Abstract
Objectives:
To investigate the effects on muscle performance after one-year cessation of 18-month low-magnitude high-frequency vibration (LMHFV) intervention in the untrained community elderly.
Methods:
This is a case-control study with 59 community elderly women (25 control without any treatment; 34 received 18-month LMHFV but discontinued for 1 year from our previous clinical study). Muscle strength, balancing ability, occurrence of fall/fracture, quality of life (QoL) were assessed 1-year after cessation of intervention. The 30-month results were compared with baseline and 18-month treatment endpoint data between groups.
Results:
At 30 months (i.e. one year post-intervention), the muscle strengths of dominant and non-dominant legs relative to baseline in treatment group were significantly better than those of control. In balancing ability test, reaction time, movement velocity and maximum excursion of treatment group (relative to baseline) remained significantly better than the control group. The muscle strength, balancing ability and quality of life at 30 months relative to 18 months did not show significant differences between the two groups.
Conclusion:
The benefits of LMHFV for balancing ability, muscle strength and risk of falling in elderly were retained 1 year after cessation of LMHFV.
Keywords: Vibration Treatment, Community Women, Muscle Strength, Balancing Ability, Sustained Effect
Introduction
Fragility fracture is one of the most prominent medico-social problems among the elderly in the community. Most fractures result from a combination of poor balance, falls, and deteriorating bone strength. Exercise training is effective in reducing risk of falling, improving balance and increasing lower extremity strength in elderly[1,2]; however, this is only beneficial to those with good compliance to exercise programs. The effects on muscle performance often disappear shortly after the cessation of the exercise training, therefore sustained adherence to falls prevention exercise programs is necessary to reduce fall risks[2]. An ideal fall prevention program should include interventions which sustainably reduce risk of falling.
In our previous study[3], low-magnitude high-frequency vibration (LMHFV) was proven to reduce fall incidences throughout 18-month intervention, together with improvement in balancing ability and muscle strength. Vibration treatment has also been demonstrated to have positive effects on muscle strength[4], postural control[5,6], balancing ability[7,8], new bone formation[6,9,10], and circulation[11]. LMHFV was proven effective and well adopted by previously untrained elderly[3,9], thus suitable to implement in elderly training programs. However, there were only limited reports on the retention effects of whole-body vibration. Previous studies focused on retention effects of short-term vibration treatment (8 weeks)[12] or vibration combined with exercise[13], but not long-term LMHFV treatment. Resistance training exercise is well proven in improving the muscle strength and physical functions, therefore it is the most common intervention in fall prevention among elderly. Some studies had also demonstrated that the beneficial effects of resistance training were sustainable after detraining[14,15], which is comparable to whole-body vibration treatment. Based on the results of previous study, long-term LMHFV is of great potential to produce sustainable effects on muscle among elderly. So there is a need to further investigate the sustained effects of long-term whole body vibration treatment on muscle performance and bone quality among postmenopausal women.
In this study, we hypothesized that 18-month LMHFV treatment could provide significant positive effect on muscle enhancement in 1 year after cessation of intervention. The results may provide new insight in designing fall prevention program with long-lasting impact.
Materials and methods
Experimental design and subjects
This is a case-control study to investigate the effect of LMHFV on muscle performance after one-year cessation of intervention. This study used muscle strength as primary outcome and power calculation indicated that n=56 could detect a significant difference between groups (please refer to following statistical part for details). Ninety-eight community elderly women, who were eligible (i.e. one year right after 18-month intervention from our previous randomized controlled trial 3 [ClinicalTrials.gov number: NCT00973167]), were approached and invited for follow-up, while 59 (60%) were willing to come back for assessments. They included 34 vibration group subjects and 25 control subjects, where vibration group had received LMHFV (35 Hz, 0.3 g where g=gravitational acceleration) for 20 min/day, 5 days/week for 18 months, while control group remained in sedentary lifestyle. Neither the control, nor the vibration group did not receive any further treatment for the last 12 months of the study (i.e. 18-30 months). They were all healthy females aged 60 years or above, independent and active in the community. All subjects were assessed at baseline, post-intervention (18-month) and follow-up (30-month) at Prince of Wales Hospital. We excluded anyone: (1) who was taking any medications/had medical conditions affecting the musculoskeletal system, e.g. bisphosphonates, (2) who participated in supervised regular exercise for twice a week or more, (3) with pace-maker in situ, (4) with malignancy, or (5) with a history of smoking or excessive alcohol use (more than 7 drinks per week)[16]. The study protocol was approved by the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee (Ref. no.: CRE-2008.067-T) and complied with the Declaration of Helsinki.
Interventions
Elderly enrolled in the vibration group, in addition to usual activities, received 18-month LMHFV treatment one year ago by standing upright without knee bending on a vibration platform providing vertical synchronous vibration at 35 Hz, 0.3 g (peak-to-peak magnitude), amplitude of 0.06 mm, 20 minutes/day, 5 days/week, where the LMHFV configuration were based on our positive findings on muscle strength in our previous clinical study[3]. Control group subjects remained in their habitual life style and participated in the normal interest group activities (e.g. card games, drama) organized by the community centers regulated by the Social Welfare Department of Hong Kong SAR Government.
Outcome measures
Fifty-nine subjects who were willing to follow-up came to Prince of Wales Hospital, The Chinese University of Hong Kong to do the following assessments. Outcome assessors and the statistician were blinded to group allocation, and participants were reminded not to tell the assessors of their original group allocation. Subjects’ medical records in the city-wide Clinical Management System (CMS) were reviewed for study eligibility and record of health conditions. Also, self-reported health conditions, physical activities level, dietary habit, and intake of supplements/over-the-counter drugs were interviewed and recorded.
Knee extensor strength
Quadriceps muscle strength was measured by instructing the subjects to perform an active extension of knee joint in a sitting position with both feet free from ground, and the hip and knee joint flexed at 90°. The peak isometric force of the knee extension was measured by a dynamometer (FallScreen©, Neuroscience Research Australia (NeuRA), Australia) attached at the malleoli level with a strap. Measurements were repeated thrice in each lower limb and the maximum force was used for analysis[17]. Leg dominance was determined by asking the subject which leg she would use to kick a ball placed in front of her[18]. The short-term coefficient of variation % is 3.39%[3].
Balancing ability
Balancing ability was assessed with the limits of stability test using the Basic Balance Master System (NeuroCom International Inc, OR, USA). Subjects were instructed to stand barefoot on the force plate and control the location of their center-of-gravity (COG) cursor by swaying and weight-shifting of her body to eight different target directions without falling or moving their feet[7]. The measured parameters of limits of stability test included reaction time (second), directional control (%), movement velocity (degrees per second), endpoint excursion (%), and maximum excursion (%). The short-term coefficient of variation % is 3.92%.
Bone mineral density
Areal bone mineral density (BMD, g/cm2) was measured at the hip of the non-dominant leg and lumbar spine (L1 to L4) by dual energy X-ray absorptiometry (DXA)(Delphi W, Hologic, Waltham, MA, USA). For consistency, one certified bone densitometry technologist performed all positioning and measurements. Calibration of DXA machine was done using bone phantom every day, which gave a precision error of 1.31% for total hip and 0.72% for spine[19].
Quality of life questionnaire
The health-related quality of life was assessed with the validated Chinese version of the 36-Item Short-Form Health Survey (SF-36)[20]. The physical component summary, mental component summary and total score of the SF-36 were analyzed. All scores range from 0 to 100 with higher scores indicating better quality of life.
Adverse events
An information sheet with our contacts and trial details was provided to every subject since our previous randomized controlled trial. All subjects were instructed to contact us and report any health problems or suspected adverse events to us. All complaints or complications from the subjects with regards to the LMHFV were documented. Also, during follow-up assessments, clinician recorded the health status of subjects (e.g. deterioration of pre-existing medical problems, newly diagnosed problems).
Power calculation and statistical analysis
This is a case-control study with muscle strength as primary outcome. From the results of our previous study[3], muscle strength difference of 2.06±2.75 kg was observed between groups after 9-month intervention. With muscle strength for sample size estimation, a total sample size of 56 had a power of 0.8 to detect a significant difference between groups using a two-sided two samples t-test with significance level of 0.05.
All results were expressed in mean ± standard deviation. The statistical analysis was performed using SPSS 20 (IBM, NY, USA). Shapiro–Wilk test was performed to test for the normality of data. For the primary outcome, one-way (examining the between-group differences in over-time changes of muscle strength of the dominant and non-dominant side) and two-way (examining the interaction effect among the effects of time, side (dominant vs. non-dominant), and group (vibration group vs. control group)) repeated measures ANOVA were further performed for analysis. Independent sample t-test (for between-group comparison) and paired t-test (for within-group comparison) were performed to compare the other outcome measurements between intervention and control groups. Bonferroni adjustment was adopted for multiple post-hoc comparisons. Significance level was set at p≤0.05 (2-tailed).
Results
The baseline characteristics of 59 subjects were summarized in (Table 1). All the follow-up assessments were scheduled 1 year after completion of 18-month intervention.
Table 1.
Baseline characteristics of study subjects.
| Characteristic* | Vibration group (n=34) | Control group (n=25) |
|---|---|---|
| Age (years) | 69.3(4.9) | 69.8(4.4) |
| Muscle strength ‡ (kg) | ||
| - Dominant leg | 6.9(2.2) | 7.3(2.2) |
| - Non-dominant leg | 6.2(2.3) | 6.9(2.1) |
| Balancing ability | ||
| - Reaction time (s) | 1.04(0.2) | 0.79(0.4) |
| - Movement velocity (°/sec) | 2.48(0.8) | 2.6(0.9) |
| - Endpoint excursion (%) | 55.6(12.8) | 57.9(11.2) |
| - Maximum point excursion (%) | 68.3(13.2) | 71.2(11.3) |
| - Directional control (%) | 70.9(8.3) | 66.0(10.2) |
| SF-36 | ||
| - Physical health component | 66.3(14.3) | 66.0(16.5) |
| - Mental health component | 79.6(14.4) | 78.8(15.4) |
| - Total | 74.6(12.8) | 73.7(14.3) |
| Bone mineral density (g/cm2) | ||
| - Total hip | 0.73(0.13) | 0.74(0.09) |
| - Total Spine | 0.79(0.16) | 0.80(0.13) |
| T-score | ||
| - Total hip | -1.45(1.16) | -1.46(0.91) |
| - Total Spine | -1.85(1.54) | -1.76(1.20) |
Values above are mean(SD).
Knee extensor strength
Analyzed by one-way repeated measures ANOVA (Figure 1 & Table 3), there was significant between-group difference in both dominant and non-dominant legs (p<0.0005 and <0.0001 respectively). Vibration group showed significant within-group increase of muscle strength at 1-year post-intervention than baseline (dominant leg: p=0.003; non-dominant: p<0.0005) but not in control group (dominant leg: p=0.507; non-dominant: p=0.872).
Figure 1.
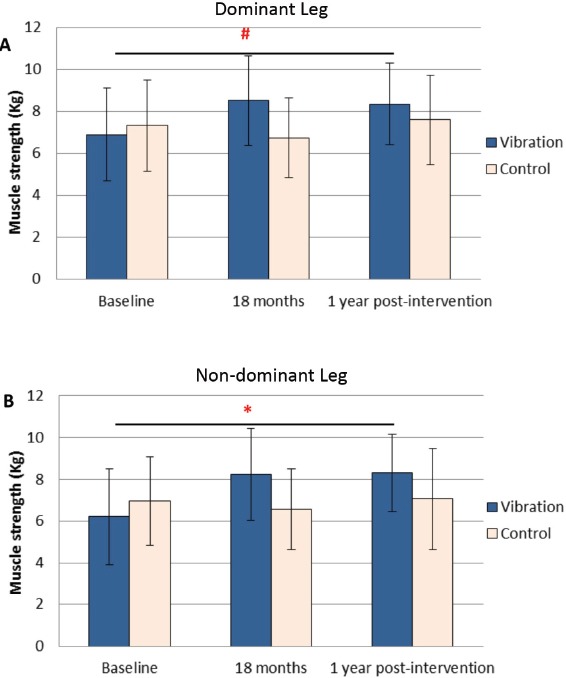
Quadriceps muscle strength measured at baseline, 18 months and post-intervention (1 year after cessation of intervention). A: Quadriceps muscle strength of dominant leg (Kg). B: Quadriceps muscle strength of non-dominant leg (Kg). The post-intervention muscle strength of vibration group remained significantly better than the baseline. (#, *: refer to (Table 3) for their significant differences).
Table 2.
Difference in secondary outcomes between vibration and control groups (Two-sample t-test analysis). (Note: the data of difference between baseline and 18 months can be referred to the previous article[3]).
| Mean difference±SD (Vibration group) | Mean difference±SD (Control group) | Difference between groups (V-C) (95%CI) | P value§ | |
|---|---|---|---|---|
| Muscle strength: | ||||
| Dominant leg (Kg) | ||||
| - Post intervention-baseline | 1.5±2.5 | -0.03±2.5 | 1.5 (0.2, 2.8) | Table 3 |
| - Post intervention-18 months | -0.2±1.8 | 0.6±2.0 | -0.7 (-1.7, 0.3) | Table 3 |
| Non-dominant leg | ||||
| - Post intervention-baseline | 2.1±2.4 | -0.2±2.3 | 2.3 (1.02, 3.5) | Table 3 |
| - Post intervention-18 months | 0.07±1.8 | 0.2±1.8 | -0.1 (-1.1, 0.8) | Table 3 |
| SF-36: | ||||
| Physical score | ||||
| - Post intervention-baseline | 2.1±14.9 | -3.8±17.3 | 5.9 (-2.6, 14.5) | 0.167 |
| - Post intervention-18 months | 9.9±11.5 | 8.7±9.8 | 1.2 (-4.6, 6.8) | 0.680 |
| Mental score | ||||
| - Post intervention-baseline | 4.1±11.5 | -0.8±16.0 | 4.9 (-2.4, 12.1) | 0.184 |
| - Post intervention-18 months | -2.3±8.7 | -3.2±16.9 | 0.92 (-6.7, 8.6) | 0.807 |
| Total score | ||||
| - Post intervention-baseline | 2.0±11.3 | -2.7±14.2 | 4.7 (-2.1, 11.4) | 0.170 |
| - Post intervention-18 months | -0.4±9.4 | -2.4±10.8 | 0.9 (-5.9, 7.7) | 0.455 |
| Bone mineral density: | ||||
| Total hip (% change) | ||||
| - Post intervention-baseline | -0.8±2.8 | -1.2±2.4 | 0.4 (-1.1, 1.8) | 0.620 |
| - Post intervention-18 months | -1.3±2.9 | -0.4±2.1 | -0.9 (-2.3, 0.5) | 0.203 |
| Total spine (% change) | ||||
| - Post intervention-baseline | 1.9±4.8 | 1.3±3.8 | 0.6 (-1.8, 2.9) | 0.632 |
| - Post intervention-18 months | 1.3±3.5 | 0.2±2.4 | 1.1 (-0.4, 2.7) | 0.150 |
| Balancing ability: | ||||
| Reaction time (Sec) | ||||
| - Post intervention-baseline | -0.09±0.3 | 0.3±0.4 | -0.4 (-0.6, -0.2) | <0.001* |
| - Post intervention-18 months | 0.04±0.2 | -0.01±0.3 | 0.05 (-0.1, 0.2) | 0.424 |
| Movement velocity (Deg/sec) | ||||
| - Post intervention-baseline | 0.9±1.2 | 0.2±0.9 | 0.7 (0.1, 1.3) | 0.024* |
| - Post intervention-18 months | 0.2±1.2 | 0.3±0.8 | -0.2 (-0.7, 0.4) | 0.576 |
| Endpoint excursion (%) | ||||
| - Post intervention-baseline | 9.8±15.3 | 4.1±18.0 | 5.8 (-3.3, 14.8) | 0.209 |
| - Post intervention-18 months | 0.6±11.0 | 4.6±13.7 | -4.0 (-10.7, 2.7) | 0.237 |
| Maximum excursion (%) | ||||
| - Post intervention-baseline | 15.1±16.1 | 4.0±15.7 | 11.0 (2.2, 19.8) | 0.015* |
| - Post intervention-18 months | 1.1±10.4 | 5.7±10.8 | -4.6 (-10.4, 1.2) | 0.121 |
| Directional control (%) | ||||
| - Post intervention-baseline | 0.7±9.4 | 4.7±14.0 | -4.0 (-11.0, 2.9) | 0.245 |
| - Post intervention-18 months | 1.0±6.2 | 2.5±9.5 | -1.5 (-5.8, 2.7) | 0.472 |
V, vibration; C, control; CI, confidence interval
From the 2-sample t-test. Using a Bonferroni-adjusted critical P value of 0.025 (n = 2 comparisons), asterisk marks significant difference
(p<0.025, n = 2 comparisons).
Table 3.
P values of muscle strength comparison of dominant and non-dominant legs analyzed by repeated measures ANOVA.
| Overall p-value | 18 months - Baseline | 1 year post-intervention - Baseline | |
|---|---|---|---|
| Dominant Leg | |||
| Within-group | |||
| - Vibration | <0.0005 | <0.0005 | 0.003 |
| - Control | 0.054 | 0.116 | 0.507 |
| - Between-group | <0.0005 | <0.0001 | 0.056 |
| Non-Dominant Leg | |||
| Within-group | |||
| - Vibration | <0.0001 | <0.0001 | <0.0005 |
| - Control | 0.397 | 0.238 | 0.872 |
| - Between-group | <0.0001 | <0.0001 | 0.003 |
Analyzed by two-way repeated measures ANOVA, there was significant difference in muscle strength between the dominant and non-dominant sides (p=0.003); however, such differences did not vary between groups (p=0.795 for interaction term side*group). There was no significant interaction between side and time (p=0.183 for interaction term side*time), indicating that over-time changes of muscle strength did not differ significantly between the dominant and non-dominant sides. There was also no significant interaction among side, time, and group (p=0.079 for interaction term side*time*group), indicating that the observed between-group differences in over-time changes of muscle strength did not depend on side of the limbs.
Balancing ability
The overall balancing ability of the vibration group remained significantly improved 1 year after cessation of intervention as compared with the control group (Figure 2 & Table 2). At 1 year post-intervention, shorter reaction time (mean between-group difference=-0.4 seconds; 95%CI=-0.6 to -0.2; p<0.001), increased movement velocity (mean between-group difference=0.7 degree/sec; 95%CI=0.1-1.3; p=0.024), and increased maximum excursion (mean between-group difference=11.0%; 95%CI=2.2-19.8; p=0.015) relative to baseline was found significant than those of control group. From 18-month intervention end point to 1-year post-intervention, there was no difference between groups in all parameters.
Figure 2.
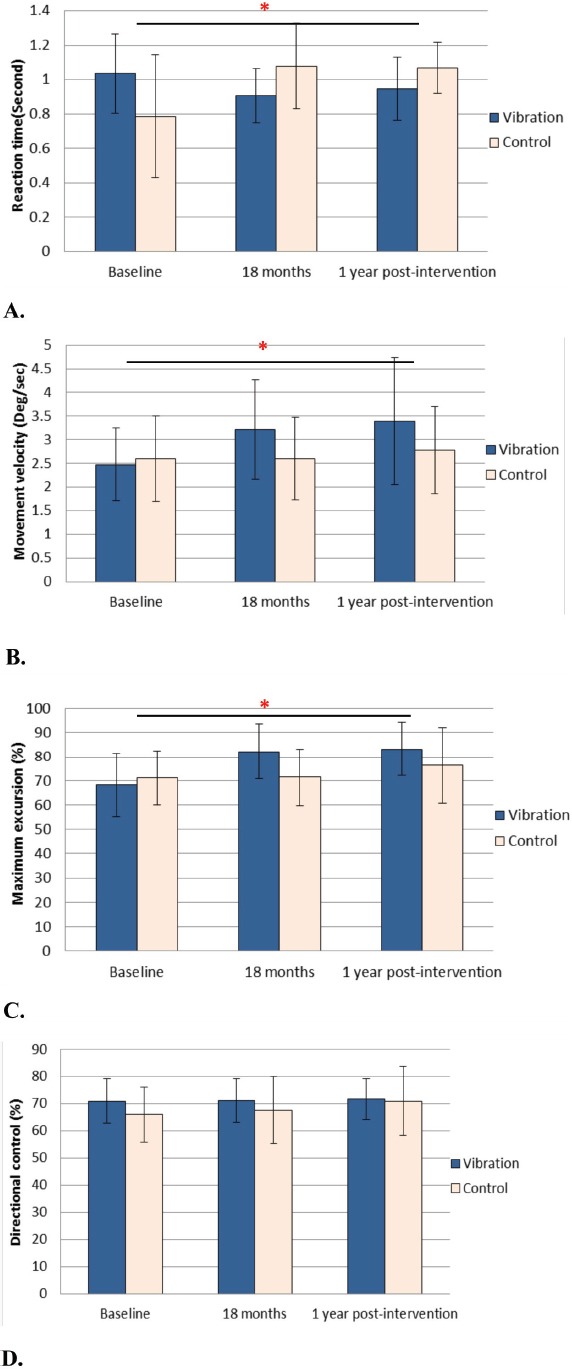
Balancing ability was assessed by the limit of stability test. A: Reaction time B: Movement velocity C: Maximum excursion D: Directional control. (*: p <0.001, independent t-test; error bar: ±SD).
Quality of life questionnaire
There was no significant difference in all SF-36 components between two groups during 1-year post-intervention (Tables 2 & 4). Compared with the baseline, there was no inter-group difference one year after cessation of intervention (95%CI=-2.1-11.4, p=0.17). From 18-month intervention end-point to 1-year post-intervention, no difference between vibration and control groups was observed either (p=0.455).
Table 4.
The results of SF-36 quality of life questionnaire, including the Physical Health component, Mental Health component and overall QoF.
| SF-36 QoF | Baseline | 18 months | 1 year post-intervention | |||
|---|---|---|---|---|---|---|
| Vibration | Control | Vibration | Control | Vibration | Control | |
| Physical Health | 66.3±14.3 | 66.0±16.5 | 66.7±16.5 | 62.1±17.3 | 68.4±17.0 | 61.8±19.5 |
| Mental Health | 79.6±14.4 | 78.8±15.4 | 85.9±8.5 | 81.3±14.1 | 83.7±12.4 | 77.6±18.4 |
| Overall QoF | 74.6±12.8 | 73.7±14.3 | 77.0±11.6 | 73.2±13.7 | 76.7±14.3 | 70.6±18.3 |
(Error bar: ±SD, Values: Mean±SD)
Bone mineral density
For total hip BMD, the mean change in the vibration group was -0.8% compared with -1.15% in control group from baseline to 1-year post-intervention (mean between-group difference=0.4%, 95%CI=-1.1-1.8, p=0.620). From 18-month intervention end-point to 1-year post-intervention, a drop of -1.3% and -0.4% total hip BMD were observed in the vibration and control groups respectively (mean between-group difference=-0.9%, 95%CI=-2.3-0.5, p=0.203). Subjects in both vibration and control groups had total hip BMD decreased throughout the study period with no significant difference between groups. The mean change of total spine (L1 to L4) BMD in the vibration group was +1.9% compared with +1.3% in the control group from baseline to 1-year post-intervention (mean between-group difference=0.6%, 95%CI=-1.8-2.9, p=0.632) without significant difference. From 18-month intervention end-point to 1-year post-intervention, there were +1.3% and +0.2% changes in spine BMD in the vibration and control groups respectively without significant difference (mean between-group difference=1.1%, 95%CI=-0.4-2.7, p=0.150) (Figure 3 & Table 2).
Figure 3.
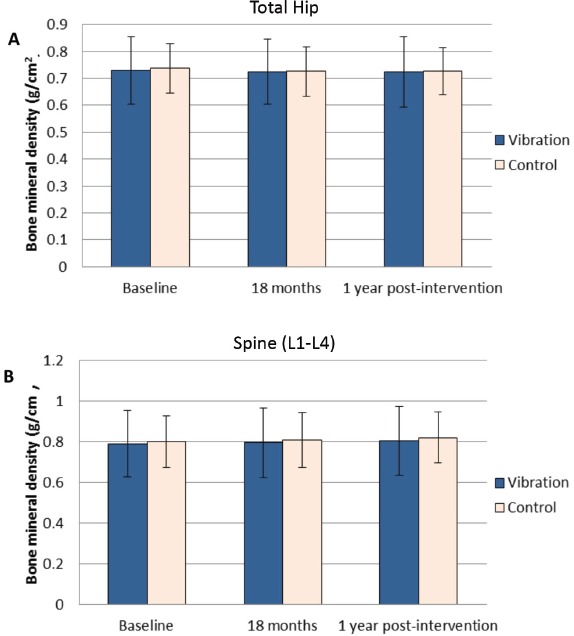
Bone mineral density measured by DXA. A: Bone mineral density of total hip. B: Bone mineral density of spine (L1 to L4). No significant differences were found in the BMD.
Discussion
Fall and fragility fractures are two closely related problems causing significant morbidity and loss of functions among elderly, therefore an intervention that can decrease the risks of fall and fracture in long term is needed. In our previous study[3], LMHFV was confirmed with its efficacy on reducing fall incidences and fracture risks, enhancing lower limb muscle strength and improving balancing ability. The beneficial effects were observed as early as 9 months after the commencement of LMHFV and sustained throughout the 18-month intervention period, but the retention effects of LMHFV was unknown.
From the results of this study, the beneficial effects on muscle performance remained significant in 1 year after the cessation of LMHFV treatment. In the vibration group, the knee extensor muscle strength of both dominant and non-dominant legs at 1-year post-intervention remained 21-33% significantly better than baseline. The reaction time, movement velocity and maximum point excursion of the vibration group also remained significantly improved from baseline to 1-year post-intervention (p<0.001, 0.024 and 0.015 respectively). When observing the change of muscle performance from the 18-month intervention end-point to 1-year post-intervention, there were no significant differences found between two groups. After cessation of LMHFV treatment, there was no significant loss of functional gains in the vibration group. Significant positive effects of LMHFV on muscle performance were confirmed in previously untrained elderly, with improved muscle strength and balancing ability for at least one year after cessation. However, there were no significant changes in quality of life and BMD in both groups throughout the study.
In this study, vibration group subjects received 18-month LMHFV for 5 days/week with an average compliance rate of 79% (approximately 4 sessions a week), and retained significant improvement in muscle performance up to 1-year post-intervention. Significant sustained effects of whole-body vibration training were also observed in older adults in previous studies[12,13]. In Marin et al study[12], only the group which received 4 days/week of vibration treatment (amplitude: 1.05-2.11 mm; 35-40 Hz) retained significant effect on muscle enhancement after 3 weeks of detraining, but not the group received vibration treatment 2 days/week. And these results echo with this study, which both studies demonstrated the sustained effects of vibration treatment with 4 sessions per week. So the results may suggest that high compliance of vibration treatment not only improves functional performance during the intervention period, but also induces longer lasting adaptions of muscle. In another study providing 1-year whole-body training of 3 days/week[13], the muscle power of vibration group remained significantly higher after 1-year detraining. Considering all the results, vibration treatment of at least 3days/week is recommended to elderly for a longer lasting improvement on lower limb functions. In contrast to previous studies[12,13], our study investigated the sole effects of low-magnitude vibration (0.3 g or displacement <0.1 mm), instead of high amplitude vibration (up to 2mm) with static or dynamic leg exercises performed on treatment platform. Therefore, the retention of functional gain induced by low-magnitude vibration in 1-year detraining period was comparable to that of high amplitude vibration (+/- exercise) which requires better balancing ability to manage. Also, this is the first study investigating the detraining effects of long-term whole-body vibration treatment up to 18 months, thus providing new insight in designing long-term fall prevention programs.
In mobility-limited elderly, muscle power failure is related to impairments in neuromuscular activation rate and poor muscle quality[21]. Prolonged LMHFV provides mechanical vibration signals to actively stimulate muscles, and this may enhance the muscle power and balancing ability with lower recruitment thresholds and increased firing rate of motor units[22,23]. The lower recruitment thresholds are important in producing rapid action (i.e. balancing) and powerful muscle contraction, while the increased firing rate may also allow more synchronous contraction of motor units which increases the overall muscle power[24]. Also, an increase in muscle fiber cross-sectional areas may contribute to the enhanced muscle strength during vibration treatment. In a previous study, relatively little effect was observed in fiber cross-sectional areas during detraining period and this may correlate to the sustained increase of maximal dynamic strength from resistance training[25]. Furthermore, vibration treatment was reported to increase evoked neurotransmitter release at neuromuscular synapses, which may translate into performance gains[26]. All these muscular and neural adaptions may explain the strong sustainable effects of LMHFV treatment in elderly, which persist for at least one year in the subjects who had completed 18 months of LMHFV treatment.
Besides fall and fracture prevention, LMHFV can be another form of biophysical stimulation as adjunctive to regular exercise and is specially indicated in the elderly whom may not be able to do regular weight bearing exercise. Previous studies also demonstrated that both whole-body vibration treatment and resistance training could provide significant and comparable benefits on muscle performance in terms of postural control, muscle strength and jumping height[4,6]. The sustained effects of whole-body vibration treatment and resistance exercise are comparable, which both produce a significant gain of muscle strength although a decreasing trend was noticed during the detraining period[13-15]. The average rate of decline in lower limb strength is up to 16% per decade in elderly[27], hence an intervention enhancing lower limb strength is the key to preserve physical functions and prevent falls. However, no significant changes were observed in BMD in both groups throughout this study. In this study, low-magnitude (0.3 g) vibration of 20 mins/day was adopted considering the generalizability in frail elderly and post-fracture patients, which is of great potential for future application in rehabilitation and sarcopenia prevention. However, vibration signal is transmitted to only the lower extremities and axial skeleton in a standing individual; the muscle and bone of upper limbs are not benefited from LMHFV treatment. Also, there is no evidence showing that LMHFV may improve cardiorespiratory fitness like various kinds of exercise (e.g. resistance training)[28].
There were limitations in this study. Only community-active female were included as female gender is a well-proven factor associated to higher risks of fall and fragility fracture; however, the effect of LMHFV on male or institutionalized elderly cannot be confirmed. Also, the change of skeletal muscle mass were not assessed, therefore our results cannot provide information of the effect of LMHFV on muscle mass and the potential association with muscle enhancement.
In conclusion, the positive effect of LMHFV on muscle performance in community elderly can last for one year after the cessation of 18-month intervention. LMHFV not only enhanced subjects’ muscle strength and balancing ability during the intervention period, but also brought significant enhanced effects one year after cessation of intervention. Implementing LMHFV of at least 3 sessions a week in training and rehabilitation programs is recommended to bring long-term improvement in muscle functions, especially for elderly who can benefit with lower fall and fracture risks. Fall and fracture prevention program with LMHFV should last for at least 9 months, and ideally for 18 months or longer to maximize the treatment effect, as well as the sustained effect. For elderly who stopped LMHFV treatment due to various reasons (e.g. acute medical conditions, travel), restarting the treatment within one year is advised for a continuous effect.
Acknowledgements
This study was supported by General Research Fund (Ref: 469508) of University Grants Committee, Hong Kong SAR Government
Footnotes
Edited by: J. Rittweger
References
- 1.Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports. 2007;17:422–30. doi: 10.1111/j.1600-0838.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Vogler CM, Menant JC, Sherrington C, Ogle SJ, Lord SR. Evidence of detraining after 12-week home-based exercise programs designed to reduce fall-risk factors in older people recently discharged from hospital. Arch Phys Med Rehabil. 2012;93:1685–91. doi: 10.1016/j.apmr.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Leung KS, Li CY, Tse YK, et al. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly-a cluster-randomized controlled trial. Osteoporos Int. 2014;25:1785–95. doi: 10.1007/s00198-014-2693-6. [DOI] [PubMed] [Google Scholar]
- 4.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52:901–8. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 5.Rogan S, Hilfiker R, Herren K, Radlinger L, de Bruin ED. Effects of whole-body vibration on postural control in elderly: a systematic review and meta-analysis. BMC Geriatr. 2011;11:72. doi: 10.1186/1471-2318-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 7.Cheung WH, Mok HW, Qin L, Sze PC, Lee KM, Leung KS. High-frequency whole-body vibration improves balancing ability in elderly women. Arch Phys Med Rehabil. 2007;88:852–7. doi: 10.1016/j.apmr.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Torvinen S, Kannus P, Sievanen H, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18:876–84. doi: 10.1359/jbmr.2003.18.5.876. [DOI] [PubMed] [Google Scholar]
- 9.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 10.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22:317–25. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 11.Stewart JM, Karman C, Montgomery LD, McLeod KJ. Plantar vibration improves leg fluid flow in perimenopausal women. Am J Physiol Regul Integr Comp Physiol. 2005;288:R623–9. doi: 10.1152/ajpregu.00513.2004. [DOI] [PubMed] [Google Scholar]
- 12.Marin PJ, Martin-Lopez A, Vicente-Campos D, et al. Effects of vibration training and detraining on balance and muscle strength in older adults. J Sports Sci Med. 2011;10:559–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Kennis E, Verschueren SM, Bogaerts A, Coudyzer W, Boonen S, Delecluse C. Effects of Fitness and Vibration Training on Muscle Quality: A 1-Year Postintervention Follow-Up in Older Men. Arch Phys Med Rehabil. 2013;94:910–8. doi: 10.1016/j.apmr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Elliott KJ, Sale C, Cable NT. Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Br J Sports Med. 2002;36:340–4. doi: 10.1136/bjsm.36.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sforzo GA, McManis BG, Black D, Luniewski D, Scriber KC. Resilience to exercise detraining in healthy older adults. J Am Geriatr Soc. 1995;43:209–15. doi: 10.1111/j.1532-5415.1995.tb07324.x. [DOI] [PubMed] [Google Scholar]
- 16.Fleming MF. Screening and brief intervention in primary care settings. Alcohol Res Health. 2004;28:57–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Qin L, Choy W, Leung K, et al. Beneficial effects of regular Tai Chi exercise on musculoskeletal system. J Bone Miner Metab. 2005;23:186–90. doi: 10.1007/s00774-004-0559-2. [DOI] [PubMed] [Google Scholar]
- 18.Alonso AC, Brech GC, Bourquin AM, Greve JM. The influence of lower-limb dominance on postural balance. Sao Paulo Med J. 2011;129:410–3. doi: 10.1590/S1516-31802011000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn HS, Lau EM, Au B, Leung PC. Bone mineral density reference norms for Hong Kong Chinese. Osteoporos Int. 2005;16:1663–8. doi: 10.1007/s00198-005-1899-z. [DOI] [PubMed] [Google Scholar]
- 20.Lam CL, Gandek B, Ren XS, Chan MS. Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF-36 Health Survey. J Clin Epidemiol. 1998;51:1139–47. doi: 10.1016/s0895-4356(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 21.Reid KF, Doros G, Clark DJ, et al. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2012;112:2289–301. doi: 10.1007/s00421-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol (1985) 2012;112:388–95. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin L, Garland SJ, Ivanova T, Gossen ER. Muscle vibration sustains motor unit firing rate during submaximal isometric fatigue in humans. J Physiol. 2001;535:929–36. doi: 10.1111/j.1469-7793.2001.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–72. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 25.Staron RS, Leonardi MJ, Karapondo DL, et al. Strength and Skeletal-Muscle Adaptations in Heavy-Resistance-Trained Women after Detraining and Retraining. J Appl Physiol. 1991;70:631–40. doi: 10.1152/jappl.1991.70.2.631. [DOI] [PubMed] [Google Scholar]
- 26.Mettlach G, Polo-Parada L, Peca L, Rubin CT, Plattner F, Bibb JA. Enhancement of neuromuscular dynamics and strength behavior using extremely low magnitude mechanical signals in mice. J Biomech. 2014;47:162–7. doi: 10.1016/j.jbiomech.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 28.Pollock ML, Franklin BA, Balady GJ, et al. Resistance exercise in individuals with and without cardiovascular disease - Benefits, rationale, safety, and prescription - An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;101:828–33. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]


