Abstract
We used an extracellular pathogen Klebsiella pneumoniae to determine the role of NLRP12 since this bacterium is associated with devastating pulmonary infections. We found human myeloid cells (neutrophils and macrophages) and non-myeloid cells (epithelial cells) show upregulation of NLRP12 in human pneumonic lungs. NLRP12 silenced human macrophages and murine Nlrp12−/− macrophages displayed reduced activation of NF-κB and MAPK and expression of HDACs following K. pneumoniae infection. NLRP12 is important for the production of IL-1β in human and murine macrophages following K. pneumoniae infection. Furthermore, host survival, bacterial clearance and neutrophil recruitment are dependent on NLRP12 following K. pneumoniae infection. Using bone marrow chimeras, we showed that hematopoietic cell driven NLRP12 signaling predominantly contributes to host defense against K. pneumoniae. Intratracheal administration of either IL-17A+ CD4 T cells or CXCL1+ macrophages rescues host survival, bacterial clearance, and neutrophil recruitment in Nlrp12−/− mice following K. pneumoniae infection. These novel findings reveal the critical role of NLRP12-IL-17A-CXCL1 axis in host defense via modulating neutrophil recruitment against this extracellular pathogen.
Introduction
Lower respiratory tract infections remain the most significant cause of worldwide infectious disease morbidity and health care costs 1. The Gram-negative extracellular bacterium, Klebsiella pneumoniae, induces lung destruction and multiple abscesses in the lung even with small inoculums. In the recent years, the extensive spread of multi-drug resistant K. pneumoniae strains has caused ≥50% mortality in the U.S. and world 2, 3.
Although over 20 members of the NLR family have been identified, the function of most of their members in contributing to host resistance against microbial infection has not been determined. Nevertheless, reports suggest a function of some NLRs or inflammasomes in sensing bacterial pathogens 4, 5. Regarding K. pneumoniae infection, thus far, only two types of inflammasomes have been reported to regulate host immunity to this aflagellated bacterium: NLR family pyrin domain containing 3 (NLRP3) 6 and NLRC4 inflammasomes 7, 8.
NLRP12 (aka NALP12/MONARCH-1/PYPAF-7) was shown as the first NLR to induce IL-1β maturation via the interaction with ASC 9(. Recent studies suggest that the NLRP12 inflammasome plays a role in intestinal homeostasis 10, 11 and tumorigenesis 11, 12. Regarding the role of NLRP12 in bacterial recognition, a recent report has shown that NLRP12 is important to contribute to antibacterial defense against Yesinia pestis following subcutaneous or intravenous challenge 13. The results also show that NLRP12-deficient (Nlrp12−/−) animals had reduced survival and enhanced bacterial burden in the spleen, along with attenuated production of IL-18, IL-1β, and IFN-γ after Y. pestis infection 13. In another study using a very high dose of K. pneumoniae, results show reduced macrophage and lymphocyte influx and attenuated TNF-α levels in the lungs following the infection although neutrophil influx and bacterial clearance were not different between WT and Nlrp12−/− mice 7.
The goal of our current investigation was to delineate the unique role of NLRP12 by invoking innate immunity against K. pneumoniae by using a human cell system and a mouse model of infection.
Results
NLRP12 expression is increased in human pneumonic lungs
To examine whether NLRP12 expression is increased in human lungs, we used lung sections from patients with pneumonia and lung injury due to bacterial infection using immunofluorescence for NLRP12 in pneumonic and uninjured (control) human lungs. Diffused NLRP12 staining was detected in pneumonic lungs whereas limited NLRP12 staining was observed in these cell types in the lung sections from control patients (Fig. 1). In particular, both myeloid cells [neutrophils (lipocalin+) and macrophages (CD68+)] and non-myeloid cells [alveolar type II epithelial cells (ProSPC+)] express NLRP12 in pneumonic/injured lungs (Fig. 1A). Furthermore, we used K. pneumoniae-infected mouse lungs to examine the expression of NLRP12 in neutrophils (Gr-1+), type II epithelial cells (Pro-SPC+) and macrohphages (F4/80+) (Fig 1B). Our findings together indicate that myeloid cells (neutrophils and macrophages) and non-myeloid cells (alveolar type II epithelial cells) show upregulation of NLRP12 in infected lungs.
Figure 1. A. NLRP12 expression is increased in myeloid cells (neutrophils and macrophages) and epithelial cells in the lung during ALI/pneumonia.
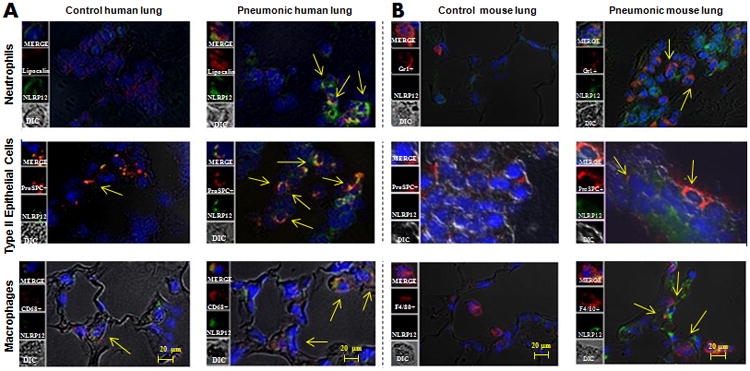
A. Immunofluorescence microscopy was performed for NLRP12 expression in normal human (control) lung tissue and human lung tissue from bacterial pneumonia. NLRP12 is indicated by green staining, neutrophils are shown by lipocalin staining, epithelial cells are shown by proSPC staining, whereas macrophages are shown by CD68 staining. B NLRP12 expression is enhanced in myeloid cells (neutrophils and macrophages) and epithelial cells in mouse lungs during pneumonia. NLRP12 is indicated by green staining, neutrophils are shown by Gr1 staining, epithelial cells are stained by proSPC staining and macrophages are shown by F4/80 staining. This is a representative image of 5 sections with similar results. Original magnification × 200.
NLRP12 regulates proinflammatory cytokines/chemokines in human and mouse macrophages
To determine the function of NLRP12 in human cells, we measured cytokines/chemokines in human peripheral blood monocyte-derived macrophages, following NLRP12 siRNA knockdown, and in Nlrp12−/− alveolar macrophages, following the infection with the Gram-negative bacterium, K. pneumoniae. Expression of proinflammatory mediators is regulated by NF-κB and MAP Kinases14. In addition, Histone deacetylases (HDACs) constitute a family of enzymes that play important roles in the epigenetic regulation of gene expression 15. Treatment with the specific HDAC inhibitor (trichostatin A) is shown to be essential to induce cytokines in human alveolar epithelial cell line A549 response to bacterial infection16. In siRNA-transfected human monocyte-derived macrophages, we found reduced NF-κB activation and attenuated levels of IL-6, IL-1β, and IL-18 (Figs. 2A-B) 3 or 6 hours after K. pneumoniae infection (Fig. 2A-B). Moreover, we found, reduced HDAC 2, 3 and 5 upregulation and decreased activation of MAPKs (p38, ERK and JNK) in NLRP12 siRNA-transfected human cells (Fig. 2C-D). In a similar fashion in murine alveolar macrophages, we observed decreased cytokines (TNF-α, IL-6, and IL-1β) and neutrophil chemokine (CXCL2/MIP-2) in Nlrp12−/− cells, 3 and 6 h after K. pneumoniae infection (supplemental data; Figs. 1A-B). Attenuated NF-κB activation, reduced HDAC 1, 2 and 3 upregulation and decreased activation of MAPKs (p38, ERK and JNK) was also observed in Nlrp12−/− cells (supplemental data; Figs. 1C-D).
Figure 2. NLRP12 is important for NF-κB activation, cytokine/chemokine production, HDAC expression and MAPK activation in human macrophages following K. pneumoniae infection.
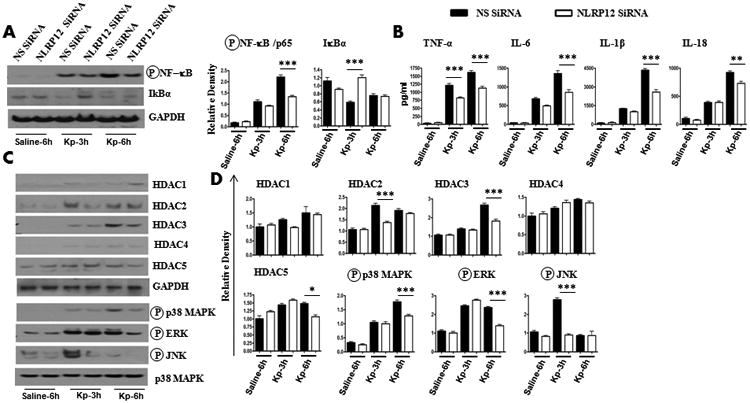
A-B. Human monocyte-derived macrophages (0.5 ×106) were transfected with 40 nM NLRP12 siRNA or scrambled siRNA (nonspecific; NS) for 48 h. Cells were then infected with 1 MOI of K. pneumoniae for 3 or 6 h and NF-κB activation was measured by western blotting (A), and cytokine/chemokine levels in supernatants (B) were measured by sandwich ELISA. Representative blots are shown from three independent experiments with identical results. Densitometry was performed using Gel Digitizing Software and normalized to GAPDH. C-D. Human macrophages were transfected with siRNA and then infected with K. pneumoniae in a similar manner, and cell lysates were used to determine HDAC expression and MAPK activation by western blotting. Representative blots are shown from three independent experiments with identical results. Densitometry was performed using Gel Digitizing Software and normalized to GAPDH. Means ± SE values were obtained from three separate experiments. NS, non-specific. *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
NLRP12 regulates host defense against K. pneumoniae infection
To assess the effect of NLRP12 activation in pulmonary defense during extracellular Gram-negative bacterial infection, WT mice and Nlrp12−/− mice were infected intratracheally (i.t.) with two (higher and lower) doses of K. pneumoniae (103 or 104 CFUs/mouse), and survival was monitored up to 15 days after the infection. The Nlrp12−/− mouse group displayed attenuated survival to both higher and lower infectious doses (Fig. 3A). To determine if reduced survival of Nlrp12−/− mice after K. pneumoniae infection is dependent on impaired bacterial clearance in the lungs or bacterial dissemination in Nlrp12−/− mice, K. pneumoniae CFU were quantified from whole lung and spleen at 24 and 48 h post-infection (lower dose). Nlrp12−/− mice demonstrated higher lung, liver and spleen CFUs as compared to their WT littermates at 48 hours (Fig. 3B).
Figure 3. NLRP12 modulates host defense against K. pneumoniae infection.
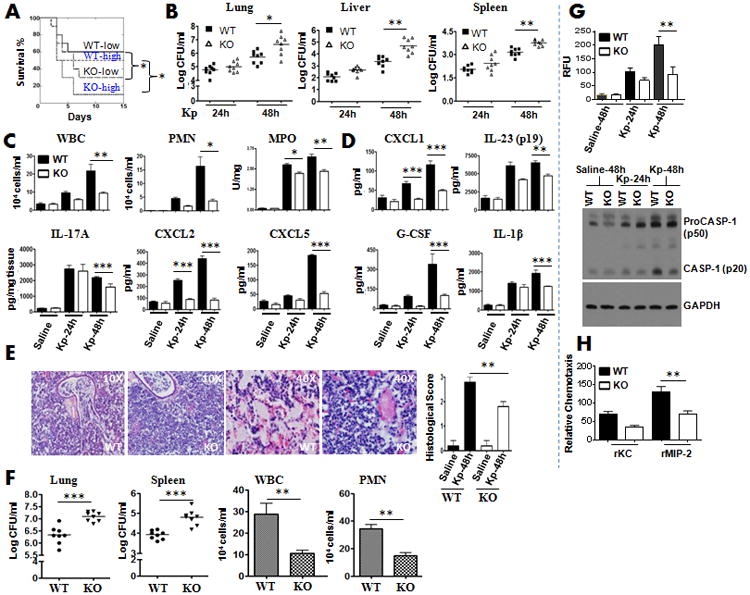
A. Nlrp12-/- and C57BL/6 (control) mice were inoculated i.t. with 103 or 104 CFUs of K. pneumoniae and mortality was monitored up to 15 days (*, p < 0.05 by log rank test). Data shown represent n = 20 mice/group from two representative independent experiments. B. Mice were infected with 1 × 103 CFUs of K. pneumoniae i.t. and lung and spleen homogenates were cultured at 24 h or 48 h for bacterial enumeration. Data shown represent mean parenchymal CFUs ± SE (*P < 0.05 comparing WT with Nlrp12-/- mice). C-D. Nlrp12-/- or C57BL/6 mice were exposed to 1 × 103 CFUs of K. pneumoniae i.t. bronchoalveolar lavage fluid (BALF) was collected and total white blood cells (C), neutrophils (C), parenchymal neutrophil influx (C) or cytokine/chemokine levels (D) were enumerated 24 h and 48 h after challenge (n = 7-10/group). E. Mice were inoculated with 1 × 103 CFUs of K. pneumoniae/mouse i.t. Lungs were obtained at 48 h post-infection and stained with H&E, and inflammatory changes in histological sections were scored. Shown are representative sections from four mice under each condition with identical results (magnification, ×200). Semiquantitative Inflammation score is a quantification of four lung sections in each group. F. Mice on an A/J background were infected with 1 × 103 CFUs of K. pneumoniae i.t. and lung and spleen homogenates were used to enumerate bacterial burden whereas BALF was used to enumerate neutrophil influx at 48 h post-infection. Data shown represent mean ± SE (n=7-9 mice/group). G. Mice were i.t. infected with 1 × 103 CFU of K. pneumoniae and lung homogenates were used to measure caspase-1 activity at 24 and 48 h post-infection (n=4-5 mice/group). Representative blots are shown from 4-5 mice with similar results. H. Migration of neutrophils toward KC and MIP-2. Neutrophil numbers in the lower chamber of a transwell were enumerated after 3 h of incubation. Data are a representation of 4 to 6 mice/group). *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
To identify mechanisms that contribute to higher bacterial CFUs in lungs and extrapulmonary organs, we assessed neutrophil recruitment to the airspaces following K. pneumoniae challenge because neutrophil influx is shown to be critical to clear K. pneumoniae infection in tissues 8, 17. Total WBC and neutrophil accumulation in the airspaces of Nlrp12−/− mice was attenuated at 48 hours compared with WT controls (Fig. 3C). Consistent with reduced neutrophil recruitment into the airspaces, we also observed reduced neutrophil influx in lung parenchyma (MPO activity in lung homogenates) of Nlrp12−/− mice as compared to WT (Fig. 3C). To determine if the decreased neutrophil influx is dependent on the production of cytokines/chemokines following K. pneumoniae infection, we measured the expression of cytokines (IL-23, IL-17A, IL-1β) and neutrophil chemoattractants (CXCL1/KC, CXCL2/MIP-2, and CXCL5/LIX) in bronchoalveolar lavage (BAL) fluid and lung homogenates 24 and 48 h following K. pneumoniae challenge (Fig. 3D). Intriguingly, IL-1β, IL-23, IL-17A and G-CSF levels in Nlrp12−/− mice in bronchoalveolar lavage fluid (BALF) or lung homogenates were attenuated at 48 h following the K. pneumoniae challenge (Fig. 3D).
Because multiple proinflammatory genes are regulated by NF-κB, HDAC and MAPKs 18, 19, we next investigated the activation of NF-κB, expression of HDACs and activation of MAPKs in the lungs of Nlrp12−/− mice following K. pneumoniae infection. As shown in supplemental data; Fig. 2A-C, NF-κB activation was reduced in Nlrp12−/− mice at 24 and 48 h following K. pneumoniae challenge. Additionally, Nlrp12−/− mice infected with K. pneumoniae exhibit reduced expression of HDAC2 (supplemental data; Fig. 2D-E) although no change in activation of p38, ERK, and JNK was observed at 24 and 48 h post-infection (supplemental data; 2F-G).
Using semiquantitative histology, WT mice showed severe suppurative bronchopneumonia, whereas Nlrp12−/− mice displayed moderate suppurative pneumonia 48 h after K. pneumoniae infection (Fig. 3E). In contrast, no significant cellular influx and alveolar edema was observed in saline-challenged (control) lungs obtained from either Nlrp12−/− or WT animals (data not shown). To demonstrate whether the effect of NLRP12 gene deficiency is mouse strain specific, we used WT and KO mice on an A/J background (after 10 generations of backcrossing) and we observed that infected Nlrp12−/− mice showed 1) more bacterial burden in the lungs and spleens; and 2) reduced neutrophil influx in BALF following the infection (Fig. 3F), suggesting that NLRP12 effects are not mouse background specific.
In prior studies, NLRP12 has been suggested to form inflammasomes in order to enhance caspase-1 activation and IL-1β and IL-18 maturation 9, 13. Therefore, despite measuring IL-1β levels in BALF following the infection, we evaluated caspase-1 activation in the infected lungs using fluorometry and western blotting. We detected a decrease in caspase-1 activity and caspase-1 cleavage in Nlrp12−/− lungs following the infection (Fig. 3G). Moreover, western blot results demonstrated that a significant amount of procaspase-1 was present in the lungs infected with K. pneumoniae (Fig. 3G).
In mice, ELR+ CXC chemokines, such as CXCL1/KC, CXCL2/MIP-2, and CXCL5/GCP-2/LIX are known neutrophil chemoattractants20. In a recent investigation, it has been shown that NLRP12 modulates dendritic cell (DC) and myeloid cell migration in a mouse model of contact hypersensitivity21. In this study, DCs and neutrophils obtained from the Nlrp12−/− mice show attenuated migration towards DC chemoattractants, such as CCL19, CCL21 and CXCL12 and neutrophil chemoattractant, such as CXCL1/KC. Our results also demonstrated attenuated neutrophil migration towards CXCL2/MIP-2, but reduced migration towards KC, which is not statistically significant (Fig. 3H).
NLRP12 mediates IL-17A differentiation of CD4+ T cells
The amount of IL-17A production is reduced in the lungs of Nlrp12−/− mice in response to K. pneumoniae (Fig. 4). Reduced number of IL-17 producing cells may be due to 1) attenuated recruitment from the bloodstream, and/or 2) augmented differentiation of Th0 cells to become Th17 cells locally. Therefore, we did an in vitro T cell differentiation assay to determine these possibilities. Our results show diminished differentiation of Th0 cells to Th17 and Th1 cells in Nlrp12−/− mice but no difference between CD4+ T cells of WT and Nlrp12−/− mice in Th2 differentiation (Fig. 4) highlighting the role of NLRP12 in Th17/Th1 differentiation.
Figure 4. Nlrp12-/- mice display attenuated Th17/Th1 differentiation of CD4 T cell population.
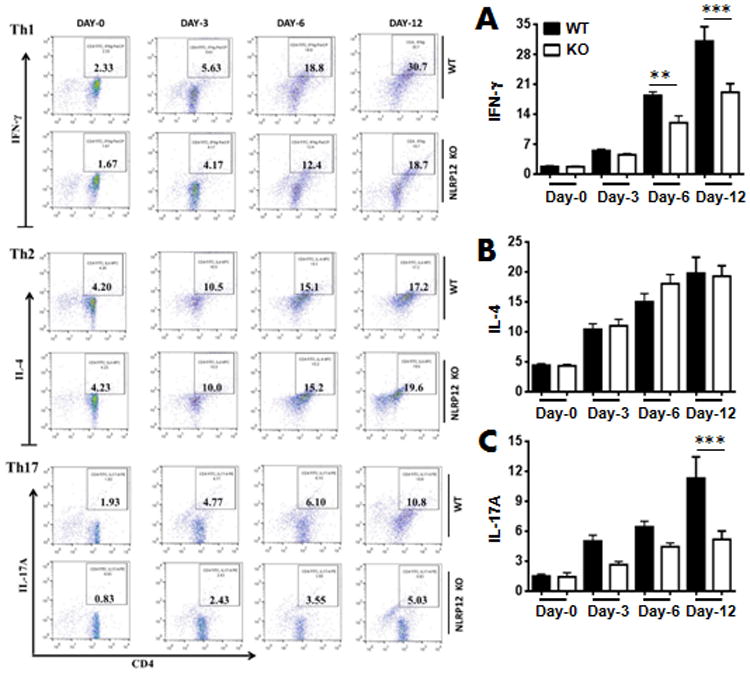
A-C right panel. Naive CD4+ T cells from WT and KO mice were isolated and stimulated with PMA as described in Materials and Methods and a representative dot blot has shown. A-C left panel. Quantitation of IL-17A producing CD4+ T cells from 3 independent experiments (n=5-8 mice/group). *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
Bone marrow-derived NLRP12 is important for host defense
Next, we asked if host defense against K. pneumoniae could be due to recruited bone marrow (hematopoietic) cells and/or resident alveolar cells. To address this hypothesis, WT or Nlrp12−/− mice were lethally-irradiated and reconstituted with bone marrow cells from donor WT or Nlrp12−/− mice to generate four groups: 1) WT mice reconstituted with WT marrow (WT→WT); 2) WT mice reconstituted with Nlrp12−/− marrow (Nlrp12−/− →WT); 3) Nlrp12−/− mice reconstituted with WT marrow (WT→ Nlrp12−/−); and 4) Nlrp12−/− mice reconstituted with Nlrp12−/− marrow (Nlrp12−/− → Nlrp12−/−). Eight weeks post-reconstitution, these bone marrow chimera mice were i.t. inoculated with K. pneumoniae, and bacterial burden in the lungs and spleens was determined. We found more bacterial burden in Nlrp12−/−→ Nlrp12−/− and Nlrp12−/−→WT chimera mice as compared with WT→WT or WT→ Nlrp12−/−chimera animals (Fig. 5A-B). Leukocye/neutrophil recruitment to the lung was attenuated in Nlrp12−/−→ Nlrp12−/− and Nlrp12−/−→WT chimera mice as compared to (WT→WT or WT → Nlrp12−/−chimera animals (Fig. 5C-D).
Figure 5. NLRP12-expressing bone marrow-derived cells are important to clear K. pneumoniae infection.
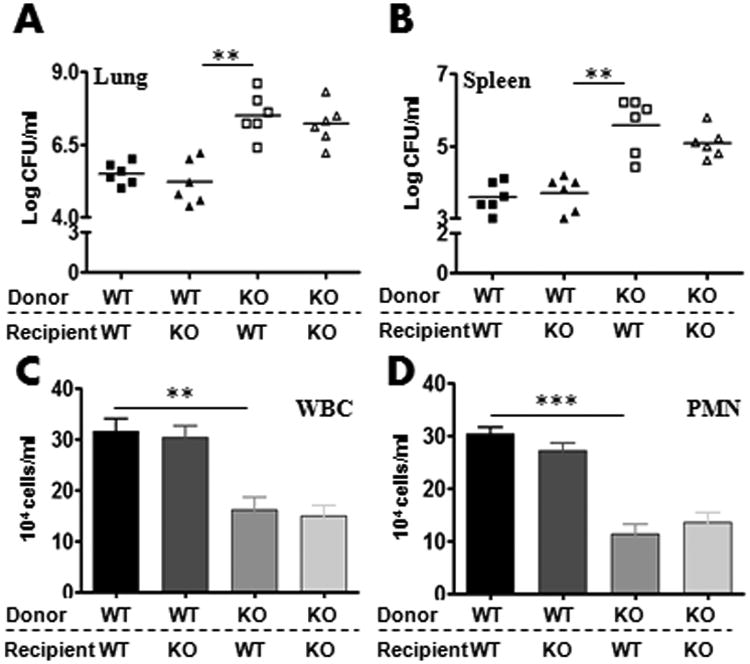
A-B. Bone marrow chimeras were generated with WT and NLRP12-/- mice. Mice were then infected with 1 × 103 CFUs of K. pneumoniae/mouse i.t. and bacterial burden in the lungs (A) and spleens (B) were assessed at 48 h post-infection (A-B). In another set of experiments, bronchoalveolar lavage fluid (BALF) was collected and total white blood cell (C) and neutrophil numbers (D) were enumerated 48 h after the infection (n = 5-8/group). *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
NLRP12 is dispensable for pyroptosis
NLRP12 has previously been shown to induce pyroptosis in response to flagellated bacterial infection, such as Burkholderia pseudomallei 22 and Salmonella typhimurium 23. Pyroptosis is induced by caspase-1 activation by proteolytic cleavage of caspase-1 24. However, annexin V stains not only pyroptotic cells but also apoptotic cells25. We detected pyroptosis and/or apoptosis using flow cytometry-based annexin V binding. As shown in Fig. 6A-B, pyroptosis or apoptosis in neutrophils (Ly6G+) or macrophages (EMR1+) in K. pneumoniae-infected lungs and spleens between WT and Nlrp12−/− mice was not dfifferent.
Figure 6. NLRP12 does not control pyroptosis in macrophages and neutrophils in the lung and spleen following K. pneumoniae infection.
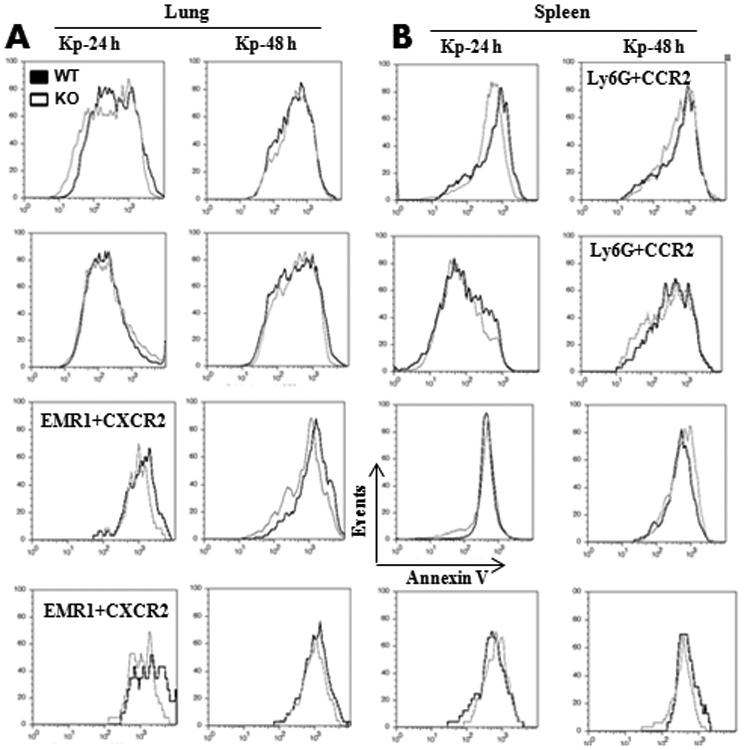
Flow cytometry was performed on CCR2+ or CXCR2+ neutrophils (Ly6G+) and macrophages (EMR1) obtained from lung and spleen homogenates at 24 and 48 h following K. pneumoniae infection as described in Materials and Methods. Representative plots from CCR2- or CXCR2-expressing neutrophils and macrophages from three independent experiments (n = 5 mice per group). Error bars represent SE.
Adoptive transfer of CD4+ T cells rescues host defense in NLRP12-/- mice
Our findings demonstrated that 1) IL-17A levels were reduced in Nlrp12−/− mice following K. pneumoniae infection (Figs. 3); and 2) CD4+ T cells from Nlrp12−/− mice show attenuated Th17 differentiation (Fig. 4). Studies unequivocally demonstrated that CD4+ T cells are an important source of IL-17A 26, 27. To examine if an increase in the number of T cells could rescue NLRP12 deficiency, we adoptively transferred 0.5 million IL-17+ CD4 T cells i.t. to Nlrp12−/− mice at the time of the infection. Transfer of mature wild-type (IL-17+/+) CD4+ T cells, but not transfer of Il-17-/- CD4+ T cells, rescued survival, bacterial load in the lungs and spleen, neutrophil influx and cytokine/chemokine expression in lungs (Fig. 7A-D).
Figure 7. IL-17A producing CD4+ T cells rescues survival, bacterial clearance, cellular recruitment and cytokine expression following K. pneumonia infection.
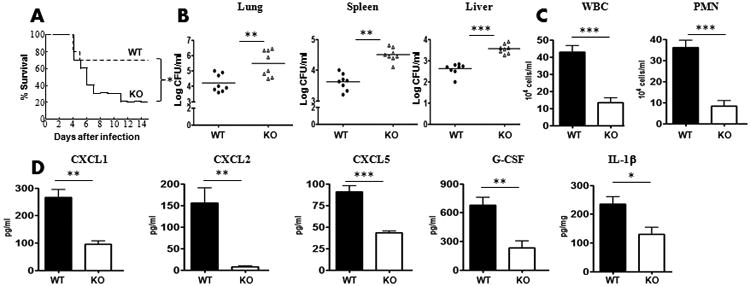
A-B. Nlrp12−/− mice administered with splenic CD4+ T cells (0.5 ×106/mouse) cells i.t. at the time of infection. Survival (A), Bacterial CFUs in the lung (B), spleen (B) and liver (B) at 48 h post-K. pneumoniae infection were enumerated. (n=8 mice/group). C-D. Leukocyte/neutrophil influx (C) and cytokine expression (D) in BALF or lung homogenates was measured at 48 h post-infection (For survival, n=20 mice/group whereas for other experiments n=6-9 mice/group). *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
Adoptive transfer of CXCL1+ macrophages restores host resistance in NLRP12-/- mice to K. pneumonia
Our data showed that CXCL1, a neutrophil chemoattractant, levels were attenuated in Nlrp12−/− mice after K. pneumoniae infection (Fig. 3). We have shown that that macrophages are an important source of CXCL1 (supplemental data; Fig. 1A). To examine if an increase in the number of CXCL1+ (WT) macrophages could rescue NLRP12 deficiency, we adoptively transferred 0.5 × 106 bone marrow macrophages i.t. to Nlrp12−/− mice at the time of the infection. Transfer of mature WT, but not transfer of Cxcl1-/- macrophages, rescued survival, bacterial burden in the lungs and spleen, neutrophil influx and cytokine/chemokine expression in lungs (Fig. 8A-D).
Figure 8. CXCL1 producing macrophages restores survival, bacterial clearance, leukocyte recruitment and cytokine production in response to K. pneumoniae infection.
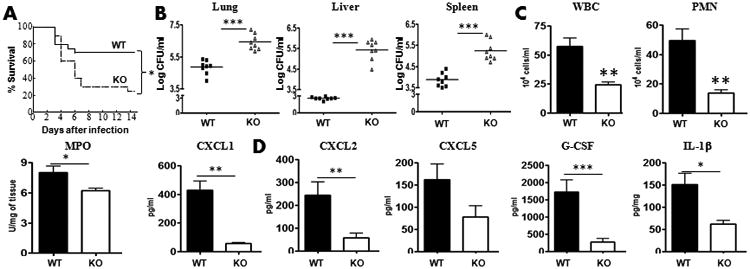
A-B. Nlrp12−/− mice administered with WT or KO bone marrow macrophages (0.5 ×106/mouse) i.t. at the time of the infection. At 48 h post-K. pneumoniae infection, survival (A), Bacterial CFUs in the lung (B), spleen (B) and liver (B) were enumerated (n=8 mice/group). C-D. Leukocyte/neutrophil recruitment (C) and cytokine production (D) in BALF or lung homogenates was measured at 48 h post-infection (For survival, n=20 mice/group whereas for other experiments n=6-10 mice/group). *, p<0.05; **, p<0.01;***, p<0.001. Error bars represent SE.
Discussion
In humans, mutations in gene encoding NLRP12 are linked to skin rashes, fevers, and joint pains that are similar to what is seen in patients with Familial Cold Autoinflammatory Syndrome (FCAS) 28, 29. These mutations seem to decrease the ability of NLRP12 protein to attenuate the inflammation, leading to the intermittent episodes of fever since these mutations may lead to increased caspase-1 activity 30, 31. The host defenses of the human lung include successful elimination of the microbes by resident alveolar macrophages 32, 33. Any defects in host defense functions in macrophages ultimately lead to infectious complications in the host by excessive bacterial colonization. Our findings conclude that NLRP12 in human macrophages has an important host defense function against a non-flagellated bacterium. This was unexpected and represents the first report of NLRP12-dependent protein induction in these unique human cells in response to K. pneumoniae infection.
In mice, both proinflammatory and anti-inflammatory functions of NLRP12 have been demonstrated 9, 11, 12. Initial findings indicate that NLRP12 regulates dendritic cell (DC) and neutrophil migration both in vitro and in vivo: (a) Isolated bone marrow dendritic cells obtained from Nlrp12−/− mice show decreased migration toward CCR7 and CXCR4 ligands; (b) the migration of Nlrp12−/− bone marrow neutrophils to CXCL1/KC was also attenuated by ∼50% in vitro; and (c) experiments also show that Nlrp12−/− DCs display a significantly reduced capacity to migrate to draining lymph nodes in a mouse model of contact hypersensitivity 21. Regarding investigations dealing with bacterial pathogens, findings reveal that NLRP12 contributes to host defense against Yesinia pestis in a mouse model of systemic (s.c and i.p) infection 13. Nevertheless, only a single report has addressed the role of NLRP12 in host resistance to infectious agents via the intrapulmonary route 7. Using a very high inoculum of K. pneumoniae (74,000 CFU/mouse), the authors show reduced macrophage and lymphocyte influx and attenuated TNF-α levels in the lungs following the infection although neutrophil influx and bacterial clearance were not different between WT (control) and Nlrp12−/− mice. However, we observed that host survival, bacterial clearance and neutrophil recruitment to the lungs are dependent on NLRP12 following K. pneumoniae infection. The discrepancy between the published study and our study could be explained by the fact that 1) both studies used different K. pneumoniae strains; 2) Allen et al. have used an extremely high dose (74,000 CFUs/mouse) and we used a low dose (1,000 CFUs/mouse) in all experiments, but used both lower and higher doses of K. pneumoniae (1000 and 10,000 CFUs/mouse) for survival experiments.
The current study is the first to demonstrate a role for NLRP12 in host resistance against both extracellular pulmonary bacterial pathogen. Redundant roles for inflammasomes may occur for optimal innate immune responses against bacteria. Thus far, two other inflammasomes have been implicated in the regulation of host immunity to K. pneumoniae: NLR family pyrin domain containing 3 (NLRP3) 6 and NLR family CARD domain containing 4 (NLRC4 or IPAF) 8 . The involvement of multiple inflammasomes for host defense against K. pneumoniae supports the emerging concept and relates to the cooperative interactions between different inflammasomes during bacterial infection in the host. Although cooperative interactions have not been explored in the lungs, interaction among different inflammasomes has been demonstrated in the gut 34, 35. Future studies using double- or triple-KO mice should explore these interactions in more detail.
It has been demonstrated that neutrophil influx is a critical event to clear K. pneumoniae in the lungs as neutrophil depletion prior to the infection enhanced susceptibility to the infection 8. Here, we demonstrate that deletion of NLRP12 leads to augmented susceptibility to intrapulmonary K. pneumoniae infection. Additional data illustrate that NLRP12 inhibits bacterial colonization in the lungs and dissemination of K. pneumoniae. This increase CFUs correlates with decreased neutrophil influx and the production of neutrophil chemoattractants, such as KC, MIP-2, or LIX in the lungs. This is also the first demonstration of an important role for NLRP12 in host resistance against pulmonary infection. Although attenuated production of neutrophil chemoattractants have contributed to reduced neutrophil influx in the lungs, our results also reveal that NLRP12-/- neutrophils have an inherent defect towards migrating neutrophil chemoattractants, such as CXCL2/MIP-2. Consistent with this speculation, a previous study shows that the migration of Nlrp12−/− bone marrow neutrophils towards CXCL1/KC was reduced by ∼50% compared with the neutrophils from control mice 21.
While hematopoietic cells in the lung produce several neutrophil chemokines − including KC 36 and MIP-2 37 − resident cells, such as alveolar epithelial type II cells, produce other neutrophil chemoattractants, such as LIX 38. We show that a requirement for NLRP12 signaling predominantly via hematopoietic cells for bacterial clearance and neutrophil accumulation in the lungs. Our findings are also consistent with earlier reports of the role of either hematopoietic cells or resident cells in infectious or noninfectious lung inflammation: (a) MyD88 derived from hematopoietic cells is more important for LPS-induced expression of TNF-α and IL-12p40 39, although both hematopoietic and resident cell-derived MyD88 signaling are essential for LPS-induced neutrophil influx 40, 41; (b) MD-2 signaling in both hematopoietic and resident cells is essential for neutrophil-mediated inflammation, and the expression of MIP-2, TNF-α, and IL-6 is mediated by both cell types in the lungs after LPS challenge 42; and (c) CXCL1/KC produced by both hematopoietic and resident cells is important for bacterial clearance and neutrophil recruitment to the lung upon K. pneumoniae infection 43. Since both hematopoietic cells and resident cells show upregulated expression of NLRP12, these results may lead to the prediction that both cell types to contribute to host defense. However, our results highlight the contribution of hematopoietic cell driven NLRP12 in host defense. Therefore, the inducible expression levels of NLRP12 in hematopoietic cells versus stromal cells in the lungs following the infection and cell-type specific responses following K. pneumoniae infection need to be determined by future studies to resolve this discrepancy.
A rapid immune response is critical to augmenting host defense. The innate immune system is crucial to rapidly detect infection. The emerging role of inflammasomes as mediators of innate immunity positions them as therapeutic targets. One of the known inflammasome activators, alum, is in widespread use as vaccine adjuvants in humans. The finding that inhibition of NLRP12 can paralyze pulmonary defense could have a deep impact on the strategies to treating and/or preventing bacterial pneumonias.
Methods
Immunohistochemistry
For immunofluorescence staining human lung sections from lung without evidence of infection, injury or other diseases (n=3) or from patients who died off bacterial infection with ALI/ARDs were obtained from a commercial source (Biochain, CA). These sections were analyzed for NLRP12 immunostaining. Briefly, deparaffinized fixed lung sections were permeabilized with the buffer containing Triton X-100 (0.1%) and then blocked with serum. Lung sections were incubated with anti-NLRP12 (ABGENT) and surface markers including anti-lipocalin Ab for PMNs (R&D), anti-proSPC Ab for type II epithelial cells (Millipore; US Biological) or anti-CD68 Ab for macrophages (BioLegend). For mouse lung sections, we used anti-Gr1 for PMNs (US Biological), anti-proSPC for type II epithelial cells or anti-F4/80 (Biolegend) as surface markers along with anti-NLRP12 Ab (ABGENT). Sections were washed and incubated with Alexa-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Tissue sections were washed and mounted using Vectashield mounting medium (Vector Laboratories, Inc., CA 94010) containing DAPI stain for nuclear staining. Images were acquired using an Axiocam digital camera (Zeiss, Thornwood, NY) connected to a Zeiss Axioskop 2 Plus research microscope.
Human macrophages
Frozen human peripheral blood mononuclear cells were obtained from Astarte Biologics (Redmond, WA) and were used as described in our previous publication 8. For monocyte/macrophage differentiation, monocytes were cultured on plates for up to 7 days in RPMI 1640, containing 5% FBS, 1% penicillin-streptomycin, and 100 ng/mL M-CSF. For knockdown experiments, a pre-validated pool of siRNA (a cocktail of 4 siRNAs) for human NLRP12 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cells (0.5×106) were transfected with either 40 nM siRNA or a control siRNA (Santa Cruz Biotechnology Inc) using TransIT-TKO Transfection Reagent from MIRUS (Madison, WI) for 48 hours. Cells were then infected with 1 MOI of K. pneumoniae for 6 hours. For cytokine/chemokine assays, supernatants were collected 24 hours post-infection. For immunoblotting experiments, macrophages were washed 3 times with PBS before lysing with Urea/Chaps/Tris buffer supplemented with protease and phosphatase inhibitors.
Mouse macrophages
All mice were on C57BL/6 background were bred in specific pathogen–free rooms within animal facilities at the Louisiana State University (LSU). Controls for each experiment were gender and age matched. Murine alveolar macrophages were isolated from BALF fluid from WT or Nlrp12-/- mice as previously described 43, 44. Mice were anesthetized and then sacrificed by cardiac exsanguination. Lungs were lavaged with 0.8 mL sterile saline each time through an intratracheal catheter as described previously 43, 45, and a total of 8 mL saline was instilled and recovered from each mouse. The lavage fluid was spun at 300×g for 10 minutes to pellet alveolar macrophages. Cells were cultured in 12-well culture plates at 37°C with 5% CO2 at a concentration of 0.5×106 cells per well in 1 ml RPMI 1640 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% FBS, 1 mM pyruvate, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. After 2 hours of incubation, non-adherent cells were removed with phosphate buffered saline (PBS), and the medium was replaced. Cells were then infected with 1 multiplicity of infection (MOI) of K. pneumoniae (ATCC 43816) for designated time points. For cytokine studies, media was collected at 3 and 6 hours following the infection. For western blotting, cells were washed three times with PBS and lysed with Urea/Chaps/Tris buffer containing protease and phosphatase inhibitors.
Mice
8- to-10-week-old female mice, genetically deficient for NLRP12 (Nlrp12-/-) 46 or age and gendet-matched WT mice weighing 22-26 grams, were used for in vivo experiments. Nlrp12-/- mice were backcrossed 10 times with age-matched C57Bl/6 or A/J mice. Mice were kept on 12:12 hour light:dark cycle with free access to food and water. All animal experiments were approved by the Louisiana State University IACUC.
Pneumonia model
K. pneumoniae serotype 2 strain (ATCC 43816) was used for a intrapulmonary challenge as described earlier 17, 43, 45. The bacteria were grown for 8 hours at 37°C in 50 mL tryptic soy broth (TSB) with shaking at 225 rpm. Bacteria were harvested by centrifuging the culture at 1200×g for 2 min and washed twice in sterile saline. The cells were resuspended in an isotonic saline at a concentration of 103 CFUs/50 μL/mouse. After anesthesia, K. pneumoniae suspension (103 CFUs in 50 μL) in 0.9% saline was inoculated via the intratracheal (intrapulmonary) route. The CFUs were enumerated by serially diluting the suspension of initial inoculums and subsequently plating 20 μL aliquots of each dilution onto a tryptic soy agar (TSA) plate and a MacConkey agar plate. Similarly, for counting bacterial CFUs in lungs and spleen, tissues were homogenized in PBS for 15 and 30 sec respectively, and 20 μL of homogenates were plated in 10-fold serial dilutions onto TSA and MacConkey agar plates. The survival of NLRP12-/- and WT mice was monitored for up to 15 days following inoculation with K. pneumoniae. For adoptive transfer experiments, 0.5 ×106 IL-17A+/IL-17A- CD4+ T cells or CXCL1+/CXCL1- bone marrow macrophages were i.t administered at the time of the infection.
BALF collection
The animals were euthanized, and the trachea was exposed and subsequently cannulated with a 20-gauge catheter as described earlier 43, 45. BAL fluid was collected 4 times by instilling 0.8 mL of PBS containing heparin and dextrose. Total leukocytes in BAL fluid were enumerated by counting on a hemocytometer, whereas BAL differential leukocyte cell counts were determined by standard light microscopy. The remaining (2 mL) of the undiluted cell-free bronchoalveolar lavage fluid (BALF) was passed through a 0.22 μm filter and used for the determination of cytokine/chemokine levels.
Lung isolation
Following the infection, the whole (non-lavaged) lungs were excised and snap frozen. For long-term storage, these lung tissues were stored at -70°C and used for cytokine/chemokine determination, western blots, and MPO activity assay. Lung tissue was briefly homogenized in 2 mL PBS supplemented with 0.1% triton X-100 and complete protease inhibitor (1 tablet/50 mL media), and the resulting homogenates were centrifuged at 12,000×g/20 min. The supernatants were harvested, passed through a 0.22 μm filter, and used as required.
MPO activity
The lung homogenates were resuspended in 50 mM potassium phosphate buffer (pH 6.0) supplemented with 0.5% hexadecyltrimethylammonium bromide (HTAB), as described in previous publications 17, 38, 42, 43, 45. Samples were then sonicated, incubated at 60°C for 2 hours, and assayed for MPO activity in a hydrogen peroxide/O-dianisidine buffer at 460 nm. Absorbance was measured at 460 nm using a spectrophotometer. The increase activity was calculated between 0 sec and 90 sec.
Cytokine/chemokine determination
Cytokines/chemokines were determined by sandwich ELISA as described earlier 17, 42, 43, 45. The minimum detection limit of the assay was 2 pg/mL of protein. For mouse lungs, TNF-α, IL-6, LIX, MIP-2, IL-23, IL-17A, IL-1β, and IL-18 concentrations were normalized to the total protein concentration in the samples measured. Results are expressed as pg/mg of total protein for lung tissue and in pg/mL for BALF.
Semiquantitative histology
The lungs were perfused from the right ventricle of heart with an isotonic saline, 24 and 48 hours post-infection, and harvested. For hematoxylin and eosin staining, lungs were fixed in 4% phosphate-buffered formalin, processed in paraffin blocks, and cut into fine sections (5 μm in thickness). Semiquantative histology was performed by a Veterinary Pathologist in a blinded fashion according to the following scoring scale: 0, no inflammatory cells (macrophages or neutrophils) are present in section; 1, <5% of section is infiltrated by inflammatory cells; 2, 5–10% of section is infiltrated by inflammatory cells; and 3, >10% of section is infiltrated by inflammatory cells, as indicated in our earlier publications 45, 47.
Caspase-1 activation
(A) Fluorometry: Infected or control lungs were used to make single cell suspension. A total of 50 cells/well were used in a 96 well plate to measure caspase-1 activity according to manufacturer's recommendation (Biovision, CA). At the end of incubation, samples were measured at a 400-nm excitation filter and 505-nm emission. Increase in florescence activity was plotted as relative fluorescence units (RFU). (B). Western blotting: The lungs were harvested at the designated times and homogenized in 1 mL of phosphate buffered saline (PBS) containing 0.1% Triton X-100 supplemented with the cocktail of complete protease and phosphatase inhibitors as described in earlier publications48. Mouse anti-caspase-1 Ab (AdipoGen, CA) at a concentration of 1 μg/ml was used.
Chemotaxis
Neutrophil transmigration assay was performed in a Transwell system using 24-well tissue culture plates with a pore size of 3.0 μm as described in a prior publication49. Chemoattractants, either recombinant KC (1 μg/0.5 ml) or MIP-2 (1 μg/0.5ml), and phosphate-buffered saline (PBS) supplemented with bovine serum albumin (BSA; 2 μg/ml) was added to each of the lower wells in the chamber. A total of 0.1 × 106 LPS-activated neutrophils (PMNs) in Dulbecco modified Eagle medium (DMEM) supplemented with 0.1% BSA was added to every well of the upper chamber. Following incubation at at 37°C 5% CO2 for 3 h, the cells from 10 fields in the lower plate were counted using an inverted microscope. The number of PMNs in lower chamber is indicated as relative chemotaxis.
Th1/Th2/Th17 differentiation
Th1/Th2/Th17 differentiation has been performed as previously described 50, 51 . Cells were washed and resuspended in PBS followed by blocking with Fc receptor blocking reagent. Cells were surface stained with anti-CD4 and intracellular with anti- IFN-γ, -IL-4 or -IL-17A. Flowjo software was used for data analysis.
Bone marrow (BM) transplantation
BM chimera experiments were performed as described in earlier publications 17, 42, 43. Recipient groups were gamma irradiated from a cesium source in two 525-rad doses separated by 3 hours. BM was flushed from tibias and femurs from donor mice, and a total of 8 × 106 BM cells were injected into the tail veins of lethally irradiated recipient mice. Reconstituted mice were treated with 0.2% neomycin sulfate for the first 2 weeks post-transplantation. Bacterial challenge experiments were performed 8 weeks after BM reconstitution. In another set of experiments, we used donor cells expressing green fluorescent protein. Blood sample was collected from recipient mice at 6 and 8 weeks after reconstitution, and hematological parameters, such as total WBC counts and differential counts were measured. Using flow cytometry, we found that more than 75-85% of blood leukocytes were derived from donor marrow at the time the mice were used for experiments (6–8 weeks post-transplantation.
Pyroptosis or apotosis
Lung or spleen digests from C57BL/6 (WT) or Nlrp12−/− mice challenged with K. pneumoniae for 24 or 48 h were used to determine cells undergoing pyroptosis as outlined in previous publication 8. Briefly, lung and spleens cell suspensions were passed through a 0.70 μ filter. Following 2 PBS washings, cells were FcR blocked and aliquoted for surface staining with conjugated PerCP anti-mouse Gr-1/Ly6G or EMR1 and APC anti-mouse CCR2 or CXCR2. Red blood cells (RBCs) were lysed by adding NH4Cl lysing buffer. Cells were resuspended in 1× binding buffer containing 5 μl of Annexin V-FITC and 5 μl of PI according to the manufacturer's protocol (Annexin V apoptosis detection kit from BD Pharmingen). The cell suspension was vortexed and incubated for 15 mins in the dark at room temperature. A total of 100 μl 1× binding buffer was added, and cells were analyzed by flow cytometry. CCR2 or CXCR2 positive Gr-1/Ly6G (neutrophils) and EMR1 (macrophages) that were positive for Annexin V-FITC and negative for PI are shown in histograms.
Adoptive transfer of CD4+ T cells and macrophages
Splenic CD4+ T cells were isolated and made single cell suspensions. Cell suspensions were washed, RBCs were lysed and CD4+ T cells were isolated by negative selection from single cell suspension using the EasySep cell separation procedure (StemCell Technologies, Vancouver, Canada). Resulting cell preparations were resuspended to a final density of 0.5×106 cells per 50 μL PBS for i.t. administration. BM from femur and tibia was flushed and marrow was passed through a 21G needle 4-6 times to dissociate the cells. RBCs were lysed using 1X RBC lysis buffer. Cell suspension was washed with PBS twice and cells were resuspended in DMEM +5% FBS+P/S containing 2 million cells/ml with M-CSF 25 ng/ml and seeded for 6-7 days. Fresh BMDM growth medium was added on day 3 and 5, and the formation of mature BMDM was evaluated after 7 days using flow cytometry analysis to detect cells expressing CD11b and F4/80. Resulting cell preparations were resuspended to a final density of 0.5×106 cells per 50 μL PBS for i.t. administration.
Statistics
Data are expressed as mean ± SEM. ANOVA, followed by Bonferroni's post hoc analysis, was performed for comparisons among multiple groups. All statistical calculations were performed using InStat software and GraphPad Prizm 4.0 (GraphPad Sotware, La Jolla, CA). All experiments were performed 3 times, with the exception of the survival experiments, which were performed twice. The survival results were compared by Wilcoxon rank sign test. A p value *, p<0.05; **, p<0.01; ***, p<0.001 was considered significant.
Supplementary Material
Acknowledgments
Supported by Scientist Awards from the Flight Attendant Medical Research Institute (CIA and YCSA to SJ; YCSA to SC and SB); and grants from the NIH (R01 HL-091958 and R01 HL-091958S1 via ARRA to SJ and R15-1R15ES023151-01 to SB. The authors thank Millennium Pharmaceuticals for providing NLRP12-/- mice. We thank the Lung Biology members Mary Leissinger, Liliang Jin, Ritwij Kulkarni, Pankaj Baral, and K. Jeyagowri for helpful discussions.
Footnotes
The authors have no conflicts of interest.
References
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3(2):e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endimiani A, Depasquale JM, Forero S, Perez F, Hujer AM, Roberts-Pollack D, et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother. 2009;64(5):1102–1110. doi: 10.1093/jac/dkp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mataseje LF, Boyd DA, Willey BM, Prayitno N, Kreiswirth N, Gelosia A, et al. Plasmid comparison and molecular analysis of Klebsiella pneumoniae harbouring bla(KPC) from New York City and Toronto. J Antimicrob Chemother. 2011;66(6):1273–1277. doi: 10.1093/jac/dkr092. [DOI] [PubMed] [Google Scholar]
- 4.Ferrand J, Ferrero RL. Recognition of Extracellular Bacteria by NLRs and Its Role in the Development of Adaptive Immunity. Front Immunol. 2013;4:344. doi: 10.3389/fimmu.2013.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leissinger M, Kulkarni R, Zemans RL, Downey GP, Jeyaseelan S. Investigating the role of nucleotide-binding oligomerization domain-like receptors in bacterial lung infection. Am J Respir Crit Care Med. 2014;189(12):1461–1468. doi: 10.1164/rccm.201311-2103PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183(3):2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen IC, McElvania-TeKippe E, Wilson JE, Lich JD, Arthur JC, Sullivan JT, et al. Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PLoS One. 2013;8(4):e60842. doi: 10.1371/journal.pone.0060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 Inflammasome-Mediated Production of IL-1beta Modulates Mucosal Immunity in the Lung against Gram-Negative Bacterial Infection. J Immunol. 2012 doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277(33):29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 10.Chen GY. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur J Immunol. 2014;44(2):321–327. doi: 10.1002/eji.201344135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36(5):742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 15.Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012;4(1):5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmeck B, Lorenz J, N'Guessan PD, Opitz B, van Laak V, Zahlten J, et al. Histone acetylation and flagellin are essential for Legionella pneumophila-induced cytokine expression. J Immunol. 2008;181(2):940–947. doi: 10.4049/jimmunol.181.2.940. [DOI] [PubMed] [Google Scholar]
- 17.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188(7):3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32(7):335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Batra S, Balamayooran G, Sahoo MK. Nuclear Factor-kappaB: a Key Regulator in Health and Disease of Lungs. Arch Immunol Ther Exp (Warsz) 2011;59(5):335–351. doi: 10.1007/s00005-011-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2012;46(5):566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185(8):4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog. 2011;7(12):e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107(7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 25.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243(1):206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HC, Velichko S, Hung LY, Wu R. IL-17A and Th17 cells in lung inflammation: an update on the role of Th17 cell differentiation and IL-17R signaling in host defense against infection. Clin Dev Immunol. 2013;2013:267971. doi: 10.1155/2013/267971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol. 2007;16(8):692–698. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 29.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105(5):1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeru I, Le Borgne G, Cochet E, Hayrapetyan H, Duquesnoy P, Grateau G, et al. Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis Rheum. 2011;63(5):1459–1464. doi: 10.1002/art.30241. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Y, Kinio A, Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein E, Lippert W, Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 34.Nunes T, de Souza HS. Inflammasome in intestinal inflammation and cancer. Mediators Inflamm. 2013;2013:654963. doi: 10.1155/2013/654963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao LY, Liu X, Franchi L. Inflammasomes in inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2013;29(4):363–369. doi: 10.1097/MOG.0b013e32836157a4. [DOI] [PubMed] [Google Scholar]
- 36.Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154(11):6048–6057. [PubMed] [Google Scholar]
- 37.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, et al. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol. 1995;58(3):359–364. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 38.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, et al. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32(6):531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, et al. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol. 2005;175(10):6861–6869. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 40.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287(1):L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 41.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38(6):699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40(6):701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol. 2010;185(10):6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Xu F, Barrett E. Metalloelastase in lungs and alveolar macrophages is modulated by extracellular substance P in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L162–170. doi: 10.1152/ajplung.00282.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183(10):6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204(13):3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun. 2011;79(7):2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin L, Batra S, Douda DN, Palaniyar N, Jeyaseelan S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J Immunol. 2014;193(7):3549–3558. doi: 10.4049/jimmunol.1401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1-/- mice. J Immunol. 2012;189(12):5849–5859. doi: 10.4049/jimmunol.1200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang Y, Yu S, Ellis JS, Sharav T, Braley-Mullen H. Comparison of sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis. J Leukoc Biol. 2010;87(6):1019–1028. doi: 10.1189/jlb.0509352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181(3):1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


