Abstract
There are numerous studies presenting the beneficial effect of bisphosphonates (BPs) on bone disease of patients suffering from beta-thalassemia major (TM). Although BPs have been widely used, adverse events have been described including atypical femoral fractures (AFF). In the present case, a male adult patient suffering from TM sustained an AFF fulfilling all major and two minor criteria. Before AFF, the patient had been treated with zoledronic acid for three years and remained another one year without osteoporosis therapy. To our knowledge, this is the first reported case of AFF in a patient suffering from TM, probably due to the small sample size of patients with thalassemia. The purpose of the present case is to increase the awareness amongst haematologists, who mainly deal with TM patients, of the adverse events of BP use.
Keywords: Thalassemia, Bone Disease, Atypical Fracture, Bisphosphonates, Adverse Event
Introduction
Beta-thalassemia major (TM) affects 0.44 per 1,000 births worldwide[1]. It is an inherited recessive of beta hemoglobin synthesis due to beta globin genes mutations on chromosome 11, leading to ineffective erythropoiesis and anaemia requiring regular blood transfusions[2]. Chronic transfusion therapy results to iron overload that cause a wide spectrum of complications due to heart, liver and endocrine glands damage[3]. Although improvement of management of patients with TM have increased life span, other comorbidities of the disease including bone disease have emerged. There are numerous studies presenting the beneficial effect of bisphosphonates (BPs) on bone disease and their use is widespread in TM patients with low bone mass (BMD) and high fracture risk[4-6].
Although bisphosphonates have been widely used, they present several adverse events including osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF)[7]. Only recently 3 cases of ONJ have been reported in these patients following BP therapy[8]. To our knowledge, this is the first reported case of a male adult patient suffering from TM that sustained an AFF after previous zoledronic acid use.
Case report
A 36-year-old male patient with beta-thalassemia major sustained a left tibial medial condyle and shaft fracture after a motor vehicle accident (Figure 1). The tibial fractures were treated with two free cannulated screws and a reamed static locked intramedullary nail. Rehabilitation started on forth postoperative day with partial weight bearing on the left leg using crutches. On the eleventh postoperative day, the patient felt a sudden acute pain, with no reported prodromal pain, when walking on his right thigh and became unable to walk on his right leg. Radiographs revealed a short oblique femoral shaft fracture without comminution, and absence of cortical thickness and cortical beaking (Figure 2). This fracture was also treated with a reamed static locked intramedullary nail.
Figure 1.
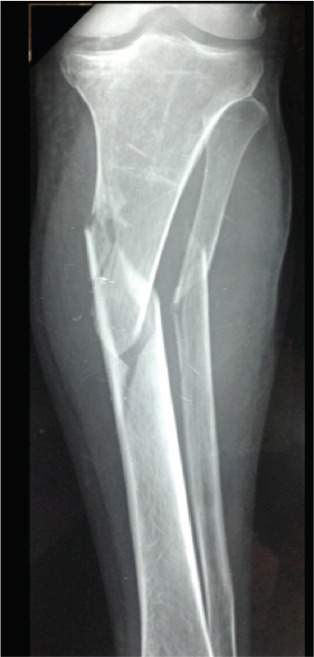
Anteroposterior radiograph of the left tibia after a motor vehicle accident presents a tibial medial condyle and shaft fracture.
Figure 2.
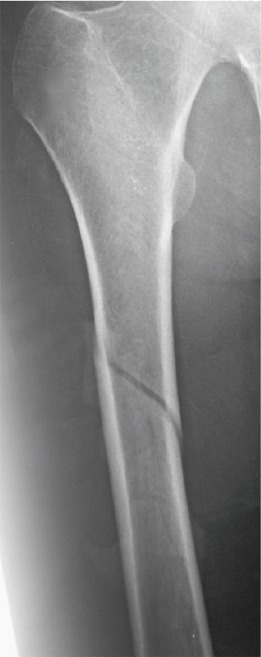
Anteroposterior radiograph of the right femur, eleven days after operation of the tibial fracture, reveals a short oblique femoral shaft fracture without comminution.
The patient suffers from beta-thalassemia major requiring transfusion twice a month since childhood and iron chelating-therapy with desferoxamine until 2007 and deferiprone since that time. Other comorbidities include type 2 diabetes mellitus, hypertension, osteoporosis with a compressive fracture of the lumbar spine (L4) sustained 6 years earlier, esophageal varices treated with banding and cholochystectomy. His medications consisted of metformin, glyclazide, magnesium, sotalol and ramipril/hydrochlorothiazide. As for the osteoporosis treatment, after the occurrence of the vertebral fracture, the patient had been treated with intranasal calcitonin 200 IU daily for one year followed by intravenous zoledronic acid 5 mg once yearly for 3 years along with calcium and vitamin D supplementation. Thus, the patient at the time of the tibial and femoral fractures had remained without osteoporosis treatment for one year. Four months postoperatively, teriparatide (TRP) treatment was initiated.
Bone densitometry measured by Lunar Prodigy dual-energy X-ray absorptiometry (DXA) was consistent with BMD lower than that expected for age in the left hip 5 and one year before tibial and femoral fracture (Z-score, -3.4 and 3.5, respectively). Biochemical evaluation revealed normal testosterone levels, acceptable levels of 25(OH) vitamin D (27 ng/mL), while bone markers were within normal range (P1NP and sCTX) three months after tibial and femoral fracture, similarly with values reported in most of the patients with AFF treated with BPs[9] (Table 1).
Table 1.
Laboratory tests of the patient three months after tibial and femoral fracture.
| Values | Normal range values | |
|---|---|---|
| Creatinine (mg/dL) | 0.65 | 0.7-1.3 |
| Calcium (mg/dL) | 8.8 | 8.4-10.2 |
| Phosphate (mg/dL) | 3.6 | 2.5-4.7 |
| ALP (IU/L) | 126 | 40-150 |
| Albumin (gr/dL) | 3.6 | 3.5-5.0 |
| Testosterone (ng/dL) | 320 | 280-1100 |
| TSH (µIU/mL) | 2.4 | 0.55-4.78 |
| 25(OH)D (ng/mL) | 27 | 30-60 (deficiency <20) |
| PTH (pg/mL) | 15.7 | 15-65 |
| PINP (ng/mL) | 26 | 15-59 |
| sCTX (ng/mL) | 0.335 | <0.584 |
ALP, alkaline phosphatase; TSH, thyroid-stimulating hormone; 25(OH)D, 25-hydroxy vitamin D; PTH, parathyroid hormone; PINP, procollagen type I N-terminal propeptide; sCTX, serum C-telopeptide cross-link of type-I collagen.
After the second operation, patient’s rehabilitation included lower-extremity isometric and range of motion exercises for 4 weeks and initiation of a weight-bearing as tolerated ambulation with bilateral axillary crutches on the first postoperative week. Six weeks postoperatively, full-weight-bearing was started. Patient returned to work and was able to perform activities of daily living on forth postoperative month.
Discussion
Thalassemia-related bone disease had been first described by Cooley et al as “peculiar bone changes” consisted by cranial, facial and limb marked deformities mainly due to bone marrow expansion[10]. The introduction of transfusion therapy in 1960 have been resulted to amelioration of such bone deformities[11]. Nowadays, bone disease is characterised by low BMD, increased risk of fractures and bone pain. Possible risk factors for fracture are age older than 20 years, male gender, hypothyroidism, hypogonadism, lack of spontaneous puberty in females, active hepatitis, heart disease and diabetes[12,13]. Most of the fractures are presented in the upper extremity and spine mainly caused by a fall[14,15].
Pathogenesis of bone disease in TM is complex and some of the underlying mechanisms are still unclear. Multiple genetic and acquired factors are involved. Already known genetic factors include polymorphisms at the Sp1 site of the collagen type Ia1 (COLIA 1) gene, at FokI and BsmI of vitamin D receptor (VDR) gene and at 713-8delC of transforming growth factor-beta (TGF-b) gene. Acquired factors include ineffective erythropoiesis and subsequent bone marrow expansion due to increased erythropoietin levels, iron overload and iron chelation therapy (particularly deferoxamine), endocrine complications such as hypogonadism, hypothyroidism, dysfunction of GH/IGF-1 axis, diabetes, calcium and vitamin C and D deficiencies, as well as decreased physical activity[12,13]. These factors lead to an imbalance in bone remodelling by inhibiting bone formation and increasing bone resorption.
General principles of management of TM patients are regular blood transfusions and iron chelation therapy with deferasirox, hormone replacement therapy, adequate calcium and vitamin D supplementation and increase of physical activity[16]. Annual BMD measurement, starting in adolescence, is recommended for the early diagnosis of BMD below the expected values for age and initiation of antiosteoporotic treatment[13]. The increased bone resorption observed in TM patients could justify the use of BPs. BPs are the most widely used drugs for the prevention and treatment of bone loss[4]. A systematic review of randomised controlled trials have concluded that reliable clinical studies in TM patients showed improved BMD, reduced bone and back pain and decreased bone turnover markers without causing any severe or unexpected adverse event after various dose regimens of an amino-BP (alendronate, neridronate, or zoledronic acid) compared to baseline and to placebo. However, long-term studies are required to answer about the reduction of fracture risk[5]. There is lack of evidence on the treatment of patients suffering from TM with other antiosteoporotic agents such as TRP. To our knowledge, there is only one case presenting encouraging results of TRP use in TM associated bone disease in the English literature[17].
According to the updated Task Force of the ASBMR, an AFF should present all the major features that include its location in the subtrochanteric region and femoral diaphysis, association with no or minimal trauma, transverse or short oblique configuration and lack of comminution. Complete AFFs extend through both cortices and incomplete only in the lateral cortex. These fractures may present also some of the minor features that include periosteal reaction of the lateral cortex, prodromal symptoms, fractures or symptoms bilaterally, delayed healing, comorbid conditions and use of certain pharmaceutical agents such as BPs[18]. Recently, AFFs have been considered as stress/ fatigue or insufficiency fractures that develop over the time and present specific features[18,19]. They have been described mostly as a potent complication of BP use[7], while the risk remains albeit decreased by about 70% per year after BP discontinuation[18,19].
Although the causal association between BP use and AFF remains uncertain, the most likely mechanisms relate to the suppression of bone turnover include increase of degree and homogenicity of mineralization, collagen maturity, microcrack accumulation, propagation and impairment of crack repair. Finally, anti-angiogenic effects of BPs may be implicated[20]. Empirical management for AFF includes application of intramedullary full-length nails, discontinuation of BP treatment, adequate supplementation with calcium and vitamin D and anabolic therapy with TRP or strontium ranelate[18,21-23].
There are many aspects needing to be addressed regarding treatment of bone disease of TM patients. Although there is evidence that BPs demonstrate beneficial effects in bone disease of TM, the dose regimen, duration of therapy and drug-free holidays have not yet been clarified[6]. Given the young age of patients, there is concern about their safety in use in reproductive age[24] and their effect on reducing morbidity and mortality of such patients. There is also lack of evidence in prediction of fracture risk by using DXA in TM patients[25,26]. Furthermore, existing data support that haemoglobin levels are positively correlated with BMD, however it is still unclear if regular blood transfusions and preservation of haemoglobin at a higher level could lead to higher BMD by reduction of hypoxia and erythropoietin levels that participate in the pathogenesis of bone disease[15].
In the present case, a male adult patient suffering from TM sustained an AFF fulfilling all major and two minor criteria (comorbid conditions and use of BPs). Biochemical evaluation revealed normal levels of bone markers three months after tibial and femoral fracture that could be a cause of concern. Before AFF, the patient had been treated with zoledronic acid for three years and remained another one year without osteoporosis therapy. To our knowledge, this is the first reported case of AFF in a patient suffering from TM, probably due to the small sample size of patients with thalassemia in the general population. The purpose of the present case is to increase the awareness amongst haematologists, who mainly deal with TM patients, of the adverse events of BP use.
Footnotes
Edited by: F. Rauch
References
- 1.Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 2.Cappellini MD. The Thalassemias. In: Goldman L, Schafer AI, editors. Goldman-Cecil Medicine: Expert Consult - Online. 25 ed. Elsevier Health Sciences; 2015. pp. 1089–95. [Google Scholar]
- 3.Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin. 2009;33(Suppl 1):S46–57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 4.Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Giusti A. Bisphosphonates in the management of thalassemia-associated osteoporosis: a systematic review of randomised controlled trials. J Bone Miner Metab. 2014;32:606–15. doi: 10.1007/s00774-014-0584-8. [DOI] [PubMed] [Google Scholar]
- 6.Terpos E, Voskaridou E. Treatment options for thalassemia patients with osteoporosis. Ann N Y Acad Sci. 2010;1202:237–43. doi: 10.1111/j.1749-6632.2010.05542.x. [DOI] [PubMed] [Google Scholar]
- 7.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee R, Bajoria R, Shah FT, Porter JB, Fedele S. High index of suspicion for early diagnosis of alendronate-induced stage zero osteonecrosis of jaw in thalassaemia major. Br J Haematol. 2014;166:292–4. doi: 10.1111/bjh.12833. [DOI] [PubMed] [Google Scholar]
- 9.Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies. Bone. 2010;47:169–80. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Cooley TB, Lee P. A series of cases of splenomegaly in children with anemia and peculiar bone changes. Trans Am Pediatr Soc. 1925;37:29–30. [Google Scholar]
- 11.Piomelli S. The management of patients with Cooley’s anemia: transfusions and splenectomy. Semin Hematol. 1995;32:262–8. [PubMed] [Google Scholar]
- 12.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24:543–57. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidar R, Musallam KM, Taher AT. Bone disease and skeletal complications in patients with beta thalassemia major. Bone. 2011;48:425–32. doi: 10.1016/j.bone.2010.10.173. [DOI] [PubMed] [Google Scholar]
- 14.Fung EB, Harmatz PR, Milet M, et al. Fracture prevalence and relationship to endocrinopathy in iron overloaded patients with sickle cell disease and thalassemia. Bone. 2008;43:162–8. doi: 10.1016/j.bone.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong P, Fuller PJ, Gillespie MT, et al. Thalassemia bone disease: a 19-year longitudinal analysis. J Bone Miner Res. 2014;29:2468–73. doi: 10.1002/jbmr.2266. [DOI] [PubMed] [Google Scholar]
- 16.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol. 2004;127:127–39. doi: 10.1111/j.1365-2141.2004.05143.x. [DOI] [PubMed] [Google Scholar]
- 17.Tournis S, Dede AD, Savvidis C, Triantafyllopoulos IK, Kattamis A, Papaioannou N. Effects of teriparatide retreatment in a patient with beta-thalassemia major. Transfusion. 2015 doi: 10.1111/trf.13237. [DOI] [PubMed] [Google Scholar]
- 18.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 19.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364:1728–37. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 20.Compston J. Pathophysiology of atypical femoral fractures and osteonecrosis of the jaw. Osteoporos Int. 2011;22:2951–61. doi: 10.1007/s00198-011-1804-x. [DOI] [PubMed] [Google Scholar]
- 21.Lampropoulou-Adamidou K, Tournis S, Balanika A, et al. Sequential treatment with teriparatide and strontium ranelate in a postmenopausal woman with atypical femoral fractures after long-term bisphosphonate administration. Hormones (Athens) 2013;12:591–7. doi: 10.14310/horm.2002.1448. [DOI] [PubMed] [Google Scholar]
- 22.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96:1627–32. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho NN, Voss LA, Almeida MO, Salgado CL, Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011;96:2675–80. doi: 10.1210/jc.2011-0593. [DOI] [PubMed] [Google Scholar]
- 24.Stathopoulos IP, Liakou CG, Katsalira A, et al. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens) 2011;10:280–91. doi: 10.14310/horm.2002.1319. [DOI] [PubMed] [Google Scholar]
- 25.Fung EB, Vichinsky EP, Kwiatkowski JL, et al. Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone. 2011;48:1305–12. doi: 10.1016/j.bone.2011.03.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong P, Fuller PJ, Gillespie MT, et al. Thalassemia bone disease: the association between nephrolithiasis, bone mineral density and fractures. Osteoporos Int. 2013;24:1965–71. doi: 10.1007/s00198-012-2260-y. [DOI] [PubMed] [Google Scholar]


