Abstract
Objective:
To investigate whether osteocytic connexin 43 (Cx43) is required for the bone response to intermittent PTH administration, and whether the connexin is involved in maintaining the bone matrix.
Methods:
Human PTH(1-34) was injected to adult male mice expressing (Cx43fl/fl) or not osteocytic Cx43 (Cx43fl/fl;DMP1-8kb-Cre) daily (100 µg/kg/d) for 14 days.
Results:
Cx43fl/fl;DMP1-8kb-Cre mice have no difference in body weight and BMD from 1 to 4 months of age. Intermittent PTH administration increased BMD and BV/TV and induced a similar increase in type I collagen, alkaline phosphatase, runx2, osteocalcin, and bone sialoprotein expression in mice from both genotypes. On the other hand, osteocytic deletion of Cx43 did not alter mRNA levels of glycosaminoglycans, proteoglycans, collagens and osteoblast-related genes. In addition, expression of collagens assessed by immunohistochemistry was not affected by deleting osteocytic Cx43. However, PTH administration increased type II collagen only in Cx43fl/fl control mice, whereas hormone increased type I collagen expression only in Cx43fl/fl;DMP1-8kb-Cre mice. Furthermore, PTH increased maturity of collagen fibers in control, but not in Cx43-deficient mice.
Conclusion:
Expression of Cx43 in osteocytes is dispensable for bone anabolism induced by intermittent PTH administration; but it can modulate, at least in part, the effect of PTH on the bone matrix environment.
Keywords: Bone Matrix, Collagen, Connexin 43, Osteocyte, PTH
Introduction
The combination of mineral, collagen, non-collagenous proteins, lipids and water provide elasticity and stiffness to the bone, contributing to bone strength and, consequently, preventing bone fractures. Osteoblasts are the bone cells responsible for extracellular bone matrix deposition, which initially is non-mineralized (osteoid) and will later become mineralized[1,2]. Osteocytes have now been implicated in maintaining the bone matrix by digesting matrix in some circumstances and acting as endocrine cells, regulating phosphate metabolism[3,4]. The two important elements that constitute the organic phase in the bone matrix are the collagen proteins, the basic unit of the matrix fiber network, and the proteoglycans[5,6]. The basic proteoglycan units include a core protein attached covalently to one or more glycosaminoglycans (GAGs) such as heparin, heparan sulfate, dermatan sulfate, keratan sulfate, and chondroitin sulfate. In addition to these GAGs, hyaluronan is also present in the bone matrix, but is different from others members of the family in that is not sulfated and does not bind to a core protein[7].
Several drugs with anti-resorptive action have been used to stop bone loss and thus prevent osteoporosis, a condition characterized by low bone mass and high risk of fractures[8]. Some of these drugs include bisphosphonates (alendronate, risedronate, pamidronate, zoledronic acid), raloxifene, denosumab, and calcitonin. Intermittent PTH administration is the only anabolic treatment currently approved by the Food and Drug Administration (FDA) to increase bone mass in patients suffering from severe osteoporosis. Daily injections of the hormone result in increased bone formation and bone mass. Part of this direct anabolic effect of PTH has been ascribed to the ability of the hormone to prolong osteoblast lifespan, resulting in accumulation of bone-forming cells with consequent increase in bone mass and mechanical strength[9-13]. In addition, intermittent PTH has indirect actions in bone, by stimulating the production of IGF-1, which leads to pro-differentiation and pro-survival effects on osteoblasts[14]. Furthermore, PTH also increases bone formation by reducing the expression of the antagonist of Wnt signaling Sost/sclerostin[15]. In addition to increasing bone mass, intermittent PTH affects the distribution of molecules in the organic bone matrix. For example, the distribution of type I collagen in cortical bone is altered in menopausal women and in estrogen-deficient rats[16,17]. Moreover, PTH can also affect the expression of hyaluronan, as shown in cultured cells and in bones from mice treated with intermittent PTH[18,19]. Depending on its molecular weight, hyaluronan can increase osteoclast recruitment and differentiation thereby increasing bone resorption (low weight)[20-23], or increase osteoblast differentiation and mineralization (high weight)[24]. Furthermore, it has been shown that PTHrP, a molecule homolog to parathyroid hormone, regulates biosynthesis of proteoglycans in bone[25], and that deletion of a proteoglycan impairs the skeletal formation and anabolic response to PTH[26]. This, together with reports that mice deficient in the proteoglycan perlecan have decreased anabolism in response to mechanical loading[27], suggests that GAGs/proteoglycans and collagen are important in mediating bone response to hormonal and mechanical stimuli.
Gap junction proteins, molecules encoded by the GJ gene family and known as connexins, mediate intercellular communications among bone cells. Six connexin molecules form a channel or connexon, which can align with a connexon on a neighboring cell to form a gap junction channel, allowing the communication between the two cells. Connexons are also present in the cell membrane to allow the communication of the cells with the extracellular environment. Connexin channels allow the passage of molecules <1.2 kDa [28,29]. The potential requirement of one of the members of the connexin family of proteins, Cx43, for bone matrix homeostasis is evidenced by the expression of a dominant negative G60S mutant form for Cx43, which leads to altered synthesis of non-collagenous molecules and increased bone resorption[30]. Furthermore, collagen maturation is defective in mice lacking Cx43 in osteoblasts and osteocytes[31]. However, whether Cx43 expression in osteocytes is required to mediate the intermittent PTH effects in bone gain and whether its absence is associated with changes in the bone matrix is unknown[31,32].
Our previous studies show that Cx43 deletion results in altered bone material strength and decreased collagen cross-linking[31], and that intermittent PTH administration affects the bone matrix composition[17]. Moreover, mice lacking Cx43 in osteoblasts and osteocytes exhibit a reduced anabolic response to PTH[32]. We therefore aimed to determine whether osteocytic Cx43 is required to enable the effect of intermittent PTH administration on bone mass and matrix composition.
The current study demonstrates that osteocytic Cx43 is not required for bone anabolism triggered by intermittent PTH administration in male mice. Moreover, PTH induced similar changes in the expression of non-collagenous components of the bone matrix in mice lacking Cx43 and littermate controls. However, the effects of the hormone on type I and type II collagen protein levels, and on collagen maturation are altered in the absence of osteocytic Cx43, suggesting that connexin is required for the effects of intermittent PTH on the bone matrix environment.
Materials and methods
Mice generation and PTH treatment
Mice harboring full length “floxed” Cx43[33] (Cx43fl/fl) were mated with Cx43fl/fl mice expressing Cre recombinase under control of a DNA fragment containing 8kb of the murine dentin matrix protein 1 promoter (DMP1) (Cx43fl/fl; DMP1-8kb-Cre)[34]. The same number of males and females from both genotypes were used as breeders. From this breeding, 2 genotypes were generated and used, as follows: Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre. Male 4-month-old mice were subcutaneously injected once a day for 14 days with vehicle (0.9% saline, 10 µM β-mercaptoethanol and 0.01% acid acetic) (Cx43fl/fl, n=10 per group and Cx43fl/fl; DMP1-8kb-Cre mice, n=4 per group) or 100 µg/kg of human PTH(1-34) (Bachem California Inc., Torrance, CA) dissolved in vehicle, as previously reported[9] (Cx43fl/fl, n=8 per group and Cx43fl/fl; DMP1-8kb-Cre mice, n=8 per group). All mice used were of C57BL/6 background. Mice were fed a regular diet and water ad libitum, and maintained on a 12h light/dark cycle. Body weight was monitored once a month, from 1 to 4 months of age (before starting PTH administration). Mice were assigned to each experimental group based on spinal BMD. All protocols involving mice were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and from Federal University of São Paulo School of Medicine (UNIFESP, process #307/11).
Bone mineral density (BMD) by dual energy x-ray absorptiometry (DXA)
Longitudinal BMD measurements were performed monthly from 1 to 4 months of age (before starting PTH injections) and after completion of 14-day PTH administration (4.5 months of age) by DXA using a PIXImus densitometer (G.E. Medical Systems, Lunar Division, Madison, WI). BMD measurements included whole body BMD (total BMD, excluding the head and tail), entire femur (femoral BMD) and L1-L6 vertebrae (spinal BMD)[35]. Calibration was performed before scanning with a standard phantom as recommended by the manufacturer. The percentage change BMD was calculated using the following formula: [(final BMD - pre-treatment BMD) / pre-treatment BMD] × 100, in which pre-treatment BMD was measured at 4 months of age (before starting PTH administration) and final BMD was measured at 4.5 month of age (at the time of sacrifice).
Micro-computed tomography (µCT) analysis
L4 vertebrae and femora from mice at 4.5 months of age were dissected, cleaned of soft tissue and frozen at -20ºC until imaging. Bones were scanned wrapped in parafilm using 60kV source, 0.5 mm AI filter, 0.7 degree rotation and two-image averaging at 6 µm pixel resolution on a Skyscan 1172 (SkyScan, Kontich, Belgium). Reconstruction and analysis were conducted by Nrecon SkyScan software using a 2 smoothing, 5 ring artifact reduction, 20 beam-hardening correction and a similar threshold (0-0.10) for all animals. BV/TV was measured in the cancellous bone (1 mm of tissue) of the L4 vertebrae. Material density in distal femur and femoral mid-diaphysis was analyzed in 1 slice located 3 mm distant from cancellous region[31,36,37]. The terminology and units used for µCT are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (ASBMR)[38].
RNA extraction and quantitative RT-PCR (qPCR)
Lumbar vertebrae (L6) were thawed and total RNA was extracted using Trizol reagent (Invitrogen), as previously reported[39]. mRNA was converted to cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). qPCR was performed using the ∆Ct method[40], with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. Primers and probes were designed using the Assay Design Center (Roche Applied Science, Indianapolis, IN) or were commercially available (Applied Biosystems, Foster City, CA).
Immunohistochemistry
Whole femora were dissected and fixed in 10% neutral-buffered formalin for 48h, followed by demineralization in 10% ethylenediaminetetraacetic acid (EDTA), pH 7.0 for 10 days, with changes of the EDTA solution every 3 days. Next, distal femora were embedded in paraffin and sectioned through the frontal plane at 5 µm thickness. Sections were then deparaffinized, rehydrated and incubated with 3% H2O2 in methanol for 5 min to inhibit endogenous peroxidase and with 1% BSA for 30 min to block nonspecific binding sites. For proteins identification and quantification, sections were incubated with primary antibody against collagen 1a1 (1:2000), collagen 2a1 (1:150) and decorin (1:300) (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS+1% BSA overnight. Negative controls were obtained by replacing the primary antibody by PBS+1% BSA. After rinsing with PBS, sections were incubated by corresponding secondary antibodies conjugated with horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min. Samples were developed with DAB substrate-chromogen system (Dako North America Inc., Carpinteria, CA) and mounted with entellan®. All sections were evaluated by two blinded investigators. For quantitative assessment, all slides were stained in one batch. Two different sections from 4-8 animals/group (400x magnification) were used, and 40 different points were analyzed within distal cortical compartment in order to calculate the average of the densitometric grey levels using an image analysis system (AxioVision Rel. 4.6., Carl Zeiss, Germany). All cortices were evaluated at 390 µm below the lowest point of the growth plate[17] in an area of 1.5 mm2. The values are expressed as arbitrary units (a.u.)[41,42].
Picrosirius red staining
Bone embedded in paraffin and sectioned through the frontal plane at 5 µm thickness were deparaffinized, rehydrated, and stained using the modified picrosirius red technique[43-45]. Histochemistry for picrosirius red was used to reveal the collagen fibers. Briefly, bone sections were incubated for 10 minutes in 0.2% aqueous phosphomolybdic acid to render the cytoplasm and elastin colorless and then stained in a 0.1% solution of Sirius red in saturated aqueous picric acid for 90 minutes, washed in 0.01N HCl for 2 minutes, dehydrated, cleaned, and mounted with entellan®. Two sections from each mouse were imaged (200x magnification) in distal femur area under a microscope Axioskop 40 microscope (Carl Zeiss, Germany) equipped with polarizing filters using an AxioCamMRc 5 digital camera (Carl Zeiss, Germany) by two blinded-investigators at 390µm below the lowest point of the growth plate (to exclude the primary spongiosa)[17]. Approximately 1.5 mm2 of cancellous and 1.2 mm of cortical area were examined. The average from two sections of 4-6 animals/group was scored from 1 to 5, with 1 indicating 100% of reddish birefringence; 2, 75% of reddish and 25% of greenish birefringence; 3, 50% of reddish and 50% of greenish birefringence; 4, 25% of reddish and 75% of greenish birefringence; and 5, 100% of greenish birefringence.
Statistical analysis
Data were analyzed by using SigmaPlot (Systat Software Inc., San Jose, CA). Multiple comparisons for the 4 groups were performed using two-way ANOVA test followed by Student-Newman-Keuls post-hoc analysis to determine the significance of the difference among treatments, and considered significant when p<0.05. All values are reported as the mean ± standard deviation (SD).
Results
Expression of Cx43 in osteocytes is not required for bone anabolism induced with intermittent PTH administration
The absence of Cx43 specifically in osteocytes of male mice did not alter body weight or total body, femoral, or spinal BMD assessed longitudinally from 1 to 4 months of age (Figure 1a). Intermittent PTH administration for 14 days increased BMD in both control Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre mice (Figure 1b). Thus, PTH administration induced a significant increase in BMD in all sites, without genotype × treatment interaction, indicating that PTH has similar effects in mice expressing or lacking osteocytic Cx43 (Table 1). This analysis also showed that mice lacking Cx43 in osteocytes and treated with vehicle lose more spinal BMD than control littermates during the 14 days of the experiment, suggesting that osteocytic Cx43 is required for maintaining spinal bone mass in adult male mice. Cancellous bone volume (BV/TV) was increased in the lumbar vertebrae in mice from both genotypes treated with intermittent PTH (Figure 1c and Table 1).
Figure 1.
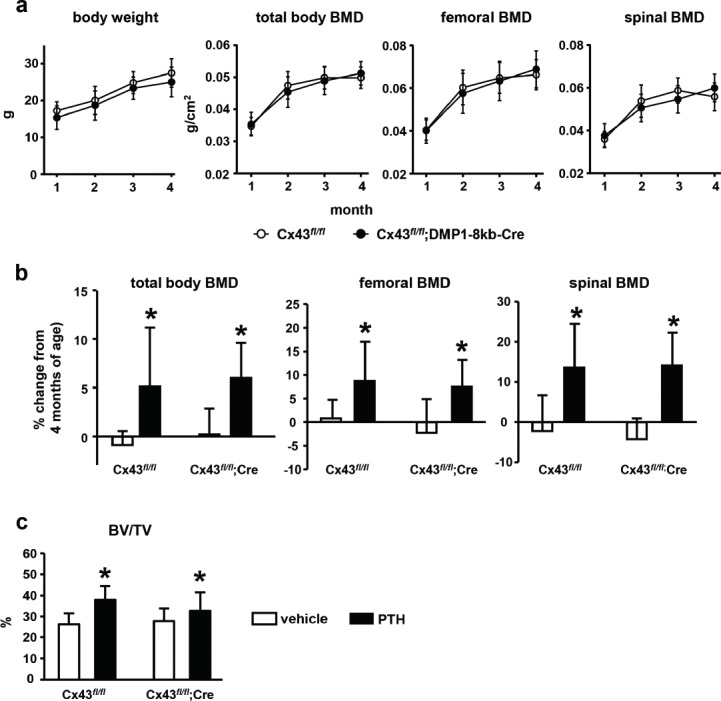
Deletion of Cx43 in osteocytes does not change bone mass and is not required for intermittent PTH-mediated anabolism. (a) Body weight and total, femoral and spinal BMD was assessed monthly from 1 to 4 months by DXA in Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre male mice. The symbols correspond to mean ± SD, n=18-23. (b) BMD percentage change was calculated using the BMD values at the end of treatment (4.5 months of age) and at the beginning of PTH treatment (4 months of age) for each animal. Bars represent mean ± SD, n=4-10. (c) Cancellous bone volume (BV/TV) was assessed by µCT in L4 vertebrae at 4.5 months of age. Bars represent mean ± SD, n=4-10. * p<0.05 versus vehicle by two-way ANOVA.
Table 1.
Statistical analysis of the effect of intermittent PTH administration on BMD in mice expressing or lacking Cx43 in osteocytes. Two-way ANOVA, followed by Student-Newman-Keuls post-hoc test.
| BMD | BV/TV | |||
|---|---|---|---|---|
| Source of variation | total body | femur | spine | vertebra |
| p value | p value | p value | p value | |
| Genotype | 0.471 | 0.162 | 0.019 | 0.767 |
| Treatment | 0.028 | <0.001 | 0.002 | <0.001 |
| Genotype × treatment | 0.654 | 0.599 | 0.088 | 0.086 |
Intermittent PTH administration increases mRNA levels of genes associated with osteoblast differentiation in vertebral bone, independently of osteocytic Cx43 expression
Targeted-deletion of Cx43 from osteocytes or intermittent PTH administration did not alter the expression of GAGs (has1, has2, chpf and chsy3) or proteoglycans (fibronectin, biglycan, decorin and fibromodulin), when compared to littermate controls (Figure 2a and b). On the other hand, mRNA expression for type I collagen (col1a1) was increased in vertebrae from both genotypes after PTH injections, but it was not altered by Cx43 deletion (Figure 2c and Table 2). We next investigated whether increased collagen synthesis was followed by collagen maturation; in a process conducted in part by lysyl oxidase (lox) family genes. The expression of lox was significantly affected by both the genotype and the treatment, with higher expression in Cx43fl/fl; DMP1-8kb-Cre mice treated with PTH. However, we did not find a significant interaction between genotype and treatment for this gene. On the other hand, the levels of loxl1 were affected by the genotype, with Cx43fl/fl; DMP1-8kb-Cre mice expressing higher levels; but not by PTH administration. In addition, mRNA levels of alkaline phosphatase (alp), runx2, osteocalcin, and bone sialoprotein were increased by intermittent PTH administration in both Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre mice (Figure 2d and Table 2).
Figure 2.
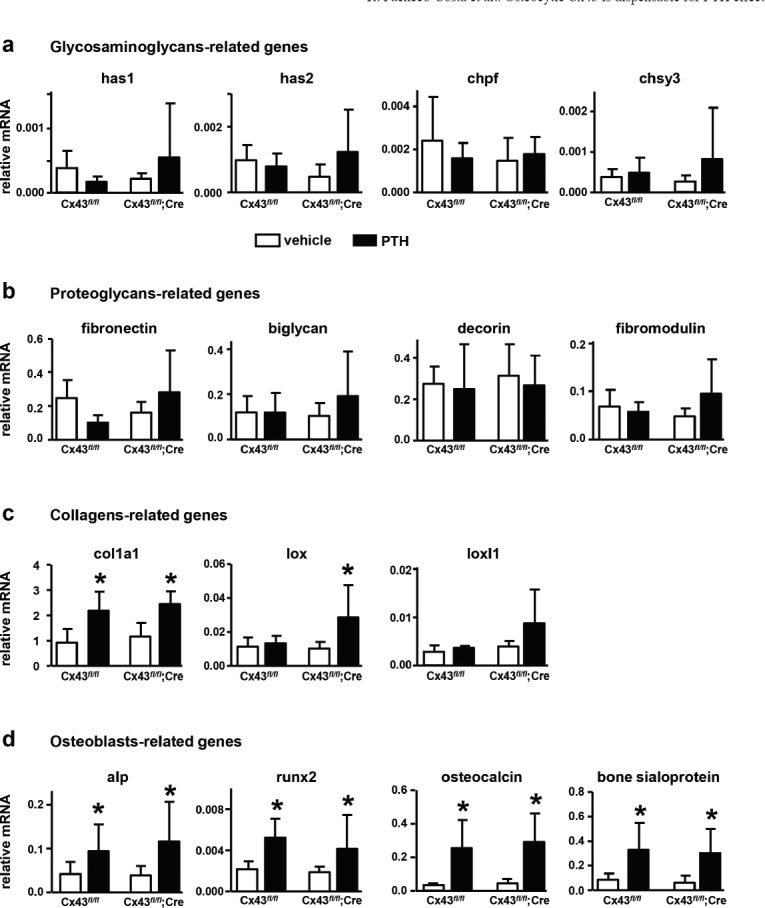
Intermittent PTH administration increases the expression of osteoblast markers, independently of osteocytic Cx43 expression. Gene expression in L6 vertebrae from Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre male mice and after treatment for 14 days with injections of PTH or vehicle control. (a) GAGs-, (b) proteoglycans-, (c) collagen-, and (d) osteoblast-related genes were measured at 4.5 months of age by qPCR and corrected by GAPDH. Bars represent mean ± SD, n=4-10. * p<0.05 versus vehicle by two-way ANOVA.
Table 2.
Statistical analysis of the effect of intermittent PTH administration on gene expression in mice expressing or lacking Cx43 in osteocytes. Two-way ANOVA, followed by Student-Newman-Keuls post-hoc test.
| collagen-related genes | osteoblast-related genes | ||||||
|---|---|---|---|---|---|---|---|
| Source of variation | col1a1 | lox | loxl1 | alp | runx2 | osteocalcin | bsp |
| p value | p value | p value | p value | p value | p value | p value | |
| Genotype | 0.176 | 0.042 | 0.028 | 0.228 | 0.260 | 0.907 | 0.520 |
| Treatment | <0.001 | 0.048 | 0.220 | <0.001 | <0.001 | <0.001 | <0.001 |
| Genotype × treatment | 0.586 | 0.138 | 0.431 | 0.462 | 0.709 | 0.084 | 0.980 |
Intermittent PTH administration increases the levels of type I collagen protein in the absence and type II collagen in the presence of osteocytic Cx43
Visual inspection of bone sections stained for type I and type II collagen and decorin suggested similar protein expression in bone sections from vehicle-treated Cx43fl/fl or Cx43fl/fl; DMP1-8kb-Cre mice (Figure 3a, c, f, h, k and m). Densitometric analysis confirmed that deletion of osteocytic Cx43 did not affect the expression of type I collagen, type II collagen or decorin, with similar levels in vehicle-treated Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre mice (Figure 3e, j and o). We also found that vehicle-treated Cx43fl/fl exhibited similar type I collagen levels when compared to the corresponding intermittent PTH-treated mice (Figure 3a and b). On the other hand, PTH-treated Cx43fl/fl; DMP1-8kb-Cre mice showed a more intense type I staining, compared to vehicle-treated mice of the same genotype (Figure 3c and d). The visual examination was confirmed by densitometric quantification of the sections, with a significant increase in type I collagen protein level by 29% only in Cx43fl/fl; DMP1-8kb-Cre mice treated with intermittent PTH, compared to vehicle-treated mice (Figure 3e), consistent with the increase in lox levels only in mice lacking Cx43 in osteocytes treated with the hormone. As for type I collagen, the expression of type II collagen was not affected by Cx43 deletion (Figure 3f and h). However, the intensity of staining for this collagen is higher in bone sections from Cx43fl/fl mice treated with intermittent PTH (Figure 3g) compared to the same mice treated with vehicle (Figure 3f). On the other hand, the expression of type II collagen in Cx43fl/fl; DMP1-8kb-Cre mice was not affected by PTH administration (Figure 3h and i). Densitometric quantification of the sections confirmed the increase in type II collagen expression induced by PTH only in Cx43fl/fl (Figure 3j). The expression of decorin, a proteoglycan associated with collagen fibrillogenesis, was not altered in bone sections from intermittent PTH-treated Cx43fl/fl or Cx43fl/fl; DMP1-8kb-Cre mice when compared to their littermates receiving vehicle injections (Figure 3k-o).
Figure 3.
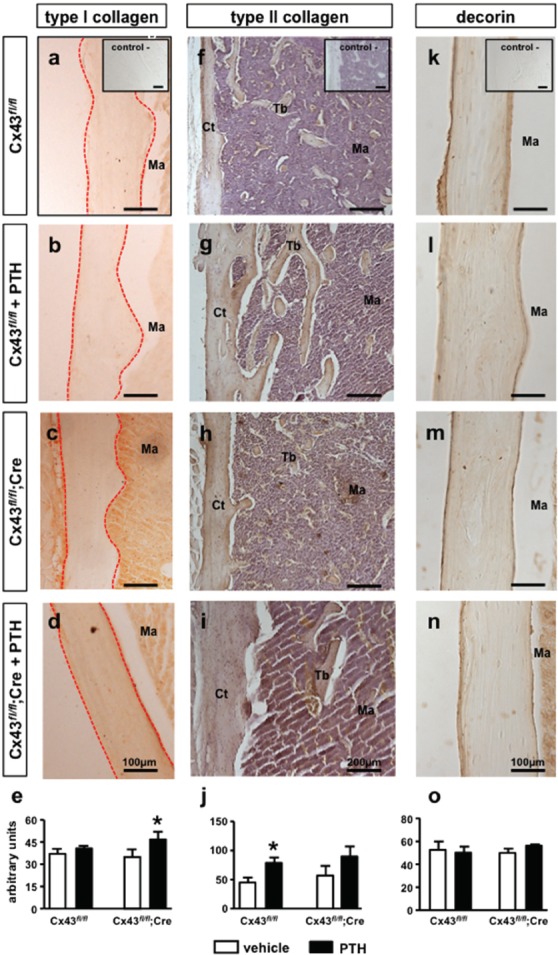
Intermittent PTH increases type I or type II collagens, depending on the absence or presence of osteocytic Cx43. Bone sections from male mice at 4.5 months of age were subjected to type I collagen immunohistochemistry (diffuse brown color). Photomicrographs show cortical region of Cx43fl/fl mice treated with vehicle (a) or with intermittent PTH (b). Inset shows a representative image from negative control for the reactions. Outlined in red shows the regions where measurements were performed. Photomicrographs from cortical region of Cx43fl/fl; DMP1-8kb-Cre mice treated with vehicle (c) or with the intermittent PTH (d) treated. Scale bars represent 100 µm. (e) Quantification of type I collagen. Bars represent mean ± SD, n= 4-8 mice per group. *p<0.05 versus corresponding vehicle by two-way ANOVA. Bone sections from male mice at 4.5 months of age stained for type II collagen (diffuse brown color). Photomicrographs from cortical and cancellous region of Cx43fl/fl mice treated with vehicle (f) or with intermittent PTH (g) are shown. Inset shows a representative image from negative control for the reactions. Photomicrographs from cortical and cancellous region of Cx43fl/fl; DMP1-8kb-Cre mice treated with vehicle (h) or with intermittent PTH (i) are shown. Scale bars represent 200 µm. (j) Quantification of type II collagen. Bars represent mean ± SD, n=4-8 mice per group. *p<0.05 versus corresponding vehicle by two-way ANOVA. Bone sections from femora from male mice at 4.5 months of age stained for decorin (diffuse brown color) are shown. Photomicrographs showing cortical region of distal femur of Cx43fl/fl mice treated with vehicle (k) or with intermittent PTH (l) are included. Inset shows a representative image from negative control for the reactions. Photomicrographs from cortical region of Cx43fl/fl; DMP1-8kb-Cre mice treated with vehicle (m) or with intermittent PTH (n) are shown. Scale bars represent 100 µm. (o) Quantification of decorin. Bars represent mean ± SD, n=4-8 mice per group. *p<0.05 versus corresponding vehicle by two-way ANOVA. Ct= cortical, Tb= trabecula, Ma= bone marrow.
Deletion of osteocytic Cx43 impairs collagen maturation induced by intermittent PTH administration
Cx43fl/fl mice treated with vehicle exhibited a slight predominance of reddish in relation to greenish birefringence in cortical (Figure 4a) and cancellous (Figure 4b) bone sections, with a relative score of approximately 2. Statistical analysis did not reveal difference in birefringence between Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre mice, indicating that collagen maturation is independent on the expression of Cx43 in osteocytes (Table 3). Intermittent PTH administration decreased greenish birefringence in the cortical bone to a score of approximately 1 in Cx43fl/fl mice (Figure 4c and Table 3), indicating the presence of more mature collagen fibers. On the other hand, the hormone did not modify the score in cancellous bone of Cx43fl/fl mice (Figure 4d), indicating changes in collagen maturation only in the cortical bone compartment. Deletion of Cx43 from osteocytes did not modify the collagen birefringence pattern in cortical (Figure 4e) or in cancellous bone (Figure 4f), when compared to control littermates (Figure 4a-b and Table 3). Furthermore, similar to Cx43fl/fl mice (Figure 4d), intermittent PTH did not alter the pattern of collagen maturation in cancellous bone in Cx43fl/fl; DMP1-8kb-Cre mice (Figure 4h). However, unlike controls expressing osteocytic Cx43 (Figure 4c), intermittent PTH administration did not alter the reddish/greenish pattern in cortical bone, with a relative score of approximately 2 in mice lacking Cx43 in osteocytes (Figure 4g and Table 3). This suggests that Cx43 expression in osteocytes is required for the effect of intermittent PTH on collagen maturation only in cortical bone.
Figure 4.
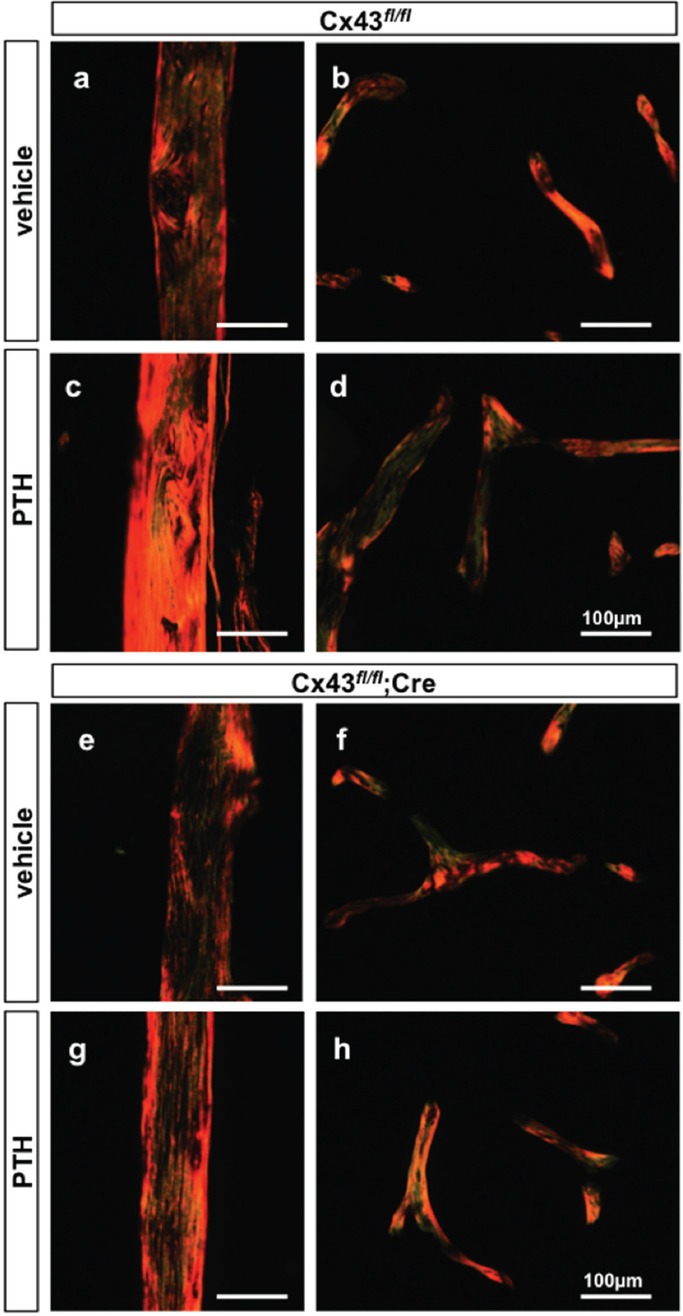
The effect of intermittent PTH on collagen birefringence in cortical bone is impaired in mice lacking osteocytic Cx43. Bone sections from femora from male mice at 4.5 months of age were stained by picrosirius red and visualized under polarized light microscopy. Photomicrographs from cortical (a) and cancellous bone (b) of Cx43fl/fl vehicle-treated mice and cortical (c) and cancellous bone (d) of intermittent PTH-treated mice. Photomicrographs from cortical (e) and cancellous bone (f) of Cx43fl/fl; DMP1-8kb-Cre vehicle-treated mice and cortical (g) and cancellous bone (h) of intermittent PTH-treated mice. The scale bar represents 100 µm. Images are representative of 4-6 mice per group.
Table 3.
Quantification of collagen birefringence in cortical and cancellous bone of mice lacking Cx43 and the corresponding littermate controls, treated with vehicle or intermittent PTH.
| Cx43fl/fl | Cx43fl/fl + PTH | Cx43fl/fl;Cre | Cx43fl/fl;Cre + PTH | |
|---|---|---|---|---|
| Cortical bone | 2.0 ± 0.0 | 1.1 ± 0.3a | 1.7 ± 0.5 | 1.9 ± 0.2 |
| Cancellous bone | 1.6 ± 0.5 | 1.8 ± 0.3 | 1.8 ± 0.5 | 1.8 ± 0.4 |
Values are mean ± SD. n=4-6. p<0.05 by two-way ANOVA.
Indicates significant difference versus vehicle-treated Cx43fl/fl mice.
Deletion of Cx43 or treatment with PTH does not change material density
We also examined the effects of genetic deletion of osteocytic Cx43 or pharmacologic administration of PTH on bone material density, an index of mineralization level of the tissue independent of bone volume, assessed by µCT[37]. Neither deletion of Cx43 from osteocytes nor intermittent PTH administration alter the material density in the distal femur and femoral mid-diaphysis from Cx43fl/fl or Cx43fl/fl; DMP1-8kb-Cre mice (Figure 5), indicating that 14-day treatment with PTH does not affect bone mineralization.
Figure 5.

Material density is not change by either genotype or treatment. Material density was assessed by µCT in cancellous bone at the distal femora and in cortical bone at the femoral mid-diaphysis from Cx43fl/fl and Cx43fl/fl; DMP1-8kb-Cre mice at 4.5 months of age. Bars represent mean ± SD, n=4-10.
Discussion
We show in the current study that expression of Cx43 in osteocytes is not required for the anabolic effect induced by intermittent PTH administration in male mice. Similarly, deletion of osteocytic Cx43 does not affect the mRNA levels of collagen and non-collagenous proteins in vertebral bone. However, it alters the effect of PTH on type I and type II collagen protein levels and blunts the effect of the hormone on collagen maturation in cortical bone.
Previous findings showed that Cx43 expression in cultured osteoblastic cells is necessary for cAMP accumulation[46,47] and for the expression of cAMP-dependent genes[47] induced by PTH treatment, thus indicating the importance of Cx43 molecule for intracellular signaling induced by the hormone. Moreover, reduced bone formation, defective osteoblast function, and, overall, decreased anabolic response to intermittent PTH administration were reported in mice lacking Cx43 in osteoblasts[32]. On the contrary, our findings show a full anabolic response of intermittent PTH administration independently of the presence (Cx43f/f mice) or the absence (Cx43fl/fl; DMP1-8kb-Cre mice) of osteocytic Cx43 in male mice, with increased total body, femoral and spinal BMD. Furthermore, cancellous bone volume in the lumbar vertebrae is also increased in mice treated with intermittent PTH, independently of osteocytic Cx43 expression. Recently, we reported that females lacking osteocytic Cx43 also respond to intermittent PTH administration[48]. Taken together with published evidence, the results reported in this manuscript indicate that Cx43 expression in osteoblasts, but not in osteocytes is required for bone formation induced by intermittent PTH administration.
The characterization of molecules in bone matrix is crucial for understanding how the matrix behaves in the absence of osteocytic Cx43, and also in the face of intermittent PTH administration, since modifications in the distribution and synthesis of collagen and other organic molecules are reflected on cell fate and fracture risk[16,49]. We dedicated part of the current study to investigate collagens and GAGs/proteoglycans, molecules abundantly expressed in the bone tissue. However, we were not able to detect pronounced changes in bone matrix components or in the level of mineralization in mice in which Cx43 was deleted from osteocytes, indicating that osteocytic Cx43 is not require for the formation of either organic or inorganic components of the bone matrix under basal (untreated) conditions.
Type I collagen fibers are the most abundant component of organic bone matrix. Studies have shown increased type I collagen expression after intermittent PTH administration in human bone tissue and type II collagen in mesenchymal stem cells[16,50]. Therefore, we used both types of collagen to verify whether PTH increases the expression of these collagens and whether they would be affected by deletion of osteocytic Cx43. Our evidence suggests a divergent role of Cx43 on the expression of collagen induced by intermittent PTH administration: while the effect of intermittent PTH on type I collagen is only present in mice lacking osteocytic Cx43, the effect of the hormone on type II collagen is only observed when osteocytic Cx43 is expressed. Further studies are required to determine the molecular basis of this disparate effect of Cx43 in osteocytes.
Altered collagen is associated with bone fragility in animals and humans[16,51-54] and the degree of collagen fibers maturation is revealed through their thickness and consequently can be assess by determining their birefringence[55]. Moreover, a relationship between collagen cross-linking and mineralization of bone matrix was reported in monkeys and humans treated with low doses of intermittent PTH for long term, indicating that PTH decreased the maturity of collagen fibers[56,57]. In the present study, we evaluated collagen birefringence behavior by picrosirius red/polarization, a well-known method to assess collagen maturation[17,43-45,58]. Immature fibers are thinner, and under light polarized show a greenish birefringence, whereas mature fibers are thicker and show a pattern of yellow to reddish birefringence[59,60]. We did not observe a difference in pattern of collagen birefringence in Cx43f/f and Cx43fl/fl; DMP1-8kb-Cre mice. This evidence contrasts with findings in mice lacking Cx43 from osteoblasts, in which disorganization of collagen was found using the same picrosirius/polarization method[61], suggesting that osteocytic Cx43 does not modulate the behavior of collagen fibers. On the other hand, the results of the current study indicate that while intermittent PTH administration induced a response in collagen maturation in cortical bone of Cx43fl/fl mice, it failed to do so in Cx43fl/fl; DMP1-8kb-Cre mice. We cannot rule out the possibility that the change of collagen birefringence is associated with changes in type II collagen, since the expression of this protein was increased in bone section of distal femora. It is also possible that Cx43fl/fl; DMP1-8kb-Cre mice exhibit a delayed response to intermittent PTH and that the hormone would induce collagen maturity in the cortical compartment given enough time.
As discussed above, we have previously reported that absence of osteocytic Cx43 is associated with impaired collagen cross-linking, and with changes in the expression of enzymes associated with collagen cross-linking[31]. Thus, we investigated whether genes related to collagen cross-linking (lox, loxl-1/4) were altered, in order to provide a potential explanation for why the effect of the hormone in the bone matrix is impaired when Cx43 is absent in osteocytes. We found that overall mRNA levels were not altered by either the genotype or the treatment in vertebral lysates. Even though the statistical analysis showed a genotype effect on the levels of lox and loxl1, the difference in vehicle-treated mice is not clear and the overall significance seems to be driven by the PTH treatment.
We also investigate the effect of the genetic (deletion of osteocytic Cx43) and pharmacologic manipulations (intermittent PTH administration) on GAGs and proteoglycans. GAGs presence is related to the development and structural consolidation of the collagen fibers in the bone matrix[62]. In addition, mice deficient of biglycan or decorin, both chondroitin-rich proteoglycans, exhibit disorganization in the shape and size of the collagen fibers[63], and removal of decorin is a potential cause for fusion of the collagen fibers[64]. Indeed, changes in GAGs/proteoglycans reflect in the collagen fibers since these elements are intrinsically associated, as reported elegantly in a morphological study showing that decorin and biglycan inhibits totally or partly the collagen fusion[65]. We therefore focused our study on decorin and biglycan expression because of their intrinsic association with collagen fibers[66-70]. Our study did not show differences in the mRNA expression of these genes or in decorin levels by immunohistochemistry, suggesting that these proteoglycans are not changed in Cx43-deficient or in intermittent PTH-treated mice. Supporting these findings, expression of biglycan is not affected in a model of PTH-related protein-deficient mice[25]. Thus, it is possible to conclude that biglycan and decorin proteoglycans are not responsible for collagen modifications when osteocytic Cx43 was deleted. Our data suggests that osteoblastic cells that still express Cx43 are able to respond to intermittent PTH administration, consequently producing proteoglycans, collagens and other molecules associated with bone formation[16,25,71,72]. In support of this notion, we detected in bone preparations increased expression of runx2, alkaline phosphatase, osteocalcin, bone sialoprotein and type I collagen, genes associated with differentiation, formation and activity of osteoblasts and known to respond to daily PTH injections[9,16,32,73-75].
In summary, the current study shows that osteocytic Cx43 expression is not required for the full anabolic response to the intermittent PTH administration in male mice; and that this connexin is involved partially in the effect of PTH on the bone matrix environment.
Acknowledgements
The authors are thankful to Iraj Hassan for µCT scanning and analysis, to Patricia Gonçalves for assistance with the immunohistochemistry and to Dr. Matthew R. Allen for his advice on µCT analysis. E.G.A received scholarships from IUPUI, Life-Health Sciences Internship Program and the CTSI - Clinical and Transitional Sciences Institute Award. R.P.C received a scholarship from Coordination of Improvement of Higher Level Personnel (CAPES), Brazil (PDEE: #1065/11-4). This research was supported by the National Institutes of Health R01-AR053643, USA to L.I.P, and by CAPES and National Council for Scientific and Technological Development - CNPq, Brazil to R.P.C, E.K and R.D.R.
Footnotes
Edited by: F. Rauch
References
- 1.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 2.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qing H, Ardeshirpour L, Pajevic PD, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–29. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–43. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287:33926–33. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamoureux F, Baud’huin M, Duplomb L, Heymann D, Rédini F. Proteoglycans: key partners in bone cell biology. Bioessays. 2007;29:758–71. doi: 10.1002/bies.20612. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–82. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Bilezikian JP, Matsumoto T, Bellido T, et al. Targeting bone remodeling for the treatment of osteoporosis: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2009;24:373–85. doi: 10.1359/jbmr.090105. [DOI] [PubMed] [Google Scholar]
- 9.Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–72. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 10.Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone. 2009;45:590–602. doi: 10.1016/j.bone.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 14.Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54:250–7. doi: 10.1016/j.bone.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascenzi MG, Liao VP, Lee BM, et al. Parathyroid hormone treatment improves the cortical bone microstructure by improving the distribution of type I collagen in postmenopausal women with osteoporosis. J Bone Miner Res. 2012;27:702–12. doi: 10.1002/jbmr.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco-Costa R, Campos JF, Katchburian E, et al. Modifications in bone matrix of estrogen-deficient rats treated with intermittent PTH. Biomed Res Int. 2015;2015:454162. doi: 10.1155/2015/454162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Midura RJ, Su X, Morcuende JA, Tammi M, Tammi R. Parathyroid hormone rapidly stimulates hyaluronan synthesis by periosteal osteoblasts in the tibial diaphysis of the growing rat. J Biol Chem. 2003;278:51462–8. doi: 10.1074/jbc.M307567200. [DOI] [PubMed] [Google Scholar]
- 19.Midura RJ, Evanko SP, Hascall VC. Parathyroid hormone stimulates hyaluronan synthesis in an osteoblast-like cell line. J Biol Chem. 1994;269:13200–6. [PubMed] [Google Scholar]
- 20.Ariyoshi W, Takahashi T, Kanno T, et al. Mechanisms involved in enhancement of osteoclast formation and function by low molecular weight hyaluronic acid. J Biol Chem. 2005;280:18967–72. doi: 10.1074/jbc.M412740200. [DOI] [PubMed] [Google Scholar]
- 21.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 22.Hirata M, Kobayashi M, Takita M, Matsumoto C, Miyaura C, Inada M. Hyaluronan inhibits bone resorption by suppressing prostaglandin E synthesis in osteoblasts treated with interleukin-1. Biochem Biophys Res Commun. 2009;381:139–43. doi: 10.1016/j.bbrc.2009.01.146. [DOI] [PubMed] [Google Scholar]
- 23.Chang EJ, Kim HJ, Ha J, et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 2007;120:166–76. doi: 10.1242/jcs.03310. [DOI] [PubMed] [Google Scholar]
- 24.Huang T, Shao Q, Macdonald A, et al. Autosomal recessive GJA1 (Cx43) gene mutations cause oculodentodigital dysplasia by distinct mechanisms. J Cell Sci. 2013;126:2857–66. doi: 10.1242/jcs.123315. [DOI] [PubMed] [Google Scholar]
- 25.Ogihara Y, Suda N, Hammond VE, Senior PV, Beck F, Yanagishita M. Biosynthesis of proteoglycan in bone and cartilage of parathyroid hormone-related protein knockout mice. J Bone Miner Metab. 2001;19:4–12. doi: 10.1007/s007740170054. [DOI] [PubMed] [Google Scholar]
- 26.Novince CM, Michalski MN, Koh AJ, et al. Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res. 2012;27:11–25. doi: 10.1002/jbmr.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Lai X, Price C, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotkin LI. Connexin 43 hemichannels and intracellular signaling in bone cells. Front Physiol. 2014;5:131. doi: 10.3389/fphys.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zappitelli T, Chen F, Moreno L, et al. The G60S connexin 43 mutation activates the osteoblast lineage and results in a resorption-stimulating bone matrix and abrogation of old-age-related bone loss. J Bone Miner Res. 2013;28:2400–13. doi: 10.1002/jbmr.1965. [DOI] [PubMed] [Google Scholar]
- 31.Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int. 2012;91:215–24. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung DJ, Castro CH, Watkins M, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–98. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 33.Theis M, de Wit C, Schlaeger TM, et al. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Bivi N, Condon KW, Allen MR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–89. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacheco-Costa R, Hassan I, Reginato RD, et al. High bone mass in mice lacking cx37 because of defective osteoclast differentiation. J Biol Chem. 2014;289:8508–20. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 37.Mulder L, Koolstra JH, Van Eijden TM. Accuracy of microCT in the quantitative determination of the degree and distribution of mineralization in developing bone. Acta Radiol. 2004;45:769–77. doi: 10.1080/02841850410008171. [DOI] [PubMed] [Google Scholar]
- 38.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–21. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Girol AP, Mimura KK, Drewes CC, et al. Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro. J Immunol. 2013;190:5689–701. doi: 10.4049/jimmunol.1202030. [DOI] [PubMed] [Google Scholar]
- 42.Hein G, Weiss C, Lehmann G, Niwa T, Stein G, Franke S. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Ann Rheum Dis. 2006;65:101–4. doi: 10.1136/ard.2004.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Florencio-Silva R, Santos MA, de Medeiros VP, et al. Effects of soy isoflavones and mechanical vibration on rat bone tissue. Climacteric. 2013;16:709–17. doi: 10.3109/13697137.2013.769096. [DOI] [PubMed] [Google Scholar]
- 44.Santos MA, Florencio-Silva R, Medeiros VP, et al. Effects of different doses of soy isoflavones on bone tissue of ovariectomized rats. Climacteric. 2014;17:393–401. doi: 10.3109/13697137.2013.830606. [DOI] [PubMed] [Google Scholar]
- 45.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 46.Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem. 1996;271:12165–71. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- 47.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–30. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacheco-Costa R, Davis HM, Sorenson C, et al. Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone. 2015;81:632–43. doi: 10.1016/j.bone.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amend SR, Uluckan O, Hurchla M, et al. Thrombospondin-1 Regulates Bone Homeostasis Through Effects on Bone Matrix Integrity and Nitric Oxide Signaling In Osteoclasts. J Bone Miner Res. 2015;30:106–15. doi: 10.1002/jbmr.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A. 2010;16:3449–55. doi: 10.1089/ten.TEA.2010.0091. [DOI] [PubMed] [Google Scholar]
- 51.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:365S–71S. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 52.Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int. 1999;65:203–10. doi: 10.1007/s002239900683. [DOI] [PubMed] [Google Scholar]
- 53.Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Biochemical changes in the collagen of human osteoporotic bone matrix. Connect Tissue Res. 1993;29:119–32. doi: 10.3109/03008209309014239. [DOI] [PubMed] [Google Scholar]
- 54.Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Post-translational modifications in the collagen of human osteoporotic femoral head. Biochem Biophys Res Commun. 1992;185:801–5. doi: 10.1016/0006-291x(92)91697-o. [DOI] [PubMed] [Google Scholar]
- 55.Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 56.Paschalis EP, Glass EV, Donley DW, Eriksen EF. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrinol Metab. 2005;90:4644–9. doi: 10.1210/jc.2004-2489. [DOI] [PubMed] [Google Scholar]
- 57.Paschalis EP, Burr DB, Mendelsohn R, Hock JM, Boskey AL. Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH(1-34) for 18 months. J Bone Miner Res. 2003;18:769–75. doi: 10.1359/jbmr.2003.18.4.769. [DOI] [PubMed] [Google Scholar]
- 58.Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74:153–6. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- 59.Retamoso LB, da Cunha TeM, Knop LA, Shintcovsk RL, Tanaka OM. Organization and quantification of the collagen fibers in bone formation during orthodontic tooth movement. Micron. 2009;40:827–30. doi: 10.1016/j.micron.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Hirshberg A, Lib M, Kozlovsky A, Kaplan I. The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol. 2007;43:278–82. doi: 10.1016/j.oraloncology.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Watkins M, Grimston SK, Norris JY, et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22:1240–51. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parry DA, Flint MH, Gillard GC, Craig AS. A role for glycosaminoglycans in the development of collagen fibrils. FEBS Lett. 1982;149:1–7. doi: 10.1016/0014-5793(82)81060-0. [DOI] [PubMed] [Google Scholar]
- 63.Corsi A, Xu T, Chen XD, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–9. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 64.Hoshi K, Kemmotsu S, Takeuchi Y, Amizuka N, Ozawa H. The primary calcification in bones follows removal of decorin and fusion of collagen fibrils. J Bone Miner Res. 1999;14:273–80. doi: 10.1359/jbmr.1999.14.2.273. [DOI] [PubMed] [Google Scholar]
- 65.Raspanti M, Viola M, Forlino A, Tenni R, Gruppi C, Tira ME. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J Struct Biol. 2008;164:134–9. doi: 10.1016/j.jsb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Vogel KG, Paulsson M, Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–97. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svensson L, Heinegård D, Oldberg A. Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4-5. J Biol Chem. 1995;270:20712–6. doi: 10.1074/jbc.270.35.20712. [DOI] [PubMed] [Google Scholar]
- 68.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Connizzo BK, Sarver JJ, Birk DE, Soslowsky LJ, Iozzo RV. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135:021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott JE, Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985;5:71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- 71.Ishikawa Y, Wu LN, Genge BR, Mwale F, Wuthier RE. Effects of calcitonin and parathyroid hormone on calcification of primary cultures of chicken growth plate chondrocytes. J Bone Miner Res. 1997;12:356–66. doi: 10.1359/jbmr.1997.12.3.356. [DOI] [PubMed] [Google Scholar]
- 72.Madiraju P, Gawri R, Wang H, Antoniou J, Mwale F. Mechanism of parathyroid hormone-mediated suppression of calcification markers in human intervertebral disc cells. Eur Cell Mater. 2013;25:268–83. doi: 10.22203/ecm.v025a19. [DOI] [PubMed] [Google Scholar]
- 73.Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007;22:64–71. doi: 10.1359/jbmr.061006. [DOI] [PubMed] [Google Scholar]
- 74.Wade-Gueye NM, Boudiffa M, Vanden-Bossche A, et al. Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge. Bone. 2012;50:1064–73. doi: 10.1016/j.bone.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 75.Sheyn D, Cohn Yakubovich D, Kallai I, et al. PTH promotes allograft integration in a calvarial bone defect. Mol Pharm. 2013;10:4462–71. doi: 10.1021/mp400292p. [DOI] [PMC free article] [PubMed] [Google Scholar]


