the mouse blastocyst: an ultrastructural study. J Embryol Exp Morphol. 1975;33:1067–1080. [PubMed] [Google Scholar]
- Finn CA, Hinchliffe JR. Reaction of the Mouse Uterus during Implantation and Deciduoma Formation as Demonstrated by Changes in the Distribution of Alkaline Phosphatase. J Reprod Fertil. 1964;8:331–338. doi: 10.1530/jrf.0.0080331. [DOI] [PubMed] [Google Scholar]
- Finn CA, Lawn AM. Transfer of cellular material between the uterine epithelium and trophoblast during the early stages of implantation. J Reprod Fertil. 1968;15:333–336. doi: 10.1530/jrf.0.0150333. [DOI] [PubMed] [Google Scholar]
- Howe CL, Granger BL, Hull M, Green SA, Gabel CA, Helenius A, Mellman I. Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci USA. 1988;85:7577–7581. doi: 10.1073/pnas.85.20.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, Danuser G, Ince TA, Brugge JS. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1:144–157. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joswig A, Gabriel HD, Kibschull M, Winterhager E. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol. 2003;1:44. doi: 10.1186/1477-7827-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, Richardson AL, King RW, Cibas ES, Schnitt SJ, et al. A nongenetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–330. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel RH. Cytological Studies of the Decidual Reaction in the Rat during Early Pregnancy and in the Production of Deciduomata. Physiol Zool. 1937;10:212–234. [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Dey SK, Andrews GK. Association of monocytes and neutrophils with early events of blastocyst implantation in mice. J Reprod Fertil. 1993;99:561–569. doi: 10.1530/jrf.0.0990561. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C, Brambilla E. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol. 1999;208:488–501. doi: 10.1006/dbio.1999.9206. [DOI] [PubMed] [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36:211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Purvanov V, Holst M, Khan J, Baarlink C, Grosse R. G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. eLife. 2014;3:e02786. doi: 10.7554/eLife.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS. Caspases: opening the boxes and interpreting the arrows. Cell Death Differ. 2002;9:3–5. doi: 10.1038/sj.cdd.4400963. [DOI] [PubMed] [Google Scholar]
- Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci USA. 2010;107:16887–16892. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang L, Xie H, Wan H, Magella B, Whitsett JA, Dey SK. Kruppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation. Proc Natl Acad Sci USA. 2012;109:1145–1150. doi: 10.1073/pnas.1118411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Chorioallantoic placenta formation in the rat. III. Granulated cells invade the uterine luminal epithelium at the time of epithelial cell death. Biol Reprod. 1993;49:38–57. doi: 10.1095/biolreprod49.1.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


