Abstract
In the current study, we present a novel fMRI protocol in which words, pseudowords, and other word-like stimuli are passively presented in a rapid, sequential fashion. In this “fast” localizer paradigm, items are presented in groups of four; within sets, words are related in orthographic, phonological, and/or semantic properties. We tested this protocol with a group of skilled adult readers (N=18). Analyses uncovered key regions of the reading network that were sensitive to different component processes at the group level; namely, left fusiform gyrus as well as the pars opercularis subregion of inferior frontal gyrus were sensitive to lexicality; several regions including left precentral gyrus and left supramarginal gyrus were sensitive to spelling-sound consistency; the pars triangularis subregion of inferior frontal gyrus was sensitive to semantic similarity. Additionally, in a number of key brain regions, activation in response to semantically similar words was related to individual differences in reading comprehension outside the scanner. Importantly, these findings are in line with previous investigations of the reading network, yet data were obtained using much less imaging time than comparable paradigms currently available, especially relative to the number of indices of component processes obtained. This feature, combined with the relatively simple nature of the task, renders it appropriate for groups of subjects with a wide range of reading abilities, including children with impairments.
Keywords: reading, component processes, fMRI, localizer, spelling-sound consistency, special populations
1. Introduction
When a reader encounters a written word, a host of brain regions work together to uncover its meaning. These regions comprise a network, or circuit, which processes the different components of a given visual word form, including its visual features (orthography), its associated spoken form (phonology), and its referent (semantics). Each of these component processes has been linked to distributed regions of cortex, including occipitotemporal, temporoparietal, and inferior frontal regions (Pugh et al., 1996; Fiebach, Friederici, Müller, & von Cramon, 2002; Simos et al., 2002). Previous investigations have shown that the functional architecture of this circuit is associated with reading skill, and differs in systematic ways between typical readers and individuals with reading impairment (Pugh et al., 2000; Shaywitz et al., 2002; Hoeft et al., 2006; Norton, Beach, & Gabrieli, 2015; Richlan, Kronbichler, & Wimmer, 2009; Richlan, Kronbichler, & Wimmer, 2011). Furthermore, even within populations of skilled readers, individual differences in the reading circuit are apparent (Seghier et al., 2004; Seghier, Lee, Schofield, & Price, 2008; Jobard, Vigneau, Simon, & Tzourio-Mazoyer, 2011; Welcome & Joanisse, 2012).
Because of the tight relationship between brain and behavior for the reading network, a snapshot of the neurobiology of this circuit is highly desirable. First, linking behavior with neurobiology allows us to gain a better understanding of the functional anatomy of the brain. Second, snapshots can be taken at multiple time points to gain insights into the mechanisms underlying longitudinal changes in behavior. Finally, characterizing the neurobiology of the reading network can feed back to the behavioral level. For example, identifying neural pathways involved in reading allows for prediction of long-term reading outcomes (Hoeft et al., 2011), or tailoring reading remediation programs to individual reader characteristics (Eden & Moats, 2002).
In the current study, we report results from a novel functional imaging paradigm involving rapid sequential presentation of words and other word-like stimuli. We have elected to focus on single word reading for several reasons. First, by characterizing the neurobiology of the reading network, this type of work helps establish a multi-level link between neurobiological theories of reading and extant computational models, which mostly concern single word reading (Seidenberg, 2012). Second, we wished to develop a tool that could be used to characterize individual differences within groups of readers, and we were motivated by previous work showing that the effect of psycholinguistic variables such as spelling-sound consistency, which is measured at the single word level, varies systematically within groups of skilled adult readers (e.g., Strain & Herdman, 1999). Finally, we wished to develop a paradigm that could potentially be used as an assay for clinical populations, and developmental dyslexia has long been associated with deficits in decoding single words (Stanovich, 1988; Snowling, 2000).
We assert that this paradigm not only allows for reliable acquisition of a snapshot of the regions associated with the component processes of single word reading, as we demonstrate with a group of skilled adult readers; it also presents a number of advantages over similar paradigms. Namely, the task is relatively simple in nature, which renders it appropriate for use with varied populations of readers, including children with impairments. Second, the administration time associated with the task is brief relative to the number of indices of component processes obtained. Because of this short duration, the task can be used in conjunction with other types of experimental paradigms within the same scanning session. Consequently, we denote the task as a “localizer” which permits an unbiased selection of functional regions of interest (ROIs) associated with individual and/or group-level effects related to component processes of reading which can then be further probed in accompanying experiments (Saxe, Brett, & Kanwisher, 2006). These accompanying experiments could potentially query how areas engaged in reading modulate according to other domains, such as memory, attention, executive function, and numeracy.
1.1 An Overview of the Reading Circuit
Single word reading has been linked with both subcortical structures (Pugh et al., 2013) as well as three cortical systems in the brain: a ventral system centered in left occipitotemporal areas, a dorsal system encompassing left temporoparietal areas, and an anterior system centered in left inferior frontal gyrus (reviewed in Sandak et al., 2012). In the ventral system, it has been argued that cortical areas process information in a hierarchical fashion, responding to progressively larger fragments of words (Vinckier et al., 2007). In alphabetic languages, these fragments include single letters, groups of two letters (bigrams), groups of three letters (trigrams), and so on. In the left hemisphere, this hierarchical processing continues into inferior occipitotemporal (OT)/fusiform gyrus, which has been associated with the invariant properties of word forms abstracted away from surface variation in font or case (McCandliss, Cohen, & Dehaene, 2003). This region, often denoted as the visual word form area (VWFA; see Dehaene & Cohen, 2011 for a review), has been argued to be pre-lexical and pre-semantic, although this view is under debate (e.g., Price & Devlin, 2003; 2011). From the VWFA, the ventral system extends into middle and inferior temporal gyrus (Tagamets, Novick, Chalmers, & Friedman, 2000), both of which have been linked to semantic processing (Fiebach et al., 2002; Simos et al., 2002). In addition, recent evidence has shown that the anterior temporal lobe (ATL) could also be an important semantic site in this pathway (Hoffman, Ralph, & Woollams, 2015).
The dorsal system includes the angular gyrus and the supramarginal gyrus (SMG) in the inferior parietal lobule (IPL), as well as posterior superior temporal gyrus (STG). These areas are thought to be involved in mapping orthographic input to phonological and semantic properties of written words (Xu et al., 2001). However, there remains debate as to the precise contributions of subregions of this circuit to cognitive operations across a number of domains, including language and number processing (Cabeza, Ciaramelli, & Moscovitch, 2012). For example, using PET, Démonet, Price, Wise, and Frackowiak (1994) observed that AG was recruited when subjects performed a word monitoring task, whereas SMG was instead recruited when subjects performed a phoneme monitoring task. These results have been taken to support the claim that SMG is specifically involved in mapping between orthographic and phonological representations, whereas the AG is instead part of the semantic network (e.g., Price, 2000; Ferreira, Göbel, Hymers, & Ellis, 2015).
Finally, the anterior system – centered in left inferior frontal gyrus – has been implicated in a number of processes including phonological recoding and semantic integration (Poldrack et al., 1999; Zhu et al., 2012; Zhu et al., 2013). For example, left IFG is sensitive to the consistency of the mapping between a visual word and its phonological form (Fiez, Balota, Raichle, & Petersen, 1999; Frost et al., 2005; Graves, Desai, Humphries, Seidenberg, & Binder, 2010); that is, left IFG is more highly engaged for inconsistent words that do not have a one to one mapping between orthography and phonology. In addition, certain subregions within left IFG are also sensitive to semantic factors such as imageability, which can be defined as the ease with which a word evokes a mental image (Frost et al., 2005; Graves et al., 2010). Based on meta-analytic work, it has been suggested that more anterior and ventral subregions of the inferior frontal gyrus (e.g., the pars orbitalis subregion, often treated as synonymous with Brodmann’s area 47) are more highly involved in semantic tasks, whereas more posterior and superior subregions (e.g., near the border of BA 44 and 6) are instead more highly involved in phonological tasks (Bookheimer, 2002).
Importantly, the relative engagement of the brain regions within these three systems has been linked to individual differences in reading skill (Hoeft et al., 2006; Pugh et al., 2013; see Pugh et al., 2010 for review). For example, meta-analytic work has shown that adults with reading impairment show hypoactivation of left hemisphere reading regions including superior temporal, middle temporal, and OT/fusiform regions, as well as hyperactivation of regions such as the left precentral gyrus and the right caudate (Richlan et al., 2011).
1.2 Localization of Regions Sensitive to Component Processes
The neurobiological systems outlined in the previous section have been characterized by a number of different functional imaging paradigms with the aim of isolating regions sensitive to the component processes of single word reading (for comprehensive reviews see Turkeltaub, Eden, Jones, & Zeffiro, 2002, and Vigneau et al., 2006). In particular, these paradigms have used a number of different types of manipulations to localize brain regions more heavily involved in certain component process compared to others. Broadly, these tasks can be distinguished along two key dimensions: the relative extent of active metalinguistic processing required for subjects to perform the task, and the amount of imaging time required to localize the reading network. As we discuss below, both of these dimensions impact the appropriateness of a particular task for different populations of readers.
1.2.1 Extent of Active Metalinguistic Processing
A classic design involving active metalinguistic processing is that detailed in Pugh et al. (1996), in which subjects were asked to perform three different types of judgments while in the scanner, each of which emphasized different properties of stimulus pairs. Namely, a case judgment emphasized orthographic properties, a rhyme judgment emphasized phonological properties, and a category judgment emphasized semantic properties. In each trial, subjects were asked to make a response only if the stimulus items in the pair shared a particular property (i.e., the stimuli were matched in case, rhymed, or belonged to the same category). This type of design has been adopted in more recent studies investigating individual differences in skilled readers (Seghier et al., 2004; Welcome & Joanisse, 2012). In addition, it has inspired similar designs such as the spelling and rhyming tasks employed by Bolger, Hornickel, Cone, Burman, and Booth (2008) and Bolger, Minas, Burman, and Booth (2008), in which children were presented with pairs of words and were asked to judge whether rime bodies within pairs either shared the same orthography or pronunciation (e.g., dime vs. lime, mint vs. pint, jazz vs. has, staff vs. gain).
An alternative design is the adaptive learning paradigm developed by Sandak et al. (2004). In this task, subjects first learned novel semantic associations for a set of pseudowords, and subsequently made judgments concerning either the orthographic, phonological, or semantic properties associated with individual items. In the scanner, subjects performed an overt naming task which included both trained and untrained items; the authors observed training-specific effects in a number of key reading-related regions which were modulated based on the type of behavioral judgment subjects made concerning these items during training. This paradigm has since been used by Zhao et al. (2014) to investigate reading of Chinese phonograms.
A third type of design is detailed in Graves et al. (2010). In this study, subjects were asked to simply read aloud single words. Importantly, the authors included dimensions such as frequency and spelling-sound consistency as covariates of interest in the general linear model, and then identified regions whose neural activity correlated with these dimensions. Using this approach, the authors identified a number of clusters within brain regions such as left IFG that showed sensitivity to one or more of these dimensions.
While these designs have certainly been highly successful, some have argued that there are benefits to examining the neurobiology of the reading system under more natural conditions, in which subjects are not required to make an overt response (e.g., McDermott, Petersen, Watson, and Ojemann, 2003). For example, McDermott et al. (2003) reported results from a paradigm in which subjects were asked to either selectively attend to sound relationships while silently reading rhyming words, or to attend to meaning relationships while silently reading semantically related words. In this way, the authors were able to segregate cortical regions involved in phonological versus semantic processing in the absence of potential confounding processes associated with metalinguistic processing demands. This design was an adaptation of an earlier study that also contrasted conditions in which subjects directed attention to meaning versus phonological segmentation (Price, Moore, Humphreys, & Wise, 1997).
Critically, some designs are more appropriate than others for localizing the reading network in special populations of readers, such as children with reading disability (RD). It has been shown that RD is often co-morbid with attention deficit hyperactivity disorder (ADHD), which is typically associated with difficulties in response inhibition (Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005). As a result, it could be difficult for children with RD to perform tasks such as Pugh et al. (1996), which requires subjects to withhold responses if items within a stimulus pair do not share a particular property (i.e., matching case, rhyming pronunciation, or belonging to the same semantic category). By contrast, designs such as Graves et al. (2010) design represent an improvement; however, even reading aloud single words while in the scanner could be overly taxing for struggling readers, and methodologically it can be challenging to collect and extract reaction times for vocal responses under conditions of scanner noise. For similar reasons, the Sandak et al. (2004) task could be difficult for struggling readers, who may have difficulty not only with reading aloud in the scanner, but also with the rigorous training schedule prior to scanning. Finally, the McDermott et al. (2003) design has the advantage of being relatively passive compared to other designs such as Pugh et al. (1996) and Bolger et al. (2008a); however, even this task might be difficult for special populations to perform because it involves switching the focus of selective attention between blocks of trials.
1.2.2 Amount of Imaging Time
Imaging time is not only a precious commodity in terms of operating costs and research productivity; it can be a highly constraining factor when working with special populations of individuals. For this reason, obtaining high quality functional imaging data in relatively little imaging time is critical when investigating the reading network in different populations of readers. As a result, the Pugh et al. (1996) design could be relatively inappropriate for special populations given that it takes about 90 minutes to administer (e.g., Welcome & Joanisse, 2012). Similarly, the Sandak et al. (2004) adaptive learning paradigm also requires 90 minutes of imaging time in addition to a 2.5-hour training session, which again could be overly demanding. The Graves et al. (2010) study of the neural systems underlying reading aloud represents an improvement, as subjects completed five runs each eight minutes in duration, for a total of forty minutes. This is considerably less than even the McDermott et al. (2003) design, which requires about sixty minutes of imaging time. Of the tasks so far discussed, the Bolger et al. (2008a) paradigm takes the least amount of time (four eight-minute runs for a total of 32 minutes). However, it only localizes regions associated with orthographic and phonological consistency and importantly does not include any semantic manipulations. In addition, even 32 minutes of performing tasks in the scanner could be too time consuming for quality data acquisition, especially for groups of children experiencing issues associated with attention deficits and/or hyperactivity.
1.2.3 “Fast” Localizer Paradigm
In the current study, we developed a novel fMRI localizer in order to overcome the limitations of previous designs. This protocol involves rapid presentation of sets of four words that vary in their orthographic, phonological, and semantic properties (cf. Pinel et al., 2007), and builds upon previous work in the priming literature investigating spelling-sound consistency (e.g., BRIBE-TRIBE versus COUCH-TOUCH; Meyer, Schvaneveldt, & Ruddy, 1974; Shulman, Hornack, & Sanders, 1978; Hanson & Fowler, 1987; Pugh, Rexer, & Katz, 1994) as well as semantic processing (e.g., BARN-SHED; Rossell, Price, & Nobre, 2003). Importantly, instead of having subjects perform trial-wise responses, this protocol instead has subjects perform a low-level recognition memory task at the end of each run. This recognition memory task motivates attention to the stimuli but removes potential confounds associated with active metalinguistic judgments and response inhibition. Furthermore, compared to other tasks, this localizer requires less administration time, especially relative to the number of indices of component processes obtained. As we show in this report, we were able to reliably localize the reading network in a group of skilled adult readers using just twenty-one minutes of imaging time per subject. These 21 minutes included presentation of two auditory conditions that we do not report here for the sake of brevity; the print conditions themselves were thus presented over the course of 16 minutes. Within this amount of time, we isolated regions sensitive to lexicality, spelling-sound consistency, and semantic similarity in accordance with previous studies. In addition, we identified regions sensitive to semantic similarity that showed individual differences related to subjects’ reading comprehension scores outside the scanner. These findings not only underscore the utility of the paradigm but also highlight its ability to contribute information of theoretical importance.
2. Methods
2.1 Subjects
Twenty-one adult native speakers of English participated in this study. All subjects were consented in compliance with Yale University’s Institutional Review Board for protection of human subjects. To assess each subject’s reading skill, we administered a behavioral battery. This included the sight word reading and phonemic decoding subtests of the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999), as well as a subsection of Form E of the Nelson-Denny reading comprehension test (Brown, Fishco, & Hanna, 1993) consisting of twenty-four multiple choice questions. The TOWRE is a timed test in which subjects read lists of familiar words or pronounceable pseudowords; it is often thought of as a measure of reading fluency, and is typically associated with phonological skills (e.g., Welcome & Joanisse, 2012). By contrast, performance on the Nelson-Denny reading comprehension test is thought to index the ability to process connected text and integrate meaning over multiple sentences within a passage, although this view has been challenged by some groups (e.g., Coleman, Lindstrom, Nelson, Lindstrom, & Gregg, 2010). Due to study attrition, TOWRE scores were not obtained from one subject and Nelson-Denny reading comprehension scores were not obtained from three subjects. Data from one subject was excluded from fMRI analyses due to below average reading scores (standard score for TOWRE phonemic decoding efficiency < 80; Nelson-Denny reading comprehension score of 50% correct), and data from two other subjects was excluded as a result of excessive motion in the scanner (greater than 20% of images were above the threshold of 3 mm point to point movement). The remaining subjects (N = 18; mean age 24; 11 F) did not report a diagnosis of a learning disability, and all self-identified as right-handed except one subject. Descriptive statistics concerning these subjects are reported in Table 1.
Table 1.
Behavioral performance on psychometric tests of reading and language for the group of subjects included in the MRI sample (N = 18; 11 F)
| Subtest | Measure | Mean (SD) | Range |
|---|---|---|---|
| TOWRE Sight Word Efficiency | Raw score out of 104 Scaled score |
95.2 (8.5) 100.3 (12.0) |
73-104 80-113 |
| TOWRE Phonemic Decoding
Efficiency |
Raw score out of 63 Scaled score |
56.8 (6.5) 105.8 (13.3) |
38-63 81-120 |
| Nelson-Denny Reading
Comprehension |
Percent correct | 88.6 (8.7) | 70.8-100 |
2.2 Stimuli
We presented subjects with both printed and spoken English monosyllables as well as non-linguistic controls. Print stimuli consisted of four word type conditions as well as a pseudoword condition and a false font condition; example stimulus sets are provided in Table 2. The four word type conditions were as follows: words with shared orthography and shared phonology (O+P+), words with shared orthography but different phonology (O+P−), semantically related words (SEM), and printed words unrelated in orthography, phonology, and semantics (UNREL). These four conditions were balanced for length [F(3,380) = 1.65, p = .17, ηp2 = .01], frequency [F(3,380) = 2.31, p = .08, ηp2 = .02], logarithmic frequency [F(3,380) = .30, p = .83, ηp2 = .002], and bigram frequency [F(3,380) = .82, p = .48, ηp2 = .01]. Importantly, the O+P+ and O+P− conditions differed in spelling-sound type consistency [t(190) = 22.92, p < .001] yet were matched for concreteness [t(190) = .60, p = .55], while the SEM and UNREL conditions were matched in spelling-sound type consistency [t(190) = − 1.45, p = .15] yet differed in concreteness [t(190) = 4.20, p < .001]. Pseudowords (PSW) were generated using the MCWord database (Medler & Binder, 2005), and were selected such that they matched the printed word conditions in length [t(478) = −1.18, p = .24] and bigram frequency [t(478) = .08, p = .93]. The false font condition (FF) consisted of sequences of characters in Wingdings font, exactly matched in length to the pseudoword condition. Audio conditions consisted of unrelated spoken words as well as vocoded speech derived from the set of unrelated spoken words. These audio conditions were included as part of an ongoing investigation in our group concerning print-speech convergence; as indicated above, these conditions are not discussed in this report for the sake of brevity.
Table 2.
Sample stimulus sets in each of the six visual conditions
| Condition | Abbreviation | Sample Stimulus Set | Length | Freq1 | Log Freq1 |
Bigram Freq2 |
Type Cons3 |
Concrete- ness4 |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unrelated | UNREL | CLAY | LAWN | FLEA | VASE | 4.33 (.07) |
100.7 (32.9) |
3.18 (.07) |
18951 (1231) |
.926 (.017) |
4.12 (.08) |
| Same Orthography; Shared Phonology |
O+P+ | BEST | NEST | PEST | VEST | 4.19 (.05) |
120.1 (30.3) |
3.11 (.07) |
18213 (816) |
.996 (.002) |
3.86 (.10) |
| Same Orthography; Different Phonology |
O+P− | DOUGH | TOUGH | COUGH | ROUGH | 4.38 (.07) |
205.0 (59.5) |
3.13 (.09) |
20141 (1150) |
.460 (.023) |
3.77 (.11) |
| Semantically Related |
SEM | FISH | BEEF | PORK | MEAT | 4.24 (.08) |
71.4 (13.5) |
3.19 (.06) |
20447 (1333) |
.888 (.020) |
4.56 (.06) |
| Pseudowords | PSW | JALL | PULE | TALM | WIBS | 4.36 (.06) |
– | – | 19321 (1290) |
– | – |
| False Font | FF |

|
|

|

|
4.36 (.06) |
– | – | – | – | – |
Note: Values in parentheses represent standard error of the mean. Freq = Frequency; Type Cons = Type Consistency
Frequency estimates are based on the SUBTLEX frequency norms (Brysbaert & New, 2009), and were obtained using the English Lexicon Project (Balota et al., 2007).
Bigram frequency was quantified using the MCWord database (Medler & Binder, 2005).
Type consistency was calculated using feedforward consistency measures in Ziegler, Stone, & Jacobs (1997).
Concreteness measures were obtained from Brysbaert, Warriner, & Kuperman (2014).
2.3 Procedure
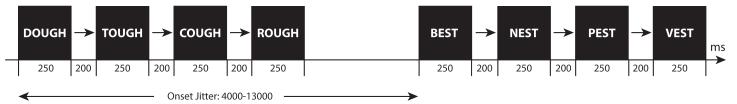
In each trial, subjects were presented with tetrads of stimuli in one of the eight stimulus conditions. For the visual conditions, items within tetrads were each present on the screen for 250 ms, separated by an ISI of 200 ms. For the auditory conditions, items within tetrads were presented with an SOA of 800 ms; the mean duration of auditory items was 536 ms (SD 110.2 ms). A sample trial sequence is illustrated in Figure 1. In total, there were 24 tetrads (96 unique items) in each condition. Across the entire experiment, subjects were presented with each item only once. For the O+P− condition, items with different pronunciations were alternated within tetrads such that the first two items within the tetrad did not rhyme with each other.
Fig 1.
A sample trial sequence illustrating key features of the “fast” localizer design.
At the beginning of the session, subjects were instructed to simply attend to the stimuli and told they would be given a short recognition memory test at the end of each run. In this recognition memory test, subjects were given six printed words, three of which were presented in the run, and three of which were not. They were asked to indicate whether or not they remembered viewing each word during the course of the run. Subjects were able to correctly remember most items, as mean accuracy on this task was 70.8% (SD 10.5%). Furthermore, accuracy on this task was not correlated with any of the three psychometric tests administered outside the scanner (all p’s > .05).
2.4 MRI Data Acquisition and Analysis
Imaging was performed using a 3T Siemens TIM-Trio scanner with a 12-channel head coil. Prior to functional imaging, sagittal localizers were prescribed (matrix size = 180 × 192; voxel size = 1.333 × 1.333 × 4 mm; FoV = 240/256 mm; TR = 15 ms; TE = 6.86 ms; flip angle = 25°). T2*-weighted images were then collected in an axial-oblique orientation parallel to the intercommissural line (32 slices; 4 mm slice thickness; no gap) using single-shot echo planar imaging (matrix size = 64 × 64; voxel size = 3.4375 × 3.4375 × 4 mm; FoV = 220 mm; TR = 2000 ms; TE = 30 ms; flip angle = 80°). To allow for stabilization of the magnetic field, the first six volumes within each run were discarded. Following the functional runs, anatomical scans were also acquired for each subject in the same orientation as the functional volumes (MPRAGE; matrix size = 256 × 256; voxel size = 1 × 1 × 1 mm; FoV = 256 mm; TR = 2530 ms; TE = 2.77 ms; flip angle = 7°).
In each functional run, subjects were presented with 48 tetrads of items (6 tetrads in each of the eight stimulus conditions) in an event-related fashion. Across all trials in the experiment, the time between trial onsets was jittered between 4 and 13 seconds. Subjects completed four runs each 316 seconds (158 volumes) in length. This translated to approximately twenty-one minutes imaging time.
Data were analyzed using AFNI (Cox, 1996). Functional images were first corrected for slice acquisition time, motion corrected using a six-parameter rigid-body transform, and normalized to Talairach space using an affine transform which also warped the data into 3 mm isotropic space. Last, all images were smoothed with a 6 mm Gaussian kernel.
At the single subject level, data were submitted to a multiple regression analysis with nuisance regressors representing temporal drift and the six movement parameters. The resulting single subject maps were then subjected to a groupwise repeated measures ANOVA to test for a main effect of stimulus type across the six types of visual stimuli. The groupwise statistical map was thresholded at a voxelwise threshold of p = .0001. To control for family-wise error rates, Monte Carlo simulations were performed (3dClustSim; 10,000 iterations) using all brain voxels within the TT_N27 template brain, and using the spherical autocorrelation function parameters concerning the error time series. This was performed in response to the latest recommendations regarding cluster correction in fMRI research (Eklund, Nichols, & Knutsson, 2016). The cluster threshold for a corrected alpha level of p = .05 was 6 voxels.
Because subregions of IFG have been shown to be differentially sensitive to different component processes of reading (e.g., Bookheimer, 2002), we divided the functionally defined cluster of IFG/left precentral gyrus according to atlas-defined anatomical subregions. Namely, this cluster was divided into three subregions, which comprised 88% of the voxels in the larger cluster: IFG pars triangularis, IFG pars opercularis, and left precentral gyrus. These anatomical regions were defined using the Eickhoff-Zilles macro labels for the N27 brain in Talairach space.
Next, for each cluster that showed a main effect of stimulus type, we performed Bonferroni corrected pairwise t-tests to contrast beta values between conditions of interest. To guard against inflation of type I error, we only performed contrasts between conditions designed to specifically isolate regions sensitive to the different component processes of reading. Namely, we performed the following four contrasts: (1) UNREL > FF; (2) PSW > UNREL; (3) O+P− > O+P+; (4) SEM > UNREL. These contrasts respectively tested for effects of: word reading, lexicality, spelling-sound consistency, and semantic similarity. Bonferroni correction was applied by multiplying resulting significance levels by a factor of four.
Last, to assess the sensitivity of the task and specific conditions to individual differences in reading, we correlated each of the three items in the behavioral battery (TOWRE sight word efficiency, TOWRE phonemic decoding efficiency, and Nelson-Denny reading comprehension) with the difference in beta weights between the conditions of interest for each of the following three contrasts: (1) PSW > UNREL; (2) O+P− > O+P+; (3) SEM > UNREL. Because we were performing nine tests for each ROI (three tests by three contrasts), Bonferroni correction was applied by multiplying all significance levels by a factor of nine. These tests were confined to the clusters that showed a main effect of condition.
3. Results
3.1 Groupwise Activation Analysis
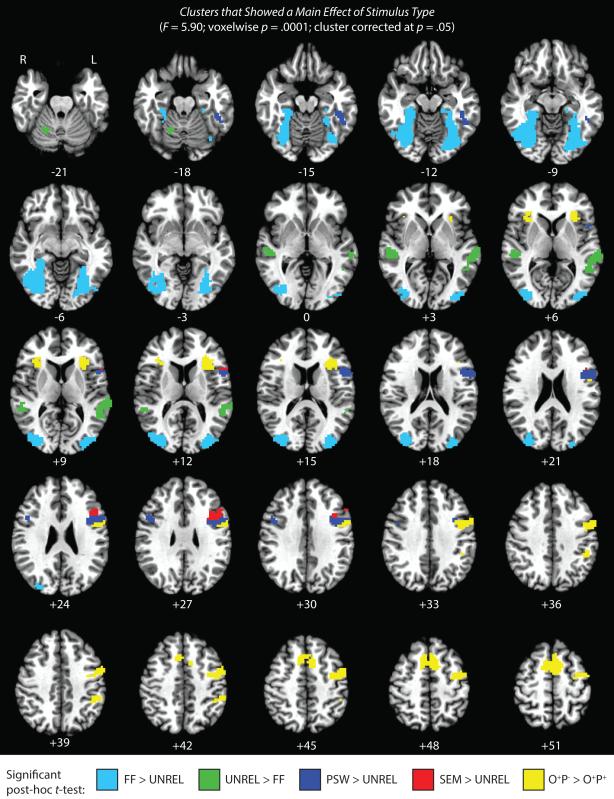
We first identified clusters that showed a main effect of stimulus type across the six visual conditions (UNREL, O+P+, O+P−, SEM, PSW, and FF), and then followed up on these by performing the following four Bonferroni corrected pairwise t-tests: (1) UNREL > FF; (2) PSW > UNREL; (3) O+P− > O+P+; (4) SEM > UNREL. The results of this analysis are illustrated in Figure 2 and detailed in Table 3; furthermore, Figure 3a, Figure 3b, and Figure 3c show beta values across conditions for the key clusters shown in the whole brain map in Figure 2. Evoked response maps for each of the six visual conditions are provided in Supplementary Figures 1 through 6.
Fig 2.
Clusters that showed a main effect of stimulus type across the six visual conditions. Colors correspond to the results of Bonferroni corrected post-hoc pairwise t-tests between conditions of interest. Note that some clusters showed significant differences between conditions for more than one critical contrast; please refer to Table 3 for details. Numerals below each slice indicate Talairach co-ordinates in the axial plane. For the sake of simplicity, only clusters larger than 25 voxels in size are shown.
Table 3.
Clusters showing a main effect of stimulus type (F = 5.90; voxelwise p = .0001; cluster corrected at p = .05)
| Region | Talairach Coordinates of
Peak |
Significant post hoc t-tests (Bonferroni corrected) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| L/R | Area | x | y | z | Extent (voxels)1 | FF > UNREL |
UNREL > FF |
PSW > UNREL |
UNREL > PSW |
O+P−
> O+P+ |
SEM > UNREL |
| L | Supramarginal gyrus | −50 | −38 | 42 | 33 | ** | |||||
| L | Precentral gyrus | −47 | −2 | 21 | 179 | *** | ** | ||||
| L/R | Supplementary motor area | −2 | −2 | 57 | 296 | *** | *** | ||||
| L | IFG/Insula | −32 | 23 | 9 | 90 | *** | ** | ||||
| R | IFG/Insula | 35 | 23 | 9 | 40 | *** | * | ||||
| L | IFG – pars triangularis | −44 | 14 | 21 | 41 | ** | * | * | |||
| L | IFG – pars opercularis | −47 | 11 | 6 | 159 | *** | * | ** | |||
| R | IFG – pars opercularis | 47 | 11 | 27 | 26 | *** | * | ||||
| L | Left OT/fusiform gyrus | −47 | −44 | −16 | 44 | *** | ** | ||||
| L | STG/MTG | −62 | −32 | 6 | 136 | *** | |||||
| R | STG/MTG | 50 | −29 | 3 | 65 | *** | |||||
| L | Anterior STG | −56 | −11 | −1 | 8 | ** | |||||
| L | IFG – pars triangularis | −50 | 29 | 6 | 13 | ** | |||||
| L | Postcentral gyrus | −59 | −20 | 24 | 23 | ** | |||||
| R | Precentral gyrus | 56 | −5 | 39 | 15 | ** | |||||
| R | Cerebellum | 20 | −59 | −19 | 26 | ** | |||||
| R | Cerebellum | 23 | −62 | −46 | 20 | ** | |||||
| R | Cerebellum | 14 | −71 | −40 | 9 | ** | |||||
| L | Putamen | −17 | 5 | 12 | 12 | ** | ** | ||||
| R | Caudate | 11 | 8 | 6 | 14 | * | * | ||||
| L | Fusiform gyrus | −32 | −62 | −13 | 292 | *** | |||||
| R | Fusiform gyrus | 29 | −62 | −13 | 646 | *** | |||||
| L | Middle occipital gyrus | −35 | −83 | 9 | 132 | *** | |||||
| R | Precuneus | 26 | −71 | 42 | 14 | ** | |||||
p < .001
p < .01
p < .05
Voxels are 3 × 3 × 3 mm, or 27 mm3, in size.
Fig 3.
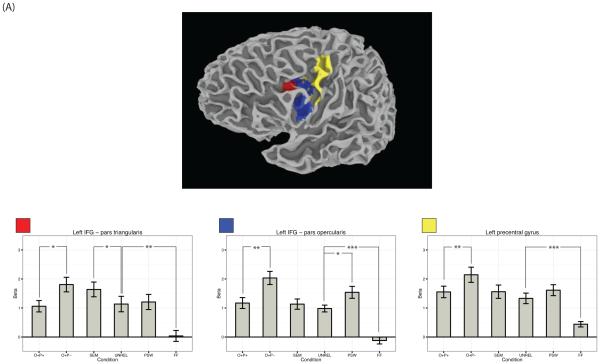
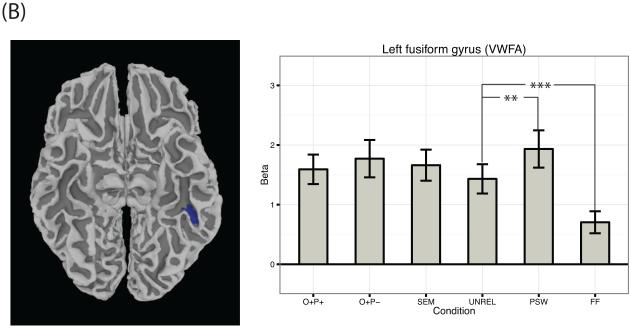
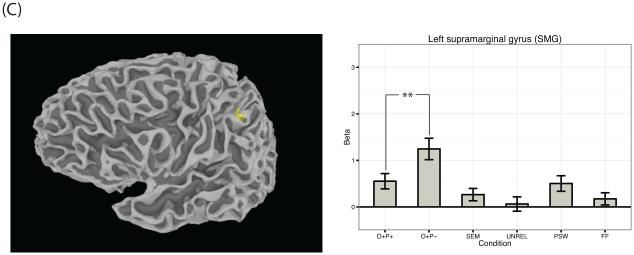
Beta values across conditions for several key reading-related regions that showed a main effect of stimulus type across the six visual conditions. Namely, beta values across conditions are plotted for (A) left IFG pars triangularis, left IFG pars opercularis, left precentral gyrus; (B) left fusiform gyrus (VWFA); (C) left SMG. Error bars represent the standard error of the mean. Asterisks represent the significance level of Bonferroni corrected post-hoc t-tests as follows: *** p < .001; ** p < .01; * p < .05.
3.1.1 Regions Involved in Word Reading
As can be seen in Figure 2, numerous areas showed greater activation for unrelated visual words compared to false font; these included key areas of the reading circuit such as the visual word form area in the left OT/fusiform gyrus (VWFA; Dehaene et al., 2002; McCandliss et al., 2003), bilateral superior temporal gyrus/middle temporal gyrus (STG/MTG), and bilateral inferior frontal gyrus (IFG). Additionally, several areas showed the reverse effect; most notably, large clusters in bilateral fusiform gyrus activated more strongly for the false font compared to the unrelated visual words.
3.1.2 Regions Sensitive to Lexicality
As shown in Figure 2, three regions showed greater activation for pseudowords compared to unrelated words: the VWFA and the pars opercularis subregion of left and right IFG. The reverse pattern held in two subcortical structures; namely, the left putamen and the right caudate. Beta values for left IFG pars opercularis and the VWFA are plotted in Figure 3a and Figure 3b respectively.
3.1.3 Regions Sensitive to Spelling-Sound Consistency
As is apparent in Figure 2, several clusters in IFG were more strongly engaged for phonologically inconsistent sets of words compared to consistent sets; these included bilateral clusters centered in pars opercularis near the insula, a large cluster of left precentral gyrus, and a cluster spanning bilateral supplementary motor area (SMA). Importantly, this same pattern was also observed in the left supramarginal gyrus (SMG). Beta values for left precentral gyrus and left SMG are plotted in Figure 3a and Figure 3c respectively.
3.1.4 Regions Sensitive to Semantic Similarity
The SEM condition showed significantly higher beta values than the UNREL condition in the pars triangularis subregion of left IFG. Beta values for this cluster are plotted in Figure 3a.
3.2 Brain-Behavior Correlations
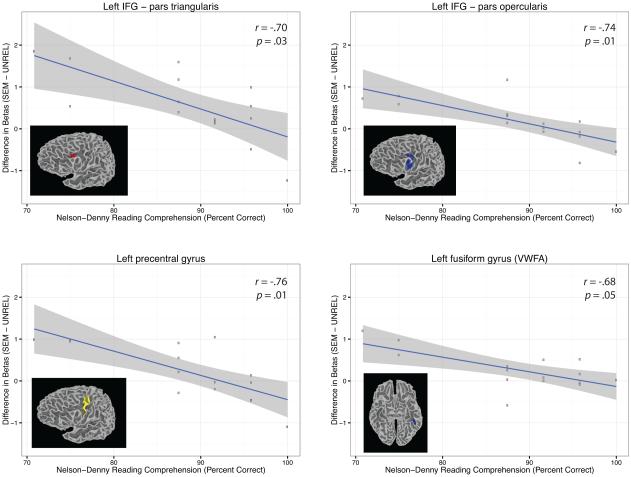
To test for individual differences in brain activation, we correlated scores for each of the three items in the behavioral battery with the difference in beta values for each of the following contrasts (1) PSW > UNREL; (2) O+P− > O+P+; (3) SEM > UNREL. As shown in Figure 4, one set of brain-behavior correlations was significant following Bonferroni correction: namely, differences in beta weights between the SEM and UNREL conditions were related to reading comprehension scores as measured using the Nelson-Denny test in a number of key regions. These regions included left IFG pars triangularis (r = −.70, p = .03), left IFG pars opercularis (r = −.74, p = .01), left precentral gyrus (r = −.76, p = .01), and the VWFA in the left fusiform gyrus (r = −.68, p = .05). The correlation was negative, as individuals with lower comprehension scores recruited these areas for semantically related words to a greater extent than they did for unrelated visual words, whereas individuals with higher comprehension scores showed either little difference in activation between these two conditions or the reverse pattern. It should be noted that these individual differences can be mostly attributed to variable activation for the semantic condition; activation for unrelated words was more comparable across individuals.
Fig 4.
Correlations between Nelson-Denny reading comprehension scores and the difference in beta values between the semantically related and unrelated conditions in regions of interest defined from the ANOVA testing for the main effect of stimulus type. The shading in each plot represents the 95% confidence interval for the line of best fit.
4. Discussion
Our aim was to develop a functional imaging paradigm that meets the following criteria: (1) allows for reliable isolation of the cortical regions involved in the component processes of reading at both the group and individual levels; (2) involves a relatively simple task and relatively little imaging time such that obtaining high quality data is feasible with different populations of readers. To do this, we developed a novel fMRI localizer that involves rapid sequential presentation of groups of four stimuli in either the auditory or visual modality. Crucially, sets of visual stimuli differed in various parameters, which allowed for identification of regions sensitive to the orthographic, phonological, and semantic properties of written words. Presentation of the stimuli was relatively passive; subjects attended to the items in order to perform a recognition memory test at the end of each run, but importantly did not make trialwise responses or metalinguistic judgments. The protocol involved only twenty-one minutes of imaging time (16 for the print conditions), which is considerably briefer (by approximately 50-80%) than previously published paradigms (e.g., Pugh et al., 1996, McDermott et al., 2003; Sandak et al., 2004; Bolger et al., 2008a; Graves et al., 2010; Welcome & Joanisse, 2012). Moreover, when comparing paradigms that probe equal numbers of component processes, the present paradigm is briefer by a mean value of 45 minutes.
4.1 Groupwise Results
As shown in this report, within a relatively small amount of imaging time, the fast localizer paradigm was able to successfully localize the component processes of reading in a group of skilled adult readers. First, a contrast between unrelated printed words and a false font of characters mimicking the properties of alphabetic font identified key regions of the reading circuit overall; second, a contrast between unrelated words and pseudowords isolated regions sensitive to lexicality; third, a contrast between phonologically consistent and inconsistent words isolated regions sensitive to phonological properties of words; fourth, a contrast between semantically related and unrelated words isolated a region sensitive to semantic similarity.
As can be seen in Figure 2, this task engaged key components of the reading circuit, including left occipitotemporal (OT)/fusiform gyrus, temporoparietal regions, and inferior frontal cortex. This circuit is highly established based on previous studies (Pugh et al., 1996; Fiebach et al., 2002; Simos et al., 2002; Sandak et al., 2004). Isolation of this region of left OT/fusiform gyrus – which some have labeled the visual word form area (VWFA) – replicates earlier work that has localized this region, whether by contrasting words and consonant strings (Cohen et al., 2002), words and scrambled words (Szwed et al., 2011), or words and false font as in the current study (Vinckier et al., 2007).
As shown in Figure 3, the VWFA activated more strongly to pseudowords than to words. This sensitivity of the VWFA to lexicality has been observed in a number of previous studies, including recent work by Bruno, Zumberge, Manis, Lu, and Goldman (2008) and Woollams, Silani, Okada, Patterson, and Price (2010), as well as prior meta-analytic work by Mechelli et al. (2003). For example, Kronbichler et al. (2004) manipulated the familiarity of printed items by presenting subjects with items of differing levels of frequency, from highly frequent real words to phonotactically legal pseudowords. The authors found that the VWFA decreased in activation during a silent reading task as items became more familiar, with pseudowords activating the VWFA to the greatest extent, and highly frequent real words the least. This effect of familiarity on activity in the VWFA has also been observed by Sandak et al. (2004), who reported attenuation of the response of the VWFA to pseudowords as a function of training. Finally, in a more recent study using a silent reading task, activation of the VWFA was again observed to be greater for pseudowords compared to words, and furthermore was modulated by another important psycholinguistic variable: orthographic neighborhood density (Braun et al., 2015).
As Dehaene and Cohen (2011) point out, many of these previous tasks entailed longer processing times and potential for top-down processing, which could have exaggerated responses to pseudowords in the VWFA. The “fast” localizer was designed with this claim in mind, as one of our secondary aims was to develop a task that could explore the functionality of the VWFA. Therefore, we opted for a task design that is rapid in nature as well as relatively passive, consistent with recommendations by Dehaene and Cohen (2011) regarding paradigms for obtaining insights into the neural coding characteristics in the VWFA. Given this emphasis on bottom-up processing, it is interesting that we observed greater activation for pseudowords than words in the VWFA in the current study. A caveat is that subjects were asked to attend to items to perform the recognition memory test; it is possible we would have observed reduced activation in the VWFA for pseudowords if the task were completely passive. It is also important to note that the different word type conditions as well as the pseudoword condition were matched in bigram frequency; this is critical given the sensitivity of the VWFA to bigram differences (Woollams et al., 2010). We would therefore argue that the current pattern of results cannot be solely attributed to potential confounds in bigram frequency (cf. Diaz & McCarthy, 2007).
Turning to the phonological contrast, a number of clusters showed sensitivity to the consistency of the mapping from orthography to phonology. Most notably, activation was greater for inconsistent compared to consistent words in bilateral IFG. This has been observed previously; however, prior studies have used very different paradigms and manipulations, such as naming and lexical decision (Fiez, Balota, Raichle, & Petersen, 1999; Frost et al., 2005; Graves et al., 2010). In the current study, we would argue that the increased engagement of bilateral IFG likely reflects the processing cost associated with integrating the four items differing in pronunciation. Because the words in the O+P+ condition all shared the same pronunciation, traces in memory associated with the phonology of these items remained partially active due to the very short lag between words, and therefore required little additional activation to cross threshold. In contrast, in the O+P− condition, readers had to inhibit incorrect pronunciations and activate correct ones; this resulted in an increased processing cost, as indexed by a greater change in the BOLD signal for these items compared to the O+P+ condition. In addition, it is likely that subjects were engaged in rehearsal of the items, as we observed greater activation for the O+P− condition compared to the O+P+ condition in regions involved in sensorimotor encoding of pronunciations such as the supplementary motor area (Démonet et al., 1994).
Similarly, the left SMG also showed greater activation for phonologically inconsistent compared to phonologically consistent sets of words. This finding reinforces the claim that this region is involved in computing the phonological form associated with a word during visual word recognition (Stoeckel, Gough, Watkins, & Devlin, 2009). The left SMG has been previously associated with the phonological store of verbal working memory (Paulesu, Frith, & Frackowiak, 1993); greater activation in this region for inconsistent sets of words could therefore have been a result of subjects keeping track of the four words differing in pronunciation. Critically, this region did not show an effect for the semantic condition in either the groupwise analyses or the brain-behavior correlations, supporting work that has shown activation in this region is driven to a greater extent by phonological as opposed to semantic processes (Démonet et al., 1994; Devlin, Matthews, & Rushworth, 2003; Mummery, Patterson, Hodges, & Price, 1998).
For the semantic contrast, activation of the pars triangularis subregion of IFG exhibited sensitivity to semantic similarity. This finding contrasts with the pattern of activation in the pars opercularis subregion, which showed greater activation for pseudowords compared to words, implicating greater involvement of this subregion in sublexical phonology. This complements work suggesting that more anterior and lateral subregions of IFG are involved in semantic as opposed to phonological processing (Poldrack et al., 1999; Bookheimer, 2002). However, a caveat is that because the semantically related and unrelated printed words differed in concreteness, we cannot dissociate concreteness effects in this region from differences in processing words overlapping in semantic features; nevertheless, it is likely these two properties are related to a common multidimensional construct such as semantic richness (Yap, Tan, Pexman, & Hargreaves, 2011).
4.2 Brain-Behavior Correlations
As shown in Figure 3, the SEM > UNREL contrast also showed individual differences. Namely, the difference in beta values between the SEM and UNREL conditions correlated with Nelson-Denny reading comprehension scores in a number of clusters including left IFG pars triangularis, left IFG pars opercularis, left precentral gyrus, and the VWFA. Importantly, these correlations were negative such that individuals with poorer reading comprehension activated these areas more strongly for semantically related words than unrelated words, whereas individuals with higher scores showed little difference between these two conditions or the reverse pattern. These negative correlations complement results from Welcome and Joanisse (2012), who also observed a negative correlation between word-reading activation and Nelson-Denny comprehension scores in left precentral gyrus.
A likely explanation of this effect is that individuals with relatively lower comprehension scores could have been more reliant on top-down semantic support to facilitate word recognition. There are two non-exclusive ways in which this could have occurred. First, the words in the current study were all highly imageable (e.g., FARM, BIKE, LIME), and it is possible that individuals with poorer comprehension abilities could have been more prone to use mental imagery to facilitate word reading (Chan, Cole, & Morris, 1990). Support for this view comes from studies such as Strain and Herdman (1999), who showed that poorer readers exhibited a greater benefit of imageability on word naming compared to skilled readers, and Pugh et al. (2008), who showed that in individuals with reading disability, the reading network more closely resembled that of typical readers for highly imageable compared to lowly imageable words. This effect could have stemmed in particular from the high degree of concreteness for the semantically related sets (e.g., FISH-BEEF-PORK-MEAT), which could have influenced the ease of mental image generation for these items. In addition, because this task involved a recognition memory component, the observed pattern of effects could also be ascribed to individual differences in the strategic use of mental imagery to aid in later retrieval. Although we cannot completely discount this possibility, we argue this is likely not the sole explanation given that accuracy on the recognition memory test did not correlate with reading comprehension scores.
A second possibility is that individuals with poorer comprehension scores may have been more prone to make use of semantic similarity when reading these sets of words. Similar to the findings described above which have shown a relation between imageability effects and reading skill, previous studies have shown that poor readers are aided by contextual information to a greater extent than skilled readers; for example, Nation and Snowling (1998) showed that children with reading impairments exhibited a greater facilitative benefit of prior sentential context on single word naming compared to their typically developing peers. Thus, greater reliance on semantic information could be a compensatory strategy in these readers, resulting in differences in semantic processing across a widely distributed set of regions (e.g., Binder, Desai, Graves, & Conant, 2009). In the current study, even though subjects were not provided with sentential context, contextual information was provided in the first few words in the set; individuals could therefore have differed in their use of the initial words to predict upcoming items. This explanation could account for the observed brain-behavior correlation in the left IFG in particular, as this region has been shown to play a role in integration of information with prior context as well as prediction of upcoming items (Zhu et al., 2013). However, this type of explanation must be interpreted with caution given that all subjects in the current sample were skilled adult readers; therefore even individuals at the lower end of the range of comprehension scores would not be considered impaired.
4.3 Limitations
We should point out that although this task does not require an overt response on each trial, subjects were still actively engaged in processing the stimuli because they were asked to remember individual items in order to perform the subsequent recognition memory test. In addition, it is likely that subjects noted the spelling-sound consistency and semantic manipulations, because these were highly salient. Therefore it is possible this task incurred a degree of active metalinguistic processing even though subjects were not asked to perform overt behavioral responses. Because we did not collect behavioral data on a trialwise basis, we cannot draw firm conclusions regarding the precise meaning of patterns of brain activity. Nevertheless, it is our view that the task is relatively simple compared to alternative tasks such as naming, lexical decision, and spelling/case/rhyme/category judgment, and that the practical benefits of this simple task for working with special populations outweigh these limitations in interpretative power.
5. Conclusions
Overall, the “fast” localizer paradigm enables acquisition of a reliable snapshot of the cortical regions involved in the component processes of reading. As we showed with a group of skilled adult readers, the localizer successfully isolated areas sensitive to lexicality, including the VWFA, a number of regions sensitive to spelling-sound consistency, including bilateral IFG and left SMG, and a subregion of IFG sensitive to semantic similarity. Furthermore, the task was sensitive to some individual differences in the processing of semantically related words, which were related to reading comprehension scores outside the scanner.
The advantages of the “fast” localizer over alternative paradigms underscore its potential to be a powerful tool for investigating the reading network in many different types of populations. Namely, the task is relatively simple in nature and allows for easy comparison across groups of subjects; furthermore, data is acquired in a brief amount of imaging time, especially relative to the number of indices of component processes obtained. Importantly, the administration time associated with the task is brief enough that the task could feasibly be used as a localizer of the reading network within the same scanning session as a second imaging study (Saxe et al., 2006). Using the localizer in this fashion not only increases the power to detect meaningful differences amongst subjects, but also permits investigations which query how areas engaged in reading modulate according to other domains, such as memory, attention, executive function, and numeracy. Given recent interest in co-morbid learning disorders, such as reading and math disability (Koepke & Miller, 2013), these types of investigations could be particularly important.
For researchers interested in implementing the “fast” localizer protocol, all programs and stimulus materials are available at the following link: http://haskins.yale.edu/datasharing/
Supplementary Material
Highlights.
We present a novel fMRI protocol for localizing the reading network
This protocol takes less imaging time than comparable paradigms currently available
The “fast” localizer is suitable for populations varying in age and skill
We demonstrate the utility of the paradigm with a group of skilled adult readers
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Numbers P01HD070837 to Georgia State University and P01HD001994 and R01HD065794 to Haskins Laboratories. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, Treiman R. The English lexicon project. Behavior Research Methods. 2007;39(3):445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Hornickel J, Cone NE, Burman DD, Booth JR. Neural correlates of orthographic and phonological consistency effects in children. Human Brain Mapping. 2008a;29(12):1416–1429. doi: 10.1002/hbm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Minas J, Burman DD, Booth JR. Differential effects of orthographic and phonological consistency in cortex for children with and without reading impairment. Neuropsychologia. 2008;46(14):3210–3224. doi: 10.1016/j.neuropsychologia.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25(1):151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braun M, Jacobs AM, Richlan F, Hawelka S, Hutzler F, Kronbichler M. Many neighbors are not silent. fMRI evidence for global lexical activity in visual word recognition. Frontiers in Human Neuroscience. 2015:9. doi: 10.3389/fnhum.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JI, Fishco VV, Hanna GS. Nelson-Denny Reading Test. Riverside; Chicago, IL: 1993. [Google Scholar]
- Bruno JL, Zumberge A, Manis FR, Lu ZL, Goldman JG. Sensitivity to orthographic familiarity in the occipito-temporal region. NeuroImage. 2008;39(4):1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, New B. Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behavior Research Methods. 2009;41(4):977–990. doi: 10.3758/BRM.41.4.977. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behavior Research Methods. 2014;46(3):904–911. doi: 10.3758/s13428-013-0403-5. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends in Cognitive Sciences. 2012;16(6):338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LK, Cole PG, Morris JN. Effects of instruction in the use of a visual-imagery strategy on the reading-comprehension competence of disabled and average readers. Learning Disability Quarterly. 1990;13(1):2–11. [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coleman C, Lindstrom J, Nelson J, Lindstrom W, Gregg KN. Passageless comprehension on the Nelson-Denny Reading Test: Well above chance for university students. Journal of Learning Disabilities. 2010;43(3):244–249. doi: 10.1177/0022219409345017. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec'H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. NeuroReport. 2002;13(3):321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Price C, Wise R, Frackowiak RSJ. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron-emission tomography study in normal human subjects. Neuroscience Letters. 1994;182(1):25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Devlin J, Matthews P, Rushworth M. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Diaz MT, McCarthy G. Unconscious word processing engages a distributed network of brain regions. Journal of Cognitive Neuroscience. 2007;19(11):1768–1775. doi: 10.1162/jocn.2007.19.11.1768. [DOI] [PubMed] [Google Scholar]
- Eden GF, Moats L. The role of neuroscience in the remediation of students with dyslexia. Nature Neuroscience. 2002;5:1080–1084. doi: 10.1038/nn946. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C, Friederici A, Müller K, Cramon DV. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound spelling-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Ferreira RA, Göbel SM, Hymers M, Ellis AW. The neural correlates of semantic richness: Evidence from an fMRI study of word learning. Brain and Language. 2015;143:69–80. doi: 10.1016/j.bandl.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Mencl WE, Sandak R, Moore DL, Rueckl JG, Katz L, Pugh KR. A functional magnetic resonance imaging study of the tradeoff between semantics and phonology in reading aloud. NeuroReport. 2005;16(6):621–624. doi: 10.1097/00001756-200504250-00021. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cerebral Cortex. 2010;20(8):1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson VL, Fowler CA. Phonological coding in word reading: Evidence from hearing and deaf readers. Memory & Cognition. 1987;15(3):199–207. doi: 10.3758/bf03197717. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Gabrieli JD. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. The Journal of Neuroscience. 2006;26(42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Gabrieli JD. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Ralph MAL, Woollams AM. Triangulation of the neurocomputational architecture underpinning reading aloud. Proceedings of the National Academy of Sciences. 2015;112(28):E3719–E3728. doi: 10.1073/pnas.1502032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Simon G, Tzourio-Mazoyer N. The weight of skill: interindividual variability of reading related brain activation patterns in fluent readers. Journal of Neurolinguistics. 2011;24(1):113–132. [Google Scholar]
- Koepke KM, Miller B. At the intersection of math and reading disabilities: Introduction to the special issue. Journal of Learning Disabilities. 2013;46(6):483–489. doi: 10.1177/0022219413498200. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. NeuroImage. 2004;21(3):946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41(3):293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini M, Price C. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An on-line orthographic database of the English language. 2005. http://www.neuro.mcw.edu/mcword/ [Google Scholar]
- Meyer DE, Schvaneveldt RW, Ruddy MG. Functions of graphemic and phonemic codes in visual word-recognition. Memory & Cognition. 1974;2(2):309–321. doi: 10.3758/BF03209002. [DOI] [PubMed] [Google Scholar]
- Mummery C, Patterson K, Hodges J, Price C. Functional neuroanatomy of the semantic system: divisible by what? Journal of Cognitive Neuroscience. 1998;10(6):766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- Nation K, Snowling MJ. Individual differences in contextual facilitation: Evidence from dyslexia and poor reading comprehension. Child Development. 1998;69(4):996–1011. [PubMed] [Google Scholar]
- Norton ES, Beach SD, Gabrieli JD. Neurobiology of dyslexia. Current Opinion in Neurobiology. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pinel P, Thirion B, Meriaux S, Jobert A, Serres J, Le Bihan D, Dehaene S. Fast reproducible identification and large-scale databasing of individual functional cognitive networks. BMC Neuroscience. 2007;8(1):91. doi: 10.1186/1471-2202-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19(3):473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9(6):727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Moore D, Della Porta G, et al. The neural basis of reading. In: Cornelissen PL, Hansen PC, Kringelbach ML, Salmelin R, editors. Mapping the word reading circuitry in skilled and disabled readers. Oxford University Press; New York: 2010. pp. 281–305. [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Rueckl JG, Constable RT, Mencl WE. Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20(7):1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D, Molfese P. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain and Language. 2013;125(2):173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Rexer K, Katz L. Evidence of flexible coding in visual word recognition. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(4):807. doi: 10.1037//0096-1523.20.4.807. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56(3):1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41(5):550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Sandak R, Frost SJ, Rueckl JG, Landi N, Mencl WE, Katz L, Pugh KR. How does the brain read words. In: Joanisse M, McRae K, Spivey M, editors. The Cambridge Handbook of Psycholinguistics. Cambridge University Press; New York: 2012. pp. 218–235. [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, Pugh KR. The neurobiology of adaptive learning in reading: a contrast of different training conditions. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(1):67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. NeuroImage. 2006;30(4):1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Human Brain Mapping. 2004;23(3):140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M. Computational models of reading: Connectionist and dual-route approaches. In: Joanisse M, McRae K, Spivey M, editors. The Cambridge Handbook of Psycholinguistics. Cambridge University Press; New York: 2012. pp. 186–203. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shulman HG, Hornak R, Sanders E. The effects of graphemic, phonetic, and semantic relationships on access to lexical structures. Memory & Cognition. 1978;6(2):115–123. [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: An integrated approach. Cerebral Cortex. 2002;12(3):297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. Dyslexia. Blackwell Publishing; 2000. [Google Scholar]
- Stanovich KE. Explaining the differences between the dyslexic and the garden-variety poor reader: The phonological-core variable-difference model. Journal of Learning Disabilities. 1988;21(10):590–604. doi: 10.1177/002221948802101003. [DOI] [PubMed] [Google Scholar]
- Stoeckel C, Gough PM, Watkins KE, Devlin JT. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45(9):1091–1096. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain E, Herdman CM. Imageability effects in word naming: an individual differences analysis. Canadian Journal of Experimental Psychology. 1999;53(4):347. doi: 10.1037/h0087322. [DOI] [PubMed] [Google Scholar]
- Szwed M, Dehaene S, Kleinschmidt A, Eger E, Valabrègue R, Amadon A, Cohen L. Specialization for written words over objects in the visual cortex. NeuroImage. 2011;56(1):330–344. doi: 10.1016/j.neuroimage.2011.01.073. [DOI] [PubMed] [Google Scholar]
- Tagamets M, Novick J, Chalmers M, Friedman R. A parametric approach to orthographic processing in the brain: an fMRI study. Journal of Cognitive Neuroscience. 2000;12(2):281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Pro-ed; Austin, TX: 1999. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55(1):143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Welcome SE, Joanisse MF. Individual differences in skilled adult readers reveal dissociable patterns of neural activity associated with component processes of reading. Brain and Language. 2012;120(3):360–371. doi: 10.1016/j.bandl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27(1):35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Silani G, Okada K, Patterson K, Price CJ. Word or word-like? Dissociating orthographic typicality from lexicality in the left occipito-temporal cortex. Journal of Cognitive Neuroscience. 2010;23(4):992–1002. doi: 10.1162/jocn.2010.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11(3):267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Yap MJ, Tan SE, Pexman PM, Hargreaves IS. Is more always better? Effects of semantic richness on lexical decision, speeded pronunciation, and semantic classification. Psychonomic Bulletin & Review. 2011;18(4):742–750. doi: 10.3758/s13423-011-0092-y. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang X, Frost SJ, Sun W, Fang SY, Mencl WE, Rueckl JG. Neural division of labor in reading is constrained by culture: A training study of reading Chinese characters. Cortex. 2014;53:90–106. doi: 10.1016/j.cortex.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Stone GO, Jacobs AM. What is the pronunciation for -ough and the spelling for /u/? A database for computing feedforward and feedback consistency in English. Behavior Research Methods, Instruments, & Computers. 1997;29(4):600–618. [Google Scholar]
- Zhu Z, Feng G, Zhang JX, Li G, Li H, Wang S. The role of the left prefrontal cortex in sentence-level semantic integration. NeuroImage. 2013;76:325–331. doi: 10.1016/j.neuroimage.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Hagoort P, Zhang JX, Feng G, Chen HC, Bastiaansen M, Wang S. The anterior left inferior frontal gyrus contributes to semantic unification. NeuroImage. 2012;60(4):2230–2237. doi: 10.1016/j.neuroimage.2012.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.