Background
While meningoencephalitis has been described as an acute or late complication of Ebola Virus Disease (EVD)1–4, detailed physical examination and brain magnetic resonance imaging (MRI) findings have not been previously reported. Here we report findings in a patient with severe EVD complicated by meningoencephalitis and multi-organ failure managed with supportive care only.
Case Report
A 34 year-old male healthcare worker in Sierra Leone was evacuated on day 7 of documented EVD to the National Institutes of Health (NIH) Clinical Research Center. Upon arrival at the NIH, he had no discrete cognitive abnormalities. Figure 1 describes his clinical course.
Figure 1.
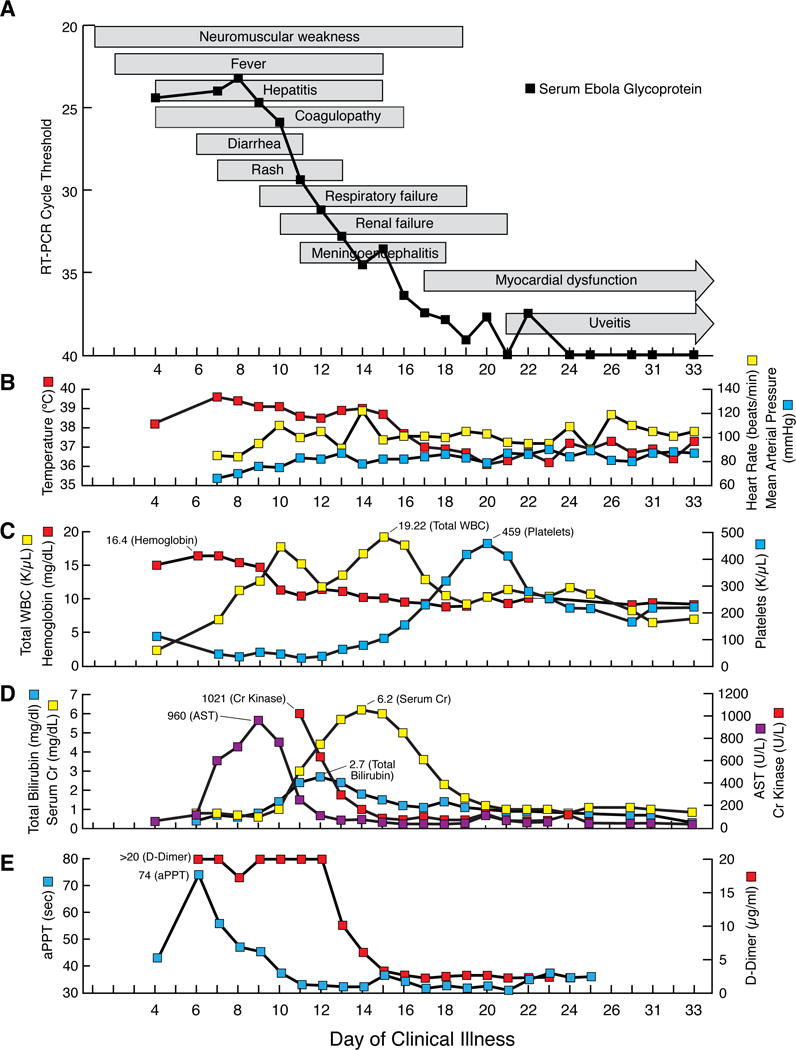
Sequential multi-organ failure in a patient with Ebola virus disease despite adequate blood pressure control: (A–E) Clinical course (A) text-boxes show approximate onset and resolution of clinical findings. Line-graph shows cycle threshold value that Ebola virus glycoprotein RNA was detected by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) in serum. Select vital signs (B) red line-graph shows maximum daily temperature, yellow line-graph shows maximum daily heart rate, and blue line-graph shows minimum daily mean arterial blood pressure. Select laboratory values (C–E). Complete blood count (C) red line-graph show hemoglobin level, yellow line-graph shows total white blood cell (WBC) count, and blue line-graph shows platelet count. Serum chemistries (D) purple line-graph shows aspartate aminotransferase (AST) level, blue line-graph shows total bilirubin level, red line-graph shows creatinine kinase level, and yellow line-graph shows serum creatinine (Cr) level. Coagulation (E) blue line-graph shows activated partial thromboplastin (aPPT) level and red line-graph shows d-dimer level.
On admission the patient was unable to walk due to profound weakness. By day 8, he was unable to move his extremities against mild resistance, roll in bed unassisted, or lift his head. Passive limb movement induced stimulus sensitive myoclonus and skin hyperesthesia. He developed delirium in the absence of detected toxic or metabolic abnormalities. Severe neuromuscular weakness contributed to respiratory failure requiring intubation on day 9 and mechanical ventilation through day 19.
When sedation was lightened, he moved all extremities to painful stimuli. His eyes were deviated rightward and unable to cross midline with Doll’s eyes maneuver. He developed Kernig’s and Brudzinski’s signs, but maintained normal side-to-side head motion. He had hyperreflexia in upper and lower extremities with a Hoffman’s sign, hyperactive pectoralis reflex, patellar clonus, sustained clonus of the ankles, flexor or equivocal plantar reflexes, and frontal release signs.
He ambulated independently the day following extubation. Persistent mild deficits included conjugate gaze with decreased smooth pursuit, postural and voice tremor, frontal release signs, wide-based gait, and bilateral limb weakness (left greater than right). He manifested hypomania, hyperphagia, pressured speech, insomnia, decreased short-term memory, and difficulty concentrating for several days. On day 21 he complained of right eye pain and light sensitivity. Topical steroids were started empirically for uveitis.
On day 33, following resolution of viral shedding, magnetic resonance imaging (MRI) showed multiple punctate lesions in the cerebral white matter on T2-weighted fluid attenuated inversion recovery (FLAIR) sequences, most prominently in the corpus callosum (Figure 2A). Some of these lesions (Figure 2B), together with a lesion abutting the left lateral wall of the fourth ventricle (Figure 2C), showed restriction of diffusion, consistent with micro-vascular occlusion and ischemia. An additional lesion, hyperintense on T2-weighted scans and hypointense on T1-weighted scans, was noted in the right lateral column of the spinal cord at the T3 vertebral body level (Figure 2D). No hemorrhage on susceptibility-weighted imaging sequences was evident. Diffusion-weighted images showed areas with restriction. Ophthalmologic examination on day 33 confirmed bilateral anterior and posterior uveitis without vasculitis.
Figure 2.
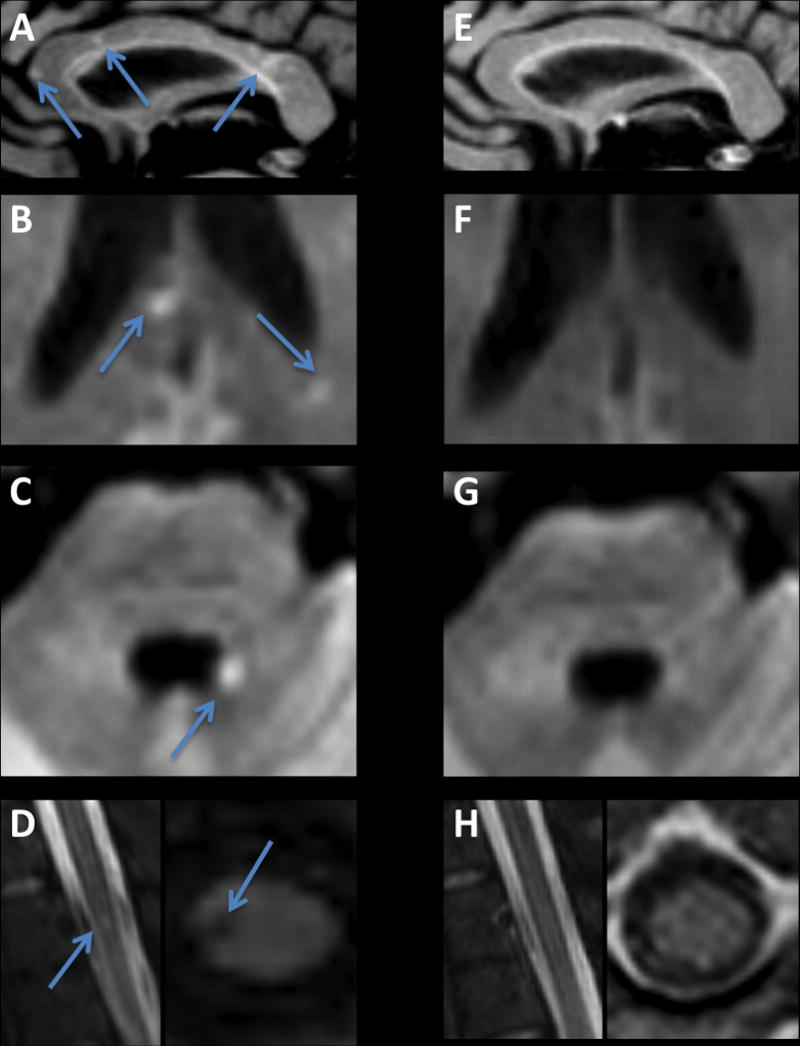
Brain MRI following recovery from Ebola meningoencephalitis: (A–D) Initial MRI, performed on day 33. (A) T2-weighted FLAIR image showing multiple punctate high signal intensity lesions in the corpus callosum. Some of the corpus callosum lesions (B), as well as a lesion on the margin of the fourth ventricle (C), showed restriction of diffusion, compatible with microvascular occlusion and ischemia. (D) A lesion in the right lateral column of the thoracic spinal cord. (E–H)
Seven months after presentation, neurologic examination showed near complete resolution of all abnormalities. Repeat MRI showed resolution of most of the previous lesions (Figure 2E–H). Ebola virus PCR testing on cerebral spinal fluid was negative. Neurocognitive assessment was normal, but the patient reported ongoing fatigue.
Discussion
Here we describe the clinical course and MRI findings of a case of EVD with sequential multi-organ failure including severe meningoencephalitis despite optimal maintenance of fluid and electrolyte balance. Profound neurological signs and symptoms indicated peripheral and CNS involvement. Generalized myopathy affected respiratory muscle function leading to respiratory failure. Florid meningeal signs suggested meningitis and prominent hyperreflexia and frontal release signs suggested encephalitis. Focal neurological signs with eye deviation and cerebellar signs were consistent with microvascular disease noted on MRI. Sensory symptoms were likely attributable to mild peripheral neuropathy, and hyperphagia and insomnia suggested hypothalamic dysfunction.
While histopathologic examination of the central nervous system in fatal EVD is unavailable, findings from two fatal cases of Marburg disease complicated by subacute encephalitis showed panencephalitis with glial nodules and perivascular infiltration5, 6. Our patient’s clinical course and MRI findings are consistent with those observations. Nonhuman primate studies of EVD do not report CNS involvement in animals that die on average eight days post-infection, but glial nodules or meningoencephalitis were observed in five of six animals surviving 21 days following experimental therapies7. Glial nodules stained positive for viral antigen suggesting microglial cells as a possible reservoir for virus in the brain. Meningoencephalitis reported as a late complication of EVD8 also suggests the CNS as a potential site for viral persistence.
In summary, we present a case of EBV characterized by severe sequential multi-organ failure including meningoencephalitis despite absence of hypotension. Recovery occurred with supportive care alone.
Acknowledgments
The successful care of this patient is attributed to the efforts of the multidisciplinary team at the NIH Clinical Center. This team was composed of volunteers and included SCSU and critical care nurses, respiratory therapists, a radiology technician, laboratory technicians, a pharmacist, a dietician, hospital epidemiologists, critical care and infectious diseases physicians, and “Watsans” from diverse backgrounds. To these individuals we express our gratitude.
References
- 1.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa–clinical manifestations and management. N Engl. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Ebola situation report-21 October 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-21-october-2015 (Accessed on October 23, 2015)
- 3.Edwards JK, Kleine C, Munster V, et al. Interpretation of Negative Molecular Test Results in Patients With Suspected or Confirmed Ebola Virus Disease: Report of Two Cases. Open Forum Infect Dis. 2015;2:ofv137. doi: 10.1093/ofid/ofv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlett P, Brown C, Helderman T, Brooks T, Lisk D, Deen G, et al. Ebola virus disease complicated by late-onset encephalitis and polyarthritis, Sierra Leone [letter] Emerg Infect Dis. 2016 Jan; doi: 10.3201/eid2201.151212. (Cited November 11, 2015). http://dx.doi.org/10.3201/eid2201.151212. [DOI] [PMC free article] [PubMed]
- 5.Bechtelsheimer H, Korb G, Gedigk P. The “Marburg-virus”-hepatitis. Studies in man and guinea pigs. Virchows Arch A Pathol Pathol Anat. 1970;351:273–90. [PubMed] [Google Scholar]
- 6.Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes. Curr Top Microbiol Immunol. 1999;235:49–75. doi: 10.1007/978-3-642-59949-1_4. [DOI] [PubMed] [Google Scholar]
- 7.Larsen T, Stevens EL, Davis KJ, et al. Pathologic findings associated with delayed death in nonhuman primates experimentally infected with Zaire Ebola virus. J Infect Dis. 2007;196(Suppl 2):S323–8. doi: 10.1086/520589. [DOI] [PubMed] [Google Scholar]


