Abstract
Introduction
Hospitalization is a unique opportunity for smoking cessation, but prior interventions have measured efficacy with narrowly defined populations. The objective of this study was to enroll smokers admitted to two “safety net” hospitals and compare the effectiveness of two post-discharge cessation interventions.
Design
Randomized comparative effectiveness trial.
Setting/participants
At two New York City public hospitals, every hospitalized patient identified as a smoker (based on admission records) was approached. Inclusion criteria were: smoked cigarettes in the past 30 days; spoke English, Spanish, or Mandarin; had a U.S. phone number; not discharged to an institution where follow-up or smoking was limited; and not pregnant/breastfeeding. Of 18,797 patients identified as current smokers between July 2011 and April 2014, a total of 3,047 (16%) were discharged before being approached, 3,273 (17%) were not current smokers, 4,026 (21%) had no U.S. phone number, 2,831 (15%) were ineligible for other reasons, and 3,983 (21%) refused participation. In total, 1,618 (9%) participants enrolled in the study. During follow-up, 69% of participants were reached at 2 months and 68% at 6 months.
Intervention
At discharge, participants were randomized to multisession telephone counseling from study staff (n=804) or referral to the state Quitline for proactive outreach and counseling (n=814).
Main outcome measures
Self-reported abstinence at 6 months. Analyses were conducted in late 2015.
Results
One quarter of participants were homeless or in unstable housing, 60% had a history of substance abuse, 43% reported current hazardous drinking, and half had a psychiatric diagnosis other than substance abuse. At follow-up, the rate of abstinence (30-day point prevalence) was higher in the intensive counseling arm than the Quitline arm at 2 months (29.0% vs 20.7%; relative risk, 1.40; 95% CI=1.13, 1.73) and 6 months (37.4% vs 31.5%; relative risk, 1.19; 95% CI=1.01, 1.40).
Conclusions
Intensive counseling was more effective than referral to the state Quitline. Long-term abstinence was excellent in both groups. Many patients were not eligible for enrollment despite minimal exclusion criteria.
Trial registration
clinicaltrials.gov Identifier: NCT01363245.
Introduction
Cigarette smoking is the leading preventable cause of death,1 particularly among low-income and minority populations.2 Although the U.S. smoking prevalence has declined from 24.1% in 1998 to 17.8% in 2013,3 it has not declined consistently across all segments of the population. Large discrepancies in tobacco use exist by level of income and education, as well as by race and ethnicity.3 Compared with white smokers and smokers above the poverty line, minority and lower-income smokers are less likely to receive cessation advice from a provider, to use proven treatment, and to quit successfully.4,5
Hospitalization is a critical opportunity to encourage cessation and offer assistance. Patients have enforced abstinence while admitted and may be newly sensitized to health-related issues. Although initiating treatment during hospitalization and continuing for at least 1 month after discharge increases long-term cessation rates,6 gaps exist in the literature. Most inpatient interventions have involved narrowly defined medical or surgical populations and high-intensity initiatives.6 Psychiatric inpatient studies have focused on staff and patient attitudes and single-center experiences with institutional smoking bans—not on treatment trials.7–13 Further, most studies have involved specially trained staff delivering intensive bedside interventions, raising questions about generalizability and feasibility.6
Public “safety net” hospitals provide ideal environments to address smoking cessation, as they care for populations with high smoking rates and less access to other sources of care. Their patients are more likely to be racial/ethnic minorities, non-English speaking, and poor.14 Increasing tobacco treatment rates at public hospitals would directly address disparities in smoking, by targeting populations that have higher rates of smoking and lower rates of treatment. As part of the Consortium of Hospitals Advancing Research on Tobacco (CHART),15 this study compared the effectiveness of two post-discharge smoking-cessation interventions at two urban safety net hospitals in New York City.
Methods
Study Design, Setting, and Participants
The CHART–New York16 study took place at Bellevue Hospital Center (part of the New York City Health and Hospitals Corporation) and the Veterans Affairs (VA) New York Harbor Healthcare System (part of the Veterans Health Administration). Both function as “safety net” hospitals, providing comprehensive care to predominantly low-income patients, and both are academically affiliated with New York University School of Medicine. The two study interventions—fax transfer to the state Quitline versus multisession counseling delivered by study staff—were selected such that they could be readily adopted by most hospitals without difficulty. This study was approved by the IRB at each institution and by the CHART Data Safety and Monitoring Board established by NIH.
Using the electronic medical record, a daily list was generated of inpatients documented as current smokers on admission screening. Assessment of smoking status is a standard nursing practice on for admitted patients, and per the team’s estimation, >90% of admitted patients were assessed for smoking status. Research assistants (RAs) reviewed the list twice daily and went to the bedside of every patient on the list. Recruitment occurred 6 days per week, including evenings. In addition to the inpatient units, RAs approached admitted patients who remained in the emergency department and patients in the intensive care units.
Inclusion criteria included anyone aged ≥ 18 years who reported smoking during the prior 30 days, had an active U.S. phone number, and spoke English, Spanish, or Mandarin. Exclusion criteria were: (1) being pregnant or breastfeeding; (2) lacking cognitive or physical ability to participate; or (3) being discharged to an institution that would limit the ability to deliver telephone counseling and where patients lacked control over their ability to smoke (e.g., jail, nursing home). Participants were not required to be interested in quitting smoking. Upon discharge, RAs verified that participants were not discharged to an institution, then logged into the study database that generated the random assignment. The randomization scheme, designed by the biostatistician, employed a computerized random number generator and stratified participants on hospital site. The target sample size was 1,612, giving 80% power to detect a 3.5% absolute difference in abstinence rates between the two arms at the 0.05 significance level (8.5% for intensive counseling arm versus 5% for Quitline arm).
Upon enrollment, participants completed a 20-minute interviewer-administered survey, which covered sociodemographics, smoking history (e.g., cigarettes per day, years smoking, prior quit attempts, other tobacco products used),17–19 readiness to quit, alcohol use,20,21 drug use,22 and healthcare utilization in the past 6 months. After discharge, RAs conducted a structured explicit chart review for the hospitalization to record admission characteristics (e.g., admitting service, length of stay), diagnoses, cessation treatment received in the hospital, and nicotine-replacement therapy (NRT) prescribed at discharge.
The 2-month telephone follow-up survey was brief and included questions on smoking status, medications and counseling offered and received since discharge, and perceptions of the counseling. The 6-month telephone follow-up survey took approximately 15 minutes and included the same measures asked on enrollment, as well as questions on smoking status and treatment received. RAs making follow-up calls were blinded to treatment group assignment. For the 2-month and 6-month follow-up survey, RAs made up to ten and 20 call attempts, respectively, including evenings and weekends. RAs also checked frequently to see if participants were in the hospital or scheduled to be in clinic, in which case they administered the survey in person. After five call attempts, they also mailed paper surveys and (for Bellevue patients) sent an e-mail about the survey.
In the intensive counseling arm, the counselors logged dates and times into a database to document call attempts and duration of counseling calls. The Quitline maintains records on each individual call and study staff contacted each state Quitline by phone or a secure online portal to ascertain the number of calls for each participant in the Quitline arm.
Interventions
Quitline arm
The project director transmitted participant information by facsimile or online referral to their state Quitline, which for 95% was the New York State Smokers’ Quitline (other states included California, Connecticut, the District of Columbia, Florida, Massachusetts, Pennsylvania, South Carolina, Tennessee, Virginia, and West Virginia). The standard Quitline protocol varies by state; in New York, they make up to five call attempts, and contacted participants receive one 15 to 20–minute counseling session with a follow-up call to assess quit status and assure any requested NRT was received. Quitline counselors are trained in motivational interviewing and the calls focus on developing a quit plan. All Quitlines involved in the study provided counseling in English or Spanish. The New York Quitline transfers Mandarin-speaking callers (all in the study were New York residents) to the Asian Smokers’ Quitline, a free nationwide Asian-language quit smoking service.
Intensive counseling arm
Study staff—Masters-level counselors with mental health training—reached out proactively to deliver seven sessions of telephone counseling in English, Spanish, or Mandarin. The structured counseling protocol was based on Motivational Interviewing23 and Problem Solving Therapy24 and addressed behavioral and cognitive issues, including motivation, self-efficacy, difficult situations, comorbid symptoms, coping strategies, medication usage, and relapse prevention. The first call was conducted in the 2 weeks after discharge (with up to ten attempts to reach the participant) and lasted approximately 15–20 minutes, and the 10 to 15–minute follow-up calls were at 1, 3, 7, 14, 30, and 42 days after the initial post-discharge contact. This call schedule was designed support a participant’s chosen quit date and to prevent relapse25; however, this schedule could be adapted based on each individual’s quit process and availability. Participants in the intensive counseling arm were also eligible for 8 weeks of NRT if they had not received an NRT prescription at discharge. The protocol was informed by evidence-based behavioral techniques recommended by the Public Health Service Guidelines for treating tobacco use and dependence.26 A similar protocol designed by the study team was more effective than warm transfer to the Quitline in patients with mental health conditions.27
Outcome Measures
As with all CHART studies, the prespecified primary outcome was self-reported 30-day point prevalence abstinence at 6-month follow-up.28 To evaluate misreporting across CHART studies, several sites (including New York) made exhaustive efforts to obtain saliva from a consecutive subsample of participants reporting abstinence and no NRT or e-cigarette use in the past 7 days at 6-month follow-up. To collect saliva, RAs sent three kits by mail, made up to five reminder calls, and offered to meet the participant in person if more convenient. Saliva samples were frozen and sent in blinded batches to Salimetrics, LLC (State College, Pennslyvania) for analysis of cotinine levels. Participants who provided saliva received $100. The final 40 people providing saliva were also asked about their smoking status in the past 7 days at the time of saliva collection.
Statistical Analysis
All analyses were conducted with SAS, version 9.3 in late 2015. Smoking-cessation studies often use either a complete case approach (only including people whose follow-up status was ascertained) or a sensitivity analysis based on the simulation of a worse-case scenario (treating all people lost to follow-up as current smokers). The North American Quitline Consortium has found a complete case analysis is more accurate than sensitivity analysis with worse-case scenario in representing true quit rates and recommends use of this calculation,29 as do other reviews.30 Therefore, the primary outcome was calculated using complete case data (i.e., those who completed follow-up surveys); sensitivity analysis outcomes are also reported. Relative risks (RRs) were calculated to compare abstinence rates between the two arms among participants at 2 months and 6 months. Lastly, the statistician evaluated whether there was an interaction between treatment arm and admitting hospital service or study site.
The costs associated with the interventions were estimated by prospectively collecting data on time spent referring patients to the Quitline, providing Quitline services, delivering telephone counseling, and providing NRT. The project manager contacted New York Quitline staff members and used the study team’s counseling records to inform estimates of call attempts (mean of 2.3 calls per patient for Quitline and 7.7 calls per patient for intensive counseling), time spent preparing for each call (not considered for Quitline because only patient contact information included in referral; mean of 10.3 minutes per patient for intensive counseling), time spent counseling patients (mean of 7.6 minutes per patient for Quitline and 46.5 minutes per patient for intensive counseling), and time spent after the call to document notes (mean of 4.3 minutes per patient for Quitline and mean 40.37 minutes per patient for intensive counseling). The cost of NRT was based on representative average wholesale prices for generics, as reported by Lexicomp.31 These cost-per-unit estimates were $0.40 for nicotine gum at a 2-mg dose, $0.42 for nicotine gum at a 4-mg dose, $2.13 for a nicotine patch at any dose, and $0.54 for a nicotine lozenge at any dose. When data on duration of therapy were unavailable, it was assumed that patients were provided with a 30-day supply. The average hourly compensation for Substance Abuse and Behavioral Disorder Counselors from the U.S. Bureau of Labor Statistics in 2014 was $18.82 per hour, and a nationally representative fringe rate was applied to estimate total hourly compensation of $27.08 per hour.32,33 Mean costs and cost effectiveness, based on the cost per quit, were derived using the ratio of intensive counseling costs to intensive counseling effectiveness ([change in cost]/[change in effectiveness]), compared with patients in the Quitline group. Then, Fieller’s theorem was used to estimate a 95% CI for the cost-per-quit ratio.34 Cost-per-quit analyses were performed from a social perspective, meaning that all identified costs were included regardless of who defrayed the cost.35 All costs were converted to 2015 U.S. dollars using the Consumer Price Index for the first two quarters of the year.
Results
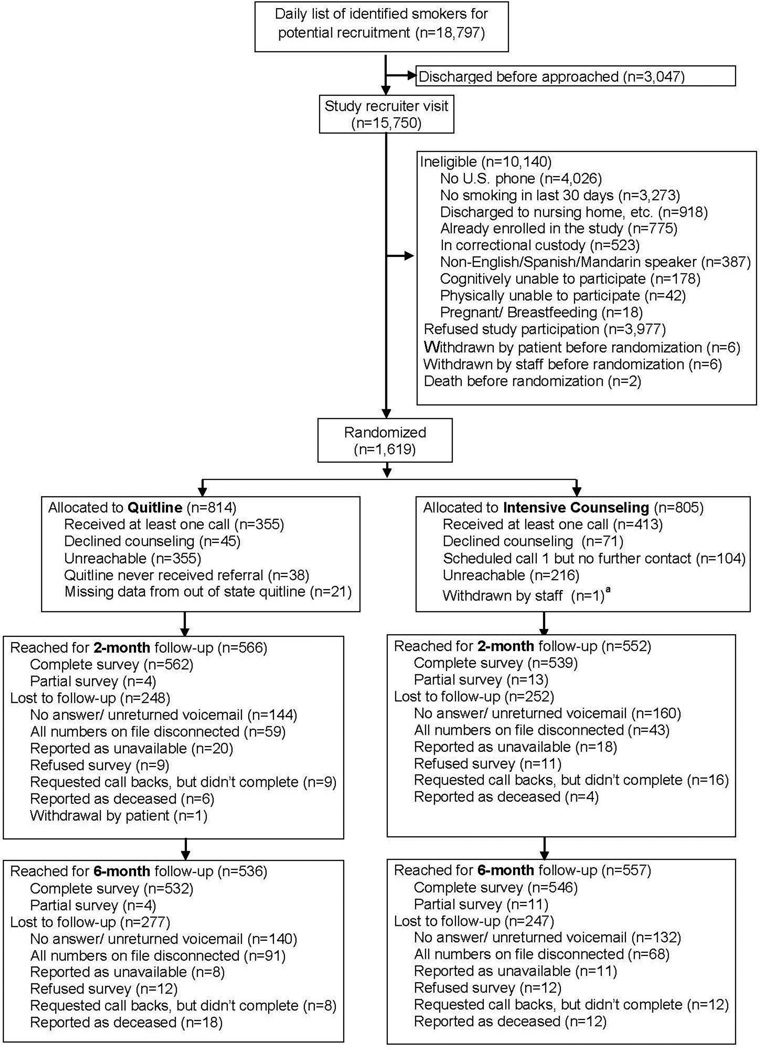
Study RAs approached all 18,797 hospitalized patients identified as current smokers between July 2011 and April 2014 (Figure 1). Approximately 3,000 were discharged before RAs could evaluate them for study eligibility. Of the remaining 15,750, approximately 10,000 were ineligible, with the main reasons being no U.S. phone number (n=4,026) or reporting no smoking in the last 30 days (n=3,273). Nearly 4,000 patients refused to participate, and 1,618 patients were enrolled.
Figure 1.
CONSORT diagram for recruitment and follow-up.
aOne VA participant was withdrawn by study staff after randomization as he was found to have been enrolled previously when he was hospitalized at Bellevue, leaving a final sample size of 1,618 participants.
Participants in both arms were well matched at baseline (Table 1). Approximately two thirds were enrolled at Bellevue, and nearly 80% were male. The majority (82%) indicated plans to quit smoking at the time of enrollment. The sample was very diverse with respect to race and ethnicity. Approximately one quarter of participants were either homeless or in unstable housing. Drug use in the last 30 days was very common: marijuana, 34%; cocaine, 21%; and illicit opioids, 17%. Sixty percent had a history of substance abuse or dependence and 43% reported current hazardous drinking. Approximately half had a psychiatric diagnosis other than substance abuse (Table 1 lists the main diagnostic categories).
Table 1.
Baseline Characteristics of Participants, by Treatment Group
| Demographics | Quitline (n=814) | Intensive counseling (n=804) |
|---|---|---|
| Bellevue | 588 (72%) | 556 (69%) |
| VA | 226 (28%) | 248 (31%) |
| Age, mean (SD), years | 48 (13) | 49 (13) |
| Male | 633 (78%) | 639 (80%) |
| Race – n (%) | ||
| White | 245 (31%) | 239 (31%) |
| Black | 295 (37%) | 297 (38%) |
| Asian | 23 (2.9%) | 27 (3.5%) |
| Native Hawaiian/Pacific Islander | 3 (0.4%) | 1 (0.1%) |
| American Indian/Alaska Native | 19 (2.4%) | 12 (1.5%) |
| Multiple races | 50 (6.3%) | 63 (8.1%) |
| Other | 156 (20%) | 140 (18%) |
| Hispanic ethnicity – n (%) | 282 (35%) | 283 (36%) |
| Education | ||
| Some high school or less | 204 (25%) | 193 (24%) |
| High school or GED | 235 (29%) | 216 (27%) |
| Associate’s or some college degree | 243 (30%) | 270 (34%) |
| 4-year college graduate or higher | 128 (16%) | 117 (15%) |
| Marital status – n (%) | ||
| Married/living with a partner | 177 (22%) | 172 (22%) |
| Separated or divorced | 238 (29%) | 235 (29%) |
| Widowed | 37 (4.6%) | 43 (5.4%) |
| Never married | 355 (44%) | 345 (43%) |
| Living situation before hospitalization – n (%) | ||
| Own/rent apartment or home | 538 (72%) | 540 (76%) |
| Transitional housing | 98 (13%) | 94 (13%) |
| Sleeping in shelters, street, etc. | 81 (11%) | 45 (6.3%) |
| Staying with family or friends | 3 (0.4%) | 8 (1.1%) |
| SRO | 13 (1.7%) | 13 (1.8%) |
| Institution (e.g. nursing home, jail, prison) | 12 (1.6%) | 12 (1.7%) |
| Employed – n (%) | 232 (29%) | 228 (29%) |
| Primary Insurance (by self-report) | ||
| No insurance or unknown | 142 (17%) | 127 (16%) |
| VA | 226 (28%) | 250 (31%) |
| Private | 62 (7.6%) | 75 (9.3%) |
| Medicare | 76 (9.3%) | 79 (9.8%) |
| Medicaid | 247 (30%) | 219 (27%) |
| Other public (e.g., Tricare, Champus) | 61 (7.4%) | 54 (6.7%) |
| Immigrant (born outside U.S.) – n (%) | 160 (20%) | 183 (23%) |
| Language for consenta | ||
| English | 770 (95%) | 760 (95%) |
| Spanish | 38 (4.7%) | 35 (4.4%) |
| Mandarin | 6 (0.7%) | 9 (1.1%) |
| Tobacco and substance use characteristics | ||
| Cigarettes per day, mean (SD) | 12.5 (10.2) | 12.3 (9.6) |
| Years Smoked, mean (SD) | 27 (14.2) | 26 (14.8) |
| Level of nicotine addiction, first cigarette within: | ||
| 5 minutes | 347 (43%) | 338 (42%) |
| 6–30 minutes | 166 (20%) | 155 (19%) |
| 31–60 minutes | 91 (11%) | 89 (11%) |
| >60 minutes | 207 (26%) | 215 (27%) |
| Other tobacco use in past 30 days | 83 (10%) | 75 (9%) |
| History of quit attempt – n (%) | 661 (82%) | 651 (82%) |
| Quit attempt in past year – n (%) | 372 (46%) | 384 (48%) |
| Plan for smoking after discharge – n (%) | ||
| Plan to stay quit | 214 (26%) | 232 (29%) |
| Plan to try to quit | 454 (56%) | 423 (53%) |
| Don’t know if going to quit | 111 (14%) | 97 (12%) |
| Do not plan to quit | 30 (3.7%) | 46 (5.8%) |
| Confidence in quitting – n (%) | ||
| Not at all confident | 80 (10%) | 74 (9%) |
| A little confident | 125 (15%) | 119 (15%) |
| Somewhat confident | 210 (26%) | 194 (24%) |
| Fairly confident | 129 (16%) | 153 (19%) |
| Very confident | 265 (33%) | 254 (32%) |
| e-cigarette use in past 30 days – n (%) | 127 (16%) | 123 (15%) |
| Marijuana use in past 30 days – n (%) | 291 (36%) | 256 (32%) |
| Cocaine use in past 30 days – n (%) | 171 (21%) | 175 (22%) |
| Illicit opioid use in past 30 days – n (%) | 135 (17%) | 148 (18%) |
| Hazardous alcohol useb – n (%) | 358 (44%) | 343 (43%) |
| Clinical characteristics | ||
| Length of stay, mean (SD), days | 7.5 (7.8) | 8.2 (10.7) |
| BMI, kg/m2, mean (SD) | 27.3 (6.6) | 27.7 (7.1) |
| Admitting service – n (%) | ||
| Psychiatry | 305 (38%) | 326 (41%) |
| Medicine | 394 (48%) | 361 (45%) |
| Other (surgery, neurology, rehab, pediatrics, gynecology) |
114 (14%) | 116 (14%) |
| Cessation counseling documented by hospital staff – n (%) |
710 (88%) | 700 (89%) |
| Smoking cessation med. during hospitalization – n (%) | 354 (44%) | 353 (44%) |
| Smoking cessation medication at discharge – n (%) | 149 (19%) | 146 (18%) |
| Cerebrovascular disease – n (%) | 33 (4.1%) | 40 (5.0%) |
| Coronary artery disease – n (%) | 141 (17%) | 129 (16%) |
| Heart failure – n (%) | 38 (4.7%) | 37 (4.6%) |
| COPD/Pulmonary disease – n (%) | 100 (12%) | 93 (12%) |
| Asthma – n (%) | 123 (15%) | 103 (13%) |
| History of alcohol or substance abuse/dependence – n (%) |
483 (59%) | 488 (61%) |
| History of psychiatric diagnosis (non-substance related) – n (%) |
387 (48%) | 410 (51%) |
| Mood disorder | 312 (38%) | 335 (41%) |
| Anxiety disorder | 123 (15%) | 113 (14%) |
| Schizophrenia/ Schizoaffective disorder | 80 (10%) | 92 (11%) |
This reflects the language in which the participant signed the informed consent form. For the counseling calls, they were assigned to a counselor who spoke Spanish or Mandarin (as appropriate for the participant) and were free to choose whether they preferred counseling in English, Spanish, Mandarin or a mixture.
Hazardous drinking was defined as a score on the AUDIT-C of ≥4 for men and ≥3 for women. VA, VA New York Harbor Healthcare System; COPD, chronic obstructive pulmonary disease; SRO, Single room occupancy hotel (which often provide temporary or transitional housing for people at risk of being homeless)
Table 2 shows the number of received counseling calls. Approximately half of participants in the intensive counseling arm did not receive any calls, one quarter received one to two calls, and the remainder received three to seven calls. Similarly, just less than half of Quitline participants did not receive any counseling calls. In a regression analysis combining both groups, intention to quit (p=0.002); being prescribed NRT at discharge (p=0.014); diagnoses of chronic obstructive pulmonary disease (p=0.007), heart failure (p=0.033) or coronary artery disease (p=0.014); no history of substance abuse (p<0.0001, lack of current substance use was similarly associated); and no history of psychiatric diagnoses (p=0.0048) were associated with having at least one counseling call.
Table 2.
Number of Telephone Counseling Calls Received, by Treatment Group
| Number of calls received |
Quitline arm | Intensive counseling arm |
Total |
|---|---|---|---|
| No calls | 400 (49%) | 391 (49%) | 791 |
| 1 call | 299 (37%) | 119 (15%) | 418 |
| 2 calls | 56 (6.9%) | 70 (8.7%) | 126 |
| 3 calls | 0 | 48 (6.0%) | 48 |
| 4 calls | 0 | 27 (3.4%) | 27 |
| 5 calls | 0 | 14 (1.7%) | 14 |
| 6 calls | 0 | 22 (2.7%) | 22 |
| 7 calls | 0 | 113 (14%) | 113 |
| Missing | 59 (7.2%) | 0 | 59 |
| Total | 814 (100%) | 804 (100%) | 1618 |
Notes: Number of calls for the Quitline is based on individual-level reports received from the New York State Smokers’ Quitline (or other Quitlines for participants who were not residents of New York State). For the intensive counseling arm, number of calls was extracted from the study counseling database.
The RAs reached 69% of participants at 2-month follow-up and 68% at 6-month follow-up, with similar rates between the two arms (Quitline: 70% at 2 months, 66% at 6 months; intensive counseling: 69% at 2 months, 69% at 6 months). Follow up rates were slightly lower among certain subgroups; RAs were able to reach 62% of participants reporting unstable housing, 62% of participants who were enrolled from the psychiatry service, and 65% of participants with current substance abuse at the 6-month time point. The self-reported 30-day point prevalence abstinence rate among participants reached for follow-up (complete case analysis) was 24.8% at 2 months and 34.4% at 6 months. At both 2 months and 6 months, the intensive counseling arm had a higher abstinence rate than the Quitline arm (Table 3). At 2-month follow-up, patients in the intensive arm were more likely to quit than those in the Quitline arm using the complete case (RR=1.40, 95% CI=1.13, 1.73) or intention-to-treat analysis (RR=1.39, 95% CI=1.11, 1.74). Similarly, the results at 6 months also favored the intensive counseling arm, using either the complete case (RR=1.19, 95% CI=1.01, 1.40) or intention-to-treat analysis (RR=1.25, 95% CI=1.05, 1.50).
Table 3.
Comparison of 2-Month and 6-Month Abstinence Rates by Intervention Arm
| 2 months | 6 months | |||||
|---|---|---|---|---|---|---|
| Analysis type | Quitline arm |
Intensive counseling arm |
Relative risk (95% CI) |
Quitline arm |
Intensive counseling arm |
Relative risk (95% CI) |
| Sensitivity analysis with worse-case scenario |
13.6% | 18.9% | 1.39 (1.11, 1.74) |
20.5% | 25.8% | 1.25 (1.05, 1.50) |
| Complete case analysis |
20.7% | 29.0% | 1.40 (1.13, 1.73) |
31.5% | 37.4% | 1.19 (1.01, 1.40) |
Note: In the sensitivity analysis, all 1,618 study participants were included; all participants with missing data were counted as smokers. In the complete case analysis, only participants who completed the 2-month (Quitline=535, intensive counseling=524) or 6-month (Quitline=530, intensive counseling=553) follow-up survey were included. Smoking status was based on self-reported 30-day point prevalence abstinence.
The between-group differences in abstinence rates at 6 months were similar across admitting hospital services (complete case data shown, sensitivity analysis with worse-case scenario results were similar): Medicine—intensive counseling 41.6% vs Quitline 33.6% (RR=1.4, 95% CI=0.995, 2.0); Psychiatry—30.3% vs 26.2% (RR=1.2, 95% CI=0.8, 1.9); all other services—40.5% vs 34.6% (RR=1.3, 95% CI=0.7, 2.4). There was no interaction between the intervention effectiveness and admitting hospital service (Medicine, Psychiatry, all other services) or study site (Bellevue versus VA).
Study RAs learned of adverse events at the time of follow-up calls or through finding the participants in the hospital on the study screening lists. During the study, in the Quitline arm, there were 16 deaths, 304 rehospitalizations among 204 participants, and 337 emergency department visits among 182 participants. Adverse events were similar in the intensive counseling arm, with 13 deaths, 332 rehospitalizations among 206 participants, and 299 emergency department visits among 180 participants. No adverse events were deemed related to the medications or the study.
For 14 months, the team attempted to biochemically validate all 195 people who reported abstinence at 6-month follow-up. Ninety-two percent (180/195) agreed to participate, and 150 samples were collected (77% of those invited, 83% of those who agreed), with 46 collected in person and 104 by mail. Participants’ reported duration of abstinence was 6 months for 79 (53%), 30 days for 53 (35%), and 7 days for 17 (11%). The salivary cotinine range was 0.09–1538.9 ng/mL (mean, 200.3). Two cotinine samples did not have sufficient quantity and were excluded. Using a 10-ng/mL cut point, 51% of self-reported quitters were verified to be nicotine free. Participants submitting samples in person were less likely to be biochemically verified nicotine free than those submitting by mail (OR=0.25, 95% CI=0.09, 0.66). Shorter duration of reported abstinence was also associated with lower likelihood of biochemically verified quit (p=0.01). The misreporting rate was the same in both arms (intensive counseling, 41/81; Quitline, 34/68). Among the final 40 participants reporting abstinence (where a brief survey asking about current smoking was also included), 32% (13/40) reported smoking in the last 7 days and testing confirmed this (12/13 were positive). Among the 68% (27/40) reporting not smoking in the last 7 days, the rate of misreporting was 30% (8/27).
Mean costs per patient were $17.84 in the Quitline arm, including $7.74 in counselor time and $10.10 in NRT, and $76.62 in the intensive counseling arm, including $37.31 in counselor time and $39.31 in NRT. The difference in per-patient costs was $58.77 (SE=$2.54) and cost per quit for intensive counseling, relative to Quitline referral, was $1,015 per patient quitting smoking (95% CI=$516, 43,013). The high degree of uncertainty for the cost-per-quit ratio is primarily attributable to the small values that comprise the lower distribution of intensive counseling’s incremental effectiveness. The number needed to treat for intensive counseling versus Quitline counseling was 17.
Discussion
The team approached nearly 19,000 smokers hospitalized at two “safety net” hospitals in New York City and enrolled 1,618 smokers. Thirty-four percent of participants reported no smoking in the prior 30 days when contacted 6 months after discharge. Approximately half the participants in each arm did not receive any counseling calls. The abstinence rate was higher in the intensive counseling arm than the Quitline arm at 2 months and 6 months (the primary outcome).
Although the intensive counseling arm was more effective, even electronic transfer to the state Quitline yielded a very good abstinence rate. These two interventions were selected as both could readily be adopted by any hospital. The effectiveness of the intervention was comparable to previous studies, most of which measured efficacy. Pooling data across 25 studies that included counseling in the hospital and for at least 1 month following discharge, Rigotti et al.6 found that 29% (1,048/3,057) were abstinent at follow-up. The cost per quit ($1,015) was reasonable in comparison with other interventions among hospitalized smokers reported in the literature, which range from $54036 to $2,67037 per quit.
The two main contributions from this study focus on effectiveness and reach. First, inpatient interventions are not only efficacious but also effective, and this is true across hospital services. Even in two safety net hospitals, with strikingly high rates of substance use disorders and homelessness, long-term abstinence rates were comparable to efficacy studies conducted in more-affluent, more-homogeneous patient populations. This is particularly important, as these vulnerable populations are often excluded from research studies and have particularly high rates of smoking.
The second key message is how challenging it is to reach the entire population of smokers in a hospital. In spite of recruiting in three languages and on evenings and weekends, only 9% of all hospitalized smokers were enrolled and telephone counseling was only delivered to half of them. Every 6 months, RAs carefully scrutinized every smoker who was discharged before they could be approached, and the team was unable to come up with practical ways to reach more of the people who were missed. Therefore, other approaches are needed to increase the reach among hospitalized smokers.
There are three potential ways to broaden the reach of an inpatient intervention. First and foremost, enrollment into post-discharge cessation treatment should probably be conducted by staff nurses as part of their routine admission tasks, as done by Duffy and colleagues38 in their study within the CHART consortium, thereby eliminating the category of smokers (n=3,047) discharged before they could be reached. Second, because more than 4,000 smokers were ineligible because of not having a U.S. phone number, studies should examine whether giving these smokers a cell phone (or connecting them to existing phone distribution programs) is feasible and effective. Third, additional research should explore ways to get more hospitalized smokers to participate in treatment, as nearly 4,000 people declined participation and only 50% of enrolled participants engaged in post-discharge counseling.
Limitations
This study has several limitations. The primary outcome was self-reported abstinence, as recommended in large, population-based studies.39 Unfortunately, misreporting was much more common than anticipated and the cotinine assay results suggest that 30%–50% of individuals in this sample had a tendency to give unreliable reports of smoking status at 6 months. Misreporting was the same in both arms, so the conclusions would be unlikely to change. Reducing the abstinence rate by 50% would lower it to 18.7% and 15.7%, still very acceptable success rates for large-scale interventions. Based on the 40 surveys added at the end of the validation period, a substantial amount of the misreporting was actually relapse during the window between 6-month survey and saliva collection. This suggests that cessation may be more unstable in hospitalized populations than in other settings. It also suggests biochemical validation is probably necessary in studies of hospitalized smokers (including an assessment of smoking status at the time of sample collection). Unfortunately, this will add significant burden to the collection practices of clinical trials enrolling hospitalized patients.
An additional limitation is that the telephone counseling was delivered by study staff, rather than medical center staff. Although the study was designed as an effectiveness study that could readily be adopted, the team felt it was important to have very consistent counseling. A hospital adopting the multisession counseling arm would likely have more variability in counseling from one patient to the next, although the Public Health Service guidelines40 would suggest the effectiveness would still be quite similar. Furthermore, only 9% of all smokers were enrolled in the study, and having hospital staff involved in the intervention could increase its reach, as suggested above, similar to the methods used by Duffy et al.38
The study also has many strengths. Safety net hospitals by definition take care of populations that have the least available resources, as was evident with the particularly high rates of homelessness, addiction, and mental health conditions. In spite of these barriers, RAs collected follow-up data from 67% of participants. Patients speaking English, Spanish, or Mandarin were enrolled and therefore only 2% of smokers were excluded for speaking another language. Finally, the team attempted to enroll all hospitalized smokers, excluding only those who were pregnant or breastfeeding, discharged to an institution, or cognitively impaired.
Conclusions
In summary, the team attempted to recruit every smoker hospitalized at two hospitals in New York City serving some of the most challenging populations for health care, and 34% of people who enrolled were abstinent 6 months later. Both interventions had excellent rates of cessation and are quite feasible for any hospital. Paraphrasing the theme song from the movie New York, New York, if one can do it here, it can be done anywhere. Hospitalization presents an important opportunity for reaching smokers and initiating a cessation intervention; post-discharge interventions are effective and should become routine care for all hospitals.
Acknowledgments
We would like to thank the outstanding team of research assistants and telephone counselors who made this study possible—Eric Arevalo, Jenny Chen, Maria Duenas, Rose Huang, Karen Gutierrez, Karishma Kurowski, Nicholas Lanzieri, Henry Lin, Rajkishen Narayanan, Daniele Ngantou, Ivelisse Rozon, Emily Sower, and Howard Wong. Henry Lin and Howard Wong also assisted with the cost analysis. Siamak Noorbaloochi, PhD provided invaluable statistical guidance. We would also like to thank Bellevue Hospital Center and VA New York Harbor Healthcare System for their unwavering support, even in the face of natural disasters such as Hurricane Sandy (which occurred during the middle of the intervention period). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of NIH or the Department of Veterans Affairs or the U.S. Government. The results of this study were presented in part at the 21st Annual Meeting of the Society for Research on Nicotine and Tobacco, Philadelphia, PA, February 25–28, 2015.
This work was supported by a grant from the National Heart, Lung and Blood Institute (NHLBI) of NIH (#1U01HL105229) and a Hurricane Sandy Supplement (#3U01HL105229-04S1), and also in part by the New York University CTSA grant UL1TR000038 from the National Center for Advancing Translational Sciences, NIH. Dr. Sherman is also supported by a grant from the National Institute on Drug Abuse (#1K24DA038345) and by the VA New York Harbor Healthcare System.
The Consortium of Hospitals Advancing Research on Tobacco was funded by NHLBI, the National Cancer Institute, the National Institute on Drug Abuse, and the Office of Behavioral and Social Sciences by cooperative agreements to a research coordinating unit (Kaiser Foundation Research Institute, Principal Investigator [PI]: Victor Stevens, PhD, U01HL52333) and six research projects (New York University School of Medicine, PI: Scott Sherman, MD, U01HL105229; University of California San Diego, PI: Shu-Hong Zhu, U01CA159533; University of Kansas Medical Center, PI: Kimber Richter, PhD, U01HL105232; University of Alabama Birmingham, PI: Kathleen Harrington, PhD, MPH, U01DA031515; University of Michigan Ann Arbor, PI: Sonia Duffy, PhD, U01HL105218; Kaiser Foundation Research Institute, PI: Jeffrey Fellows, PhD, U01HL105231). An additional project (Massachusetts General Hospital, PI: Nancy Rigotti, MD, RC1HL099668) has been included in the consortium. NIH Project Scientists on this project have included Lawton Cooper, MD, Sarah Duffy, PhD, Debra Grossman, PhD, Glen Morgan, PhD, William Riley, PhD, Catherine Stoney, PhD, and Xin Tian, PhD.
SS, ER, DS, and EG conceived of and designed the study. JL helped refine the evaluation. SS was principal investigator, oversaw all aspects of the study, and led the writing of this manuscript. EG was project director. AL was project manager. ER was intervention director. PK oversaw the telephone counseling in the intensive counseling arm. YF and BW were the trial statisticians and did the final statistical analyses of the results. All authors had full access to all of the data (including statistical reports and tables) in the study. SS takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to report.
No financial disclosures were reported by the authors of this paper.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. http://dx.doi.org/10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Cigarette smoking among adults - United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(45):1221–1226. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 4.Houston TK, Scarinci IC, Person SD, Greene PG. Patient Smoking Cessation Advice by Health Care Providers: The Role of Ethnicity, Socioeconomic Status, and Health. Am J Public Health. 2005;95(6):1056–1061. doi: 10.2105/AJPH.2004.039909. http://dx.doi.org/10.2105/AJPH.2004.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A Nationwide Analysis of U.S. Racial/Ethnic Disparities in Smoking Behaviors, Smoking Cessation, and Cessation-Related Factors. Am J Public Health. 2011;101(4):699–706. doi: 10.2105/AJPH.2010.191668. http://dx.doi.org/10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub3. http://dx.doi.org/10.1002/14651858.cd001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solty H, Crockford D, White WD, Currie S. Cigarette smoking, nicotine dependence, and motivation for smoking cessation in psychiatric inpatients. Can J Psychiatry. 2009;54(1):36–45. doi: 10.1177/070674370905400107. [DOI] [PubMed] [Google Scholar]
- 8.Campion J, Lawn S, Brownlie A, Hunter E, Gynther B, Pols R. Implementing smoke-free policies in mental health inpatient units: learning from unsuccessful experience. Australas Psychiatry. 2008;16(2):92–97. doi: 10.1080/10398560701851976. http://dx.doi.org/10.1080/10398560701851976. [DOI] [PubMed] [Google Scholar]
- 9.Kitabayashi Y, Narumoto J, Shibata K, et al. Effect of institutional smoking prohibition on Japanese inpatients with chronic schizophrenia. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2006;41(2):128–133. [PubMed] [Google Scholar]
- 10.Shmueli D, Fletcher L, Hall SE, Hall SM, Prochaska JJ. Changes in psychiatric patients’ thoughts about quitting smoking during a smoke-free hospitalization. Nicotine Tob Res. 2008;10(5):875–881. doi: 10.1080/14622200802027198. http://dx.doi.org/10.1080/14622200802027198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melamed Y, Peres D, Gelkopf M, Noam S, Bleich A. Psychiatric inpatient and staff attitudes regarding smoking reduction. Isr J Psychiatry Relat Sci. 2007;44(3):231–233. [PubMed] [Google Scholar]
- 12.Iglesias C, Lopez G, Alonso MJ. Effects of smoking ban in a general hospital psychiatric unit. Actas Esp Psiquiatr. 2008;36(1):60–62. [PubMed] [Google Scholar]
- 13.Prochaska JJ, Gill P, Hall SM. Treatment of tobacco use in an inpatient psychiatric setting. Psychiatr Serv. 2004;55(11):1265–1270. doi: 10.1176/appi.ps.55.11.1265. http://dx.doi.org/10.1176/appi.ps.55.11.1265. [DOI] [PubMed] [Google Scholar]
- 14.Gaskin DJ, Hadley J. Population characteristics of markets of safety net and non-safety net hospitals. J Urban Health. 1999;76(3):351–370. doi: 10.1007/BF02345673. http://dx.doi.org/10.1007/BF02345673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley WT, Stevens VJ, Zhu S-H, Morgan G, Grossman D. Overview of the consortium of hospitals advancing research on tobacco (CHART) Trials. 2012;13:122. doi: 10.1186/1745-6215-13-122. http://dx.doi.org/10.1186/1745-6215-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman E, Shelley D, Braithwaite RS, et al. Effectiveness of smoking-cessation interventions for urban hospital patients: study protocol for a randomized controlled trial. Trials. 2012;13:126. doi: 10.1186/1745-6215-13-126. http://dx.doi.org/10.1186/1745-6215-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski L, Frecker RC, et al. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoker per day. Br J Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 18.Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychol. 2007;26:278–287. doi: 10.1037/0278-6133.26.3.278. http://dx.doi.org/10.1037/0278-6133.26.3.278. [DOI] [PubMed] [Google Scholar]
- 19.California Tobacco Surveys. La Jolla: University of California, San Diego; 1999. [Accessed February 2010]. http://ssdc.ucsd.edu/tobacco. [Google Scholar]
- 20.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. http://dx.doi.org/10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 21.Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med. 2008;23(6):781–787. doi: 10.1007/s11606-008-0594-0. http://dx.doi.org/10.1007/s11606-008-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. http://dx.doi.org/10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 23.Rubak S, Sandboek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305–312. [PMC free article] [PubMed] [Google Scholar]
- 24.D'Zurilla TJ, Nezu AM. Problem solving therapy: A social competence approach to clinical intervention. 2nd. New York, NY: Springer; 1999. [Google Scholar]
- 25.Zhu SH, Pierce JP. A New Scheduling Method for Time-Limited Counseling. Prof Psychol Res Pr. 1995;26(6):624–625. http://dx.doi.org/10.1037/0735-7028.26.6.624. [Google Scholar]
- 26.Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. DHHS, Public Health Service; 2008. [Google Scholar]
- 27.Rogers ES, Smelson DA, Gillespie CC, et al. Telephone Smoking-Cessation Counseling for Smokers in Mental Health Clinics: A Patient-Randomized Controlled Trial. Am J Prev Med. 2016;50(4):518–527. doi: 10.1016/j.amepre.2015.10.004. http://dx.doi.org/10.1016/j.amepre.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Riley WT, Stevens VJ, Zhu S-H, Morgan G, Grossman D. Overview of the consortium of hospitals advancing research on tobacco (chart) Trials. 2012;13:122. doi: 10.1186/1745-6215-13-122. http://dx.doi.org/10.1186/1745-6215-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North American Quitline Consortium. Measuring Quit Rates. [Accessed February 8, 2014];2009 www.naquitline.org. [Google Scholar]
- 30.Gupta S. Intention-to-treat concept: A review. Biostatistics. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. http://dx.doi.org/10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lexicomp. Nicotine (Lexi-Drugs) [Accessed November 25, 2015];2015 http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7365?hl=6650. [Google Scholar]
- 32.U.S. Bureau of Labor Statistics. Occupational Employment Statistics: May 2014 National Occupational Employment and Wage Estimates United States. [Accessed November 14, 2015];2014 www.bls.gov/oes/current/oes_nat.htm.
- 33.U.S. Bureau of Labor Statistics. Employer Costs for Employee Compensation News Release. [Accessed November 25, 2015];2015 www.bls.gov/news.release/ecec.htm.
- 34.Briggs AH, O'Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. http://dx.doi.org/10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 35.Cost-effectiveness in health and medicine: a report to the U.S. Public Health Service. Washington, DC: U.S. Public Health Service. Office of Disease Prevention and Health Promotion; 1996. Panel on Cost-effectiveness in Health and Medicine U.S. [Google Scholar]
- 36.Ladapo JA, Jaffer FA, Weinstein MC, Froelicher E. Projected Cost-effectiveness of Smoking Cessation Interventions in Patients Hospitalized With Myocardial Infarction. Arch Intern Med. 2011;171(1):39–45. doi: 10.1001/archinternmed.2010.479. http://dx.doi.org/10.1001/archinternmed.2010.479. [DOI] [PubMed] [Google Scholar]
- 37.Rigotti NA, Regan S, Levy DE, et al. Sustained Care Intervention and Postdischarge Smoking Cessation Among Hospitalized Adults: A Randomized Clinical Trial. JAMA. 2014;312(7):719–728. doi: 10.1001/jama.2014.9237. http://dx.doi.org/10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy SA, Ronis DL, Titler MG, et al. Dissemination of the nurse-administered Tobacco Tactics intervention versus usual care in six Trinity community hospitals: study protocol for a comparative effectiveness trial. Trials. 2012;13:125. doi: 10.1186/1745-6215-13-125. http://dx.doi.org/10.1186/1745-6215-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. http://dx.doi.org/10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 40.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. DHHS Public Health Service; 2008. [Google Scholar]