Abstract
Catheter ablation is an established and widespread treatment for atrial fibrillation (AF). Contemporary electroanatomical mapping systems (EAMs) have been developed to facilitate mapping processes but remain limited by spatiotemporal and processing restrictions. Advanced mapping systems emerged from the need to better understand and ablate complex AF substrate, by improving the acquisition and illustration of electrophysiological information. In this review, we present you the recently advanced mapping systems for AF ablation in comparison to the established contemporary EAMs.
Keywords: Electroanatomic Mapping, Atrial Fibrillation, High Density Mapping
Introduction
Atrial fibrillation (AF) is the most common arrhythmia with an increasing prevalence and a high socio-economical health burden. Catheter ablation (CA) is an established and widespread AF treatment. After the initial discovery and abolishment of focal pulmonary vein (PV) activity as AF triggers,[1-3] CA treatment has undergone considerable improvement over the last years aiming always for better results with faster, safer and easier procedures.
Electrical pulmonary vein isolation (PVI) is the cornerstone of AF treatment.[4-6] In patients with paroxysmal AF, recovered PV conduction is the most common reason for recurrence and can be successfully treated by a new ablation session.[7,8] In patients with chronic AF though, success-rates are lower and AF triggers from a diseased left atrium (LA) are more common, requiring additional substrate modification, defragmentation or linear ablations.[9,10] Multiple atrial wavelets, macro-reentries, and localized sources (drivers) have been reported to contribute to this substrate.[11,12]
Achieving electrically continuous, transmural lesions in a beating heart is challenging and requires a reliable three-dimensional (3D) navigation, in order to avoid complications (PV stenosis, perforation, phrenic nerve or esophageal injury). In order to facilitate this task with less radiation than plain fluoroscopy, electroanatomical-mapping systems (EAMs) have been developed, enabling the tracking of intracardiac electrodes in 3D maps and the navigation of catheter ablation.
Conventional mapping systems though cannot adequately detect localized AF drivers due to their sequential spatiotemporal characteristics, their intermittent firing and spatial meandering.[13] For this reason advanced mapping tools have been developed to visualize and better understand the AF-maintaining drivers. These systems have shown promising results for AF ablation and could path the way to a new era of substrate characterization and individual ablation strategies. In this context, the current article aims to review the modern advanced mapping systems for AF ablation in comparison to the established contemporary EAMs.
Contemporary Mapping Systems
All mapping systems are based on non-fluoroscopic visualization of mapping catheters and a 3D reconstruction created by the manipulation of a mapping catheter. Electrical information at map points is recorded and can be used for the color-coded display of the electrical activation sequence known as “activation mapping”, the display of post-pacing intervals known as “entrainment mapping” or the display of unipolar/bipolar electrograms as part of “fractionation” or “voltage mapping”.[14] The most common EAMs for AF ablation are the Carto (Biosense Webster, Baldwin Park, CA, USA) and the EnsiteNavX system (St. Jude Medical, St. Paul, MN, USA).
The latest version of Carto system is based on a hybrid of magnetic and current-based catheter localization technology and enables visualization of multiple catheters simultaneously. Three active magnetic fields generated by a location pad placed underneath the patient act on mini-sensors embedded in the catheter tip providing information about its exact position and orientation, in relation to a reference sensor on the skin. Additionally, six electrode patches positioned at the patient’s back and chest, screen a unique current emitted from different catheter electrodes.[15] Multipolar mapping catheters can be used for fast anatomical mapping (FAM) by registering and reentering 3D models. Respiratory gating is possible through thoracic impedance measurement, but patient movement or dislocation of the location pad may lead to uncorrectable map shifts. In order to enhance recognition of anatomical variations, integration of pre-aquired CT/MRI data or intraprocedural inctracardiac echocardiography (ICE, CartoSound®, Biosense Webster) is possible through merging of the 3D models.[16]
The EnsiteNavX Velocity system is based on an impedance-based tracking technology, capable of tracking intracardiac electrodes as well as tagging points in a high-frequency (8 kHz) electric field produced by six skin electrodes. The 3D-localization of the catheters is calculated based on an impedance gradient in relation to a reference electrode.[17] A process called field-scaling aims to correct for the body’s non-linear impedance and the use of intracardiac refrence-catheters reduces motion artifacts. However, dislocation of the reference catheter may lead to uncorrectable map shifts. EnsiteNavX allows for visualization of multiple catheters from different manufacturers and simultaneous collection of anatomical and electrophysiological data from all electrodes of any catheter.[18] Integration of CT/MRI data though requires an extensive registration called fusion.[19]
Both of these EAMs have been proven to reduce radiation and procedural duration[20-22] and in combination with pre-acquired imaging data can lead to less complications and better results.[23,24] Additionally, integration of electrode-tissue contact force data by special catheters (SmartTouch, Biosense Webster or TactiCath, St. Jude Medical) can provide feedback for lesion creation and improve efficacy, reduce risks and procedural parameters.[25-29] The most important contribution of these systems though is the characterization of the AF substrate through fractionation (quality and temporal characteristics of the electrical signals) or voltage mapping (amplitude of electrical signals), which has been the stimulus for further mapping developments. These tools aim to identify additional ablation targets and allow a patient-tailored approach.
Fractionation Mapping
Complex fractionated atrial electrograms (CFAEs) are regarded as surrogates of asynchronous activation of myocyte bundles through a fibrotic myocardium. They are defined as atrial electrograms with low voltage (≤0.15 mV) signals with ≥2 deflections/perturbations of the baseline with continuous deflection of a prolonged activation complex; and/or a very short cycle length (≤120 milliseconds), with or without multiple potentials. The mechanisms of CFAEs creation has been related to factors which perpetuate AF, but it has been also been considered to be passive consequences of near-by rapid AF drivers.[30] Contemporary EAMS integrate automated algorithms that provide CFAEs maps, but this has not been proved superior to conventional CFAE mapping and ablation.[31] Despite the initally encouraging results, recent studies showed a higher rate of resulting atrial tachycardias and failed to reveal a benefit of additional CFAE ablation.[32,33]
Voltage Mapping
Voltage mapping is based on the correlation of low-voltage areas (<0.5 mV) in the left atrium with endocardial scar and/ or structural defects as a substrate that can diminish success rates after AF ablation. [34-38] Supplementary ablation of low-voltage zones as an additional target to PVI serves as an individualized substrate modification (similar to unstable ventricular tachycardias). According to our experience such low-voltage areas are found in 35% of patients with persistent AF and in 10% of patients with paroxysmal AF, most commonly in the septal, anterior, or posterior LA wall. Patients with low-voltage substrate have lower success rates after AF ablation (23% after PVI only) that can be significantly improved by targeting these in a patient-tailored approach (70% after a year). Moreover, this strategy could spare the majority of patients (2/3 of those with persistent AF) from additional ablation lesions and potential complications, without compromising the ablation outcomes.[39] Prospective, randomized clinical studies are needed to clarify the role of a voltage-based AF ablation in comparison to established strategies.
Advanced Mapping Systems
Contemporary EAMs have been very valuable for the navigation of AF ablation, but have some limitations. The integrated automated mapping algorithms are susceptible to annotation and interpolation errors that require a manual point-by-point verification of annotated points. This is a time-consuming process that is prone to incorrect judgment regarding signal selection, the window-of-interest and the presence of fragmented/double potentials or areas of verylow continuous potentials. Moreover, spatiotemporal analysis and registration of electrograms on a map as well as the creation of a new map in case of tachycardia change, remains a slow process limited by the speed of signal acquisition. The need to overcome these disadvantages and to improve illustration of the underlying AF mechanisms, has led to the development of advanced mapping systems.
Advanced mapping systems for AF ablation have focused on improving signal quality (high-resolution), acquisition and processing time, precision of annotation and development of automated algorithms that visualize electrophysiologic information. These efforts refer once again to the core principle of electrophysiology: the electrical signals, which guide AF ablation, should be reliable (with high resolution and low noise), appropriately acquired and processed in a timely manner. In this sense, new diagnostic catheters and novel mapping technics have been developed and will be presented here.
Ripple Mapping
Ripple Mapping is a novel technique that displays time-voltage data as dynamic bars on Carto surface shells.[40] Electrograms are visualized as color bars on 3D models, changing colors and dimensions according to the voltage-time relationship, time-gated to a preselected electrograms (reference). The operator has the impression of a “wave-like” movement of the propagation, without any manual or automatic annotations. This way ripple mapping compensates for isolated annotation and interpolation errors and as recently reported, demonstrates higher diagnostic accuracy for atrial tachycardias compared to conventional activation mapping.[41] Although it is an offline system that requires time for post-processing, ripple mapping has the potential to simplify mapping and minimize operatordependence. Further evaluation and comparison with other systems is needed to prove if this technology will be integrated in “real-time” clinical practice.
High Dominant Frequencies Mapping
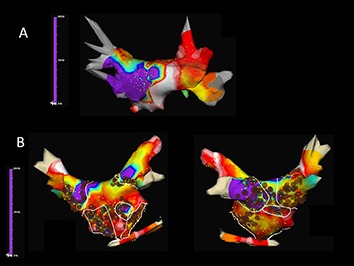
Dominant frequency (DF) maps derive by high-resolution analysis of the Fourier power spectrum and enable the color-coded hierarchical visualization of frequencies in combination with contemporary catheters and EAMs.[42] High DF sites are defined by 20% frequency gradient relative to the surrounding tissue and represent localized reentrant sources (ablation-targets). Multiple DF sites are usually found in a patient with variable distribution (predominantly PVsites in paroxysmal and more atrial sites for persistent AF) and intraprocedural spatiotemporal stability, which has raised some concern about their role as AF drivers. Ablation of DF sites may result in significant slowing of AF cycle length, reduction of AF inducibility, and AF termination especially in paroxysmal AF patients.[43,44] The RADAR-AF study compared DF ablation vs. circumferential PVI and found no incremental value for persistent AF but a noninferiority for paroxysmal AF ([Fig. 1]).[45] However, more clinical studies are needed to further evaluate the role of DF ablation.
Figure 1. Time-frequency analysis of atrial fibrillation (AF) with Dominant Frequency (DF) maps showing A. a patient with paroxysmal AF in whom DF guidead isolation of the left inferior pulmonary vein antrum lead to AF termination and B. a patient with persistent AF in whom a combined ablation approach with both high-frequency source ablation and circumferential pulmonary vein isolation was performed (Courtesy of Felipe Atienza, Hospital General Universitario Gregorio Marañón, Madrid, ES).
Focal Impulse and Rotor Mapping
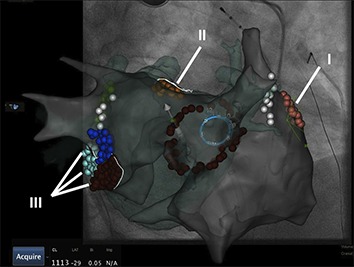
In order to improve the identification and abolishment of local reentrant sources a novel computational approach with the concept of focal impulse and rotor modulation (FIRM) has been developed.[46] For this technique, a dedicated 64-pole basket catheter (8 splines with 8 electrodes per spline) is used for panoramic intra-cardiac mapping during AF. Automated intra-procedural processing by the RhythmView mapping system (Topera, Menlo Park, CA, USA) enables the depiction of AF propagation maps projected onto grids. These maps are then used to guide ablation of AF drivers (usually 2-3 rotors or focal impulses per patient). Rotors are defined as stable and sustained spiral activation around a center of rotation, whereas focal impulses are defined by centrifugal activation from a source. Target sites are located by their electrode coordinates and radiofrequency ablation with a conventional catheter is usually applied for 15–30 sec up to 10 min, aiming for slowing or termination of AF. Conventional EAMs can integrate tracking of the basket catheter, annotation of target and ablation sites and simultaneous creation of atrial geometries, which may then be used for PV isolation ([Fig. 2]). PVI with additional direct or coincidental FIRM ablation has been shown to improve mid-term and long-term AF ablation outcome.[46-48] Similar to other technologies though, which are used to supplement conventional AF ablation, additional costs and processing time remain an issue and remain to be proofed for their clinical value.
Figure 2. Electroanatomical map (Carto 3, UniVu®) with annotation of AF rotors located by the RhythmView mapping system (Topera) on the right (grey shell, red points; I) and the left atrium (fused with a green 3D CT shell): close to the left atrial appendage (orange, II) and the mitral isthmus region (red, purple, blue, III). A circular mapping catheter (blue) is placed in the right superior pulmonary vein and an ablation catheter is at the posterior wall. The white points show the area of phrenic nerve capture.
Non-Invasive Body Surface Mapping
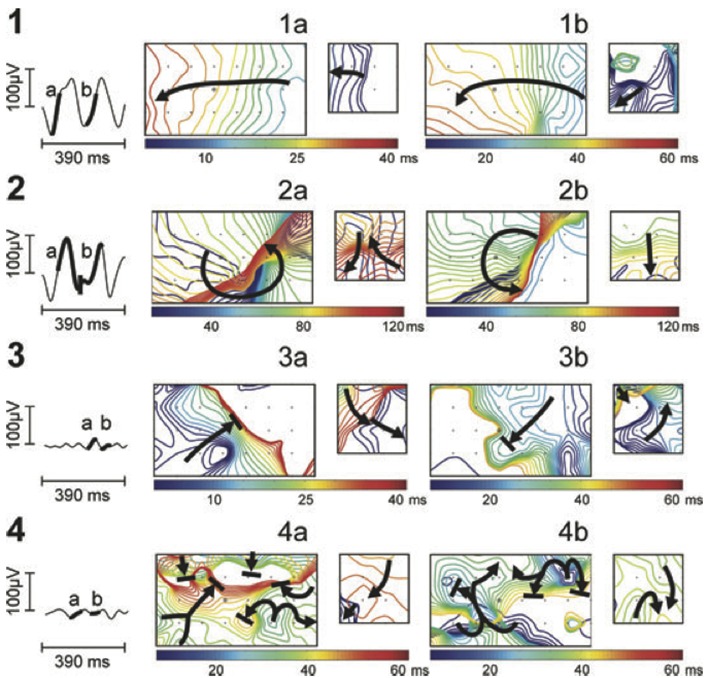
Body surface mapping (BSM) is a non-invasive bedside mapping system that aims to identify AF drivers by using an array of multiple surface electrodes and by projecting this information on a preacquired CT/MRI-based 3D model of the atria. Initial research revealed that using a 56-electrode vest around the patient’s torso, non-invasive mapping could depict wavefront propagation maps and identify specific patterns like single wavefronts, wave-breakages/ splitting or multiple simultaneous wavefronts ([Fig. 3]).[49] Further development of this kind of mapping led to a 252-electrode vest connected to a special system (ECVUE, CardioInsight Technologies Inc, Cleveland, OH) that records unipolar surface potentials. Biatrial unipolar electrograms are then automatically reconstructed from torso potentials and epicardial activation maps are computed by using the intrinsic deflection-based method. The windows with long ventricular pauses (spontaneous or diltiazem-provoked) are usually randomly selected for AF electrogram analysis. Maps of AF are generated by algorithms with a combination of signal filtering and phase mapping.[50-53] Wave propagation is then depicted color-coded on a beat-to-beat basis and spatiotemporal density maps are analyzed to identify active driver regions (classified as focal or reentrant) and the repetition of this activity. In contrast to focal impulse rotor mapping, AF drivers by BSM are usually (2-3) repetitive reentries clustering in the LA and increase with the duration of continuous AF. Their elimination could lead to AF termination (especially in paroxysmal AF) with a shorter procedural time in comparison to conventional ablation techniques.[54] Despite the need for additional off-line analysis, BSM allows for pre-procedural non-invasive AF mapping and preparation of an individual ablation strategy. Further clinical studies are needed though to elucidate the utility of this system.
Figure 3. Noninvasive AF mapping using body surface recordings from a uniform grid-torso placed around a patient with persistent AF. On the left, tracings correspond to V1. Panels a and b correspond to wavefront propagation maps of two intervals of the same segment in a color scale. On the left in each panel, maps correspond to the front part of the thorax and on the right, maps correspond to the back. Each electrode position is labelled with a ’+’ sign and V1 by a circle. Wavefront propagation lines are drawn every 2 ms, drawn blue when appearing first or red when appearing last. Arrows indicate the direction of propagation of each wavefront.49.
High Density Mapping
The concept of high-density mapping refers to the simultaneous acquisition and annotation of multiple electrograms, including activation and voltage information, which are then analyzed by automated algorithms in order to generate precise activation and substrate (voltage) maps. These algorithms were initially applied for macro-reentrant tachycardias, but they have been further developed and adapted for complex arrhythmias like AF, providing us with new insights and a better understanding. In order to achieve this novel mapping catheters have been developed; multiple electrodes serve for fast acquisition of data whereas a smaller electrode size and a shorter inter-electrode distance provide a better signal quality with less noise to far field ratio.
The PentaRay (Biosense-Webster) is a two-dimensional catheter with 20-poles arranged in 5 soft radiating splines (1-mm electrodes separated by 4-mm interelectrode spacing) laid out flat to cover an area with a diameter of 3.5 cm. The multi-branch configuration provides a broader access to information with high resolution.[55] It can be used with conventional EAMs and simplify the identification of focal or microreentry sources, scar borders and critical electrical pathways for the abolishment of macroreentrant tachycardias.[56,57] Recently, 3D high-density maps are made possible by using a specially-designed 64-pole basket array (8 splines with 8 electrodes per spline, 0,4 mm2 electrode size and 2.5-mm interelectrode spacing) attached to a bi-directional deflectable catheter (IntellaMap Orion® High Resolution Mapping Catheter) in combination with a novel EAM system (Rhythmia Mapping, Boston Scientific, Marlborough, Massachusetts, USA). The Rhythmia system uses a hybrid of magnetic-based tracking for a sensor at the catheter tip and impedance-based tracking for all 64 electrodes for catheter navigation and geometry creation. The greatest advantage of this system is the rapid and automatic acquisition of maps with high spatiotemporal resolution and without the need for extensive manual annotation. Activation maps with thousands of electrograms can be created within minutes.[58-61] Post-processing is not necessary and map-reconstruction (in case of tachycardia change or after lesion deployment) is very fast.
This is accomplished through integrated automated algorithms that meticulously select cardiac beats (based on stability of cycle length, timing, location and respiratory cycle) and filter-out points with discrepancy in comparison to those of close proximity. Far-field components are reduced by combining unipolar and bipolar electrograms. Moreover, the low noise level in the system (0.01 mV) allows the recording of very low-amplitude potentials indicative of scarred atrial myocardium.[62] As a result, the improved differentiation of signals enables depiction of narrow activation waves with high precision. Adjustment of the window of interest in an activation map can reveal early local potentials or eliminate far-field noise on the map. Similarly, changing the voltage scale can reveal electrical gaps through low-voltage areas or a breakthrough in ablation lines and it can be used to achieve the continuity of lesions ([Fig. 4A]).
Figure 4A. Voltage base verification of linear lesions: High-density voltage map of the roof region during a redo case with previous box lesion. Note high voltage regions within the box lesion (corresponding local electrograms shown in the box).
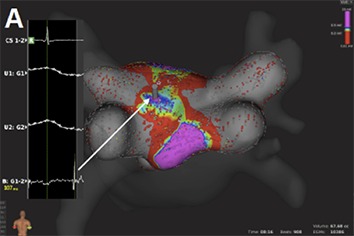
Figure 4B. Voltage base verification of linear lesions: re-map of the same region after additional ablation shows elimination of high voltage potentials.
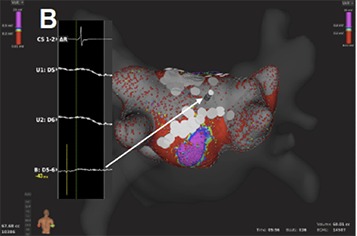
To further evaluate the application of this technology, our group has performed feasibility and efficacy studies in patients with supraventricular tachycardias, including AV nodal reentrant tachycardias, atrial flutter and fibrillation.[63,64] The initial experience of pulmonary vein mapping and ablation in a porcine model has now been expanded to human atria and pulmonary vein ablation.[65,66] Resent studies have provided more confirming results about the use of the mini-basket catheter alone to sufficiently determine PV isolation. Along with improved recording of PV potentials after incomplete ablation, this catheter also registers “PV-like” potentials from neighboring structures. In these cases, pacing maneuvers are helpful to determine PVI and avoid excessive ablation.[67] These results though support the safety of the system and encourage further clinical evaluation.
Conclusion
Contemporary EAMs provided the 3D navigation for AF ablation in order to reduce radiation and improve safety, procedural time and efficacy. Image integration and tools, like fast mapping and contactforce feedback, act complementary towards that goal. Based on EAMs, fractionation and voltage mapping evolved and provided the stimulus for further developments that focused more and more on the visualization and analysis of the myocardial electrical signals. Advanced mapping systems emerged from the need to better understand and ablate complex AF substrate. These efforts tried to overcome the spatiotemporal and processing limitations of contemporary EAMs and focused on improving the acquisition and illustration of electrophysiological information. Innovative mapping approaches like ripple mapping may someday allow experienced operators to create maps of complex atrial tachycardias without assisting experts. Mapping technics that aim to visualize AF drivers through depiction of dominant frequency areas and characterization of rotors or focal impulses during (intracardiac) or prior (noninvasive) to the procedure, have shown promising results in terms of AF termination and will be further evaluated.[68] The improved electrical signals produced by narrow-spaced catheters and the automated high-density maps may also prove valuable for scar-based ablation strategies.
Characterization and redefinition of AF substrate is a key-element for future mapping systems and personalized AF ablation. Ideally, future mapping-systems would allow visualization of the atrial anatomy and pathophysiology, in order to individualize and monitor lesion formation in a real-time fluoroscopy-free environment, like in the MRI suite. [69-71] Although there is a long way ahead, it remains an exciting time with many improvements and a bright future for AF mapping systems.
Disclosures
None.
References
- 1.Haïssaguerre M, Marcus F I, Fischer B, Clémenty J. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J. Cardiovasc. Electrophysiol. 1994 Sep;5 (9):743–51. doi: 10.1111/j.1540-8167.1994.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997 Feb 4;95 (3):572–6. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 4.Arentz Thomas, Weber Reinhold, Bürkle Gerd, Herrera Claudia, Blum Thomas, Stockinger Jochem, Minners Jan, Neumann Franz Josef, Kalusche Dietrich. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007 Jun 19;115 (24):3057–63. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 5.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Camm A John, Lip Gregory Y H, De Caterina Raffaele, Savelieva Irene, Atar Dan, Hohnloser Stefan H, Hindricks Gerhard, Kirchhof Paulus. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012 Nov;33 (21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang Feifan, Tilz Roland, Chun Julian, Schmidt Boris, Wissner Erik, Zerm Thomas, Neven Kars, Köktürk Bulent, Konstantinidou Melanie, Metzner Andreas, Fuernkranz Alexander, Kuck Karl-Heinz. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010 Dec 7;122 (23):2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang Feifan, Antz Matthias, Ernst Sabine, Hachiya Hitoshi, Mavrakis Hercules, Deger Florian T, Schaumann Anselm, Chun Julian, Falk Peter, Hennig Detlef, Liu Xingpeng, Bänsch Dietmar, Kuck Karl-Heinz. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005 Jan 18;111 (2):127–35. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 9.Hunter R J, Berriman T J, Diab I, Baker V, Finlay M, Richmond L, Duncan E, Kamdar R, Thomas G, Abrams D, Dhinoja M, Sporton S, Earley M J, Schilling R J. Long-term efficacy of catheter ablation for atrial fibrillation: impact of additional targeting of fractionated electrograms. Heart. 2010 Sep;96 (17):1372–8. doi: 10.1136/hrt.2009.188128. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill Mark D, Wright Matthew, Knecht Sébastien, Jaïs Pierre, Hocini Mélèze, Takahashi Yoshihide, Jönsson Anders, Sacher Frédéric, Matsuo Seiichiro, Lim Kang Teng, Arantes Leonardo, Derval Nicolas, Lellouche Nicholas, Nault Isabelle, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur. Heart J. 2009 May;30 (9):1105–12. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]
- 11.Konings K T, Kirchhof C J, Smeets J R, Wellens H J, Penn O C, Allessie M A. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994 Apr;89 (4):1665–80. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 12.Haïssaguerre Michel, Hocini Mélèze, Sanders Prashanthan, Takahashi Yoshihide, Rotter Martin, Sacher Frederic, Rostock Thomas, Hsu Li-Fern, Jonsson Anders, O'Neill Mark D, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006 Feb 7;113 (5):616–25. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 13.Rostock Thomas, Rotter Martin, Sanders Prashanthan, Takahashi Yoshihide, Jaïs Pierre, Hocini Mélèze, Hsu Li-Fern, Sacher Fréderic, Clémenty Jacques, Haïssaguerre Michel. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006 Jan;3 (1):27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Knackstedt Christian, Schauerte Patrick, Kirchhof Paulus. Electro-anatomic mapping systems in arrhythmias. Europace. 2008 Nov;10 Suppl 3 ():iii28–34. doi: 10.1093/europace/eun225. [DOI] [PubMed] [Google Scholar]
- 15.Gepstein L, Hayam G, Ben-Haim S A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997 Mar 18;95 (6):1611–22. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Masaomi, Sasaki Shingo, Owada Shingen, Horiuchi Daisuke, Sasaki Kenichi, Itoh Taihei, Ishida Yuji, Kinjo Takahiko, Okumura Ken. Validation of accuracy of three-dimensional left atrial CartoSound™ and CT image integration: influence of respiratory phase and cardiac cycle. J. Cardiovasc. Electrophysiol. 2013 Sep;24 (9):1002–7. doi: 10.1111/jce.12170. [DOI] [PubMed] [Google Scholar]
- 17.Wittkampf F H, Wever E F, Derksen R, Wilde A A, Ramanna H, Hauer R N, Robles de Medina E O. LocaLisa: new technique for real-time 3-dimensional localization of regular intracardiac electrodes. Circulation. 1999 Mar 16;99 (10):1312–7. doi: 10.1161/01.cir.99.10.1312. [DOI] [PubMed] [Google Scholar]
- 18.Eitel Charlotte, Hindricks Gerhard, Dagres Nikolaos, Sommer Philipp, Piorkowski Christopher. EnSite Velocity cardiac mapping system: a new platform for 3D mapping of cardiac arrhythmias. Expert Rev Med Devices. 2010 Mar;7 (2):185–92. doi: 10.1586/erd.10.1. [DOI] [PubMed] [Google Scholar]
- 19.Govil Ashul, Calkins Hugh, Spragg David D. Fusion of imaging technologies: how, when, and for whom? J Interv Card Electrophysiol. 2011 Dec;32 (3):195–203. doi: 10.1007/s10840-011-9616-7. [DOI] [PubMed] [Google Scholar]
- 20.Khaykin Yaariv, Oosthuizen Richard, Zarnett Lauren, Wulffhart Zaev A, Whaley Bonnie, Hill Carol, Giewercer David, Verma Atul. CARTO-guided vs. NavX-guided pulmonary vein antrum isolation and pulmonary vein antrum isolation performed without 3-D mapping: effect of the 3-D mapping system on procedure duration and fluoroscopy time. J Interv Card Electrophysiol. 2011 Apr;30 (3):233–40. doi: 10.1007/s10840-010-9538-9. [DOI] [PubMed] [Google Scholar]
- 21.Estner Heidi Luise, Deisenhofer Isabel, Luik Armin, Ndrepepa Gjin, von Bary Christian, Zrenner Bernhard, Schmitt Claus. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX). Europace. 2006 Aug;8 (8):583–7. doi: 10.1093/europace/eul079. [DOI] [PubMed] [Google Scholar]
- 22.Rotter Martin, Takahashi Yoshihide, Sanders Prashanthan, Haïssaguerre Michel, Jaïs Pierre, Hsu Li-Fern, Sacher Fréderic, Pasquié Jean-Luc, Clementy Jacques, Hocini Mélèze. Reduction of fluoroscopy exposure and procedure duration during ablation of atrial fibrillation using a novel anatomical navigation system. Eur. Heart J. 2005 Jul;26 (14):1415–21. doi: 10.1093/eurheartj/ehi172. [DOI] [PubMed] [Google Scholar]
- 23.Martinek Martin, Nesser Hans-Joachim, Aichinger Josef, Boehm Gernot, Purerfellner Helmut. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2007 Oct;30 (10):1215–23. doi: 10.1111/j.1540-8159.2007.00843.x. [DOI] [PubMed] [Google Scholar]
- 24.Kistler Peter M, Rajappan Kim, Jahngir Mohammed, Earley Mark J, Harris Stuart, Abrams Dominic, Gupta Dhiraj, Liew Reginald, Ellis Stephen, Sporton Simon C, Schilling Richard J. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Oct;17 (10):1093–101. doi: 10.1111/j.1540-8167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Masaomi, Sasaki Shingo, Owada Shingen, Horiuchi Daisuke, Sasaki Kenichi, Itoh Taihei, Ishida Yuji, Kinjo Takahiko, Tomita Hirofumi, Okumura Ken. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm. 2014 Jun;11 (6):984–91. doi: 10.1016/j.hrthm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Stabile Giuseppe, Solimene Francesco, Calò Leonardo, Anselmino Matteo, Castro Antonello, Pratola Claudio, Golia Paolo, Bottoni Nicola, Grandinetti Giuseppe, De Simone Antonio, De Ponti Roberto, Dottori Serena, Bertaglia Emanuele. Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace. 2014 Mar;16 (3):335–40. doi: 10.1093/europace/eut262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy Vivek Y, Shah Dipen, Kautzner Josef, Schmidt Boris, Saoudi Nadir, Herrera Claudia, Jaïs Pierre, Hindricks Gerhard, Peichl Petr, Yulzari Aude, Lambert Hendrik, Neuzil Petr, Natale Andrea, Kuck Karl-Heinz. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012 Nov;9 (11):1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama Katsuaki, Nakagawa Hiroshi, Shah Dipen C, Lambert Hendrik, Leo Giovanni, Aeby Nicolas, Ikeda Atsushi, Pitha Jan V, Sharma Tushar, Lazzara Ralph, Jackman Warren M. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008 Dec;1 (5):354–62. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]
- 29.Marijon Eloi, Fazaa Samia, Narayanan Kumar, Guy-Moyat Benoit, Bouzeman Abdeslam, Providencia Rui, Treguer Frederic, Combes Nicolas, Bortone Agustin, Boveda Serge, Combes Stephane, Albenque Jean-Paul. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J. Cardiovasc. Electrophysiol. 2014 Feb;25 (2):130–7. doi: 10.1111/jce.12303. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto Koji, Tsuchiya Takeshi, Nagamoto Yasutsugu, Yamaguchi Takanori, Narita Sumito, Ando Shin-Ichi, Hayashida Kiyoshi, Tanioka Yoshito, Takahashi Naohiko. Characterization of bipolar electrograms during sinus rhythm for complex fractionated atrial electrograms recorded in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010 Apr;12 (4):494–501. doi: 10.1093/europace/euq033. [DOI] [PubMed] [Google Scholar]
- 31.Verma Atul, Novak Paul, Macle Laurent, Whaley Bonnie, Beardsall Marianne, Wulffhart Zaev, Khaykin Yaariv. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008 Feb;5 (2):198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Wu Shao-Hui, Jiang Wei-Feng, Gu Jun, Zhao Liang, Wang Yuan-Long, Liu Yu-Gang, Zhou Li, Gu Jia-Ning, Xu Kai, Liu Xu. Benefits and risks of additional ablation of complex fractionated atrial electrograms for patients with atrial fibrillation: a systematic review and meta-analysis. Int. J. Cardiol. 2013 Oct 25;169 (1):35–43. doi: 10.1016/j.ijcard.2013.08.083. [DOI] [PubMed] [Google Scholar]
- 33.Providência Rui, Lambiase Pier D, Srinivasan Neil, Ganesh Babu Girish, Bronis Konstantinos, Ahsan Syed, Khan Fakhar Z, Chow Anthony W, Rowland Edward, Lowe Martin, Segal Oliver R. Is There Still a Role for Complex Fractionated Atrial Electrogram Ablation in Addition to Pulmonary Vein Isolation in Patients With Paroxysmal and Persistent Atrial Fibrillation? Meta-Analysis of 1415 Patients. Circ Arrhythm Electrophysiol. 2015 Oct;8 (5):1017–29. doi: 10.1161/CIRCEP.115.003019. [DOI] [PubMed] [Google Scholar]
- 34.Mahnkopf Christian, Badger Troy J, Burgon Nathan S, Daccarett Marcos, Haslam Thomas S, Badger Christopher T, McGann Christopher J, Akoum Nazem, Kholmovski Eugene, Macleod Rob S, Marrouche Nassir F. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm. 2010 Oct;7 (10):1475–81. doi: 10.1016/j.hrthm.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marrouche Nassir F, Wilber David, Hindricks Gerhard, Jais Pierre, Akoum Nazem, Marchlinski Francis, Kholmovski Eugene, Burgon Nathan, Hu Nan, Mont Lluis, Deneke Thomas, Duytschaever Mattias, Neumann Thomas, Mansour Moussa, Mahnkopf Christian, Herweg Bengt, Daoud Emile, Wissner Erik, Bansmann Paul, Brachmann Johannes. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014 Feb 5;311 (5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 36.Oakes Robert S, Badger Troy J, Kholmovski Eugene G, Akoum Nazem, Burgon Nathan S, Fish Eric N, Blauer Joshua J E, Rao Swati N, DiBella Edward V R, Segerson Nathan M, Daccarett Marcos, Windfelder Jessiciah, McGann Christopher J, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009 Apr 7;119 (13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma Atul, Wazni Oussama M, Marrouche Nassir F, Martin David O, Kilicaslan Fethi, Minor Stephen, Schweikert Robert A, Saliba Walid, Cummings Jennifer, Burkhardt J David, Bhargava Mandeep, Belden William A, Abdul-Karim Ahmad, Natale Andrea. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J. Am. Coll. Cardiol. 2005 Jan 18;45 (2):285–92. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Takanori, Tsuchiya Takeshi, Nagamoto Yasutsugu, Miyamoto Koji, Murotani Kenta, Okishige Kaoru, Takahashi Naohiko. Long-term results of pulmonary vein antrum isolation in patients with atrial fibrillation: an analysis in regards to substrates and pulmonary vein reconnections. Europace. 2014 Apr;16 (4):511–20. doi: 10.1093/europace/eut265. [DOI] [PubMed] [Google Scholar]
- 39.Rolf Sascha, Kircher Simon, Arya Arash, Eitel Charlotte, Sommer Philipp, Richter Sergio, Gaspar Thomas, Bollmann Andreas, Altmann David, Piedra Carlos, Hindricks Gerhard, Piorkowski Christopher. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Oct;7 (5):825–33. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 40.Linton Nick W F, Koa-Wing Michael, Francis Darrel P, Kojodjojo Pipin, Lim Phang Boon, Salukhe Tushar V, Whinnett Zachary, Davies D Wyn, Peters Nicholas S, O'Neill Mark D, Kanagaratnam Prapa. Cardiac ripple mapping: a novel three-dimensional visualization method for use with electroanatomic mapping of cardiac arrhythmias. Heart Rhythm. 2009 Dec;6 (12):1754–62. doi: 10.1016/j.hrthm.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Jamil-Copley Shahnaz, Linton Nick, Koa-Wing Michael, Kojodjojo Pipin, Lim Phang Boon, Malcolme-Lawes Louisa, Whinnett Zachary, Wright Ian, Davies Wyn, Peters Nicholas, Francis Darrel P, Kanagaratnam Prapa. Application of ripple mapping with an electroanatomic mapping system for diagnosis of atrial tachycardias. J. Cardiovasc. Electrophysiol. 2013 Dec;24 (12):1361–9. doi: 10.1111/jce.12259. [DOI] [PubMed] [Google Scholar]
- 42.Sanders Prashanthan, Berenfeld Omer, Hocini Mélèze, Jaïs Pierre, Vaidyanathan Ravi, Hsu Li-Fern, Garrigue Stéphane, Takahashi Yoshihide, Rotter Martin, Sacher Fréderic, Scavée Christophe, Ploutz-Snyder Robert, Jalife José, Haïssaguerre Michel. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005 Aug 9;112 (6):789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 43.Atienza Felipe, Almendral Jesús, Jalife José, Zlochiver Sharon, Ploutz-Snyder Robert, Torrecilla Esteban G, Arenal Angel, Kalifa Jérôme, Fernández-Avilés Francisco, Berenfeld Omer. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009 Jan;6 (1):33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haïssaguerre Michel, Sanders Prashanthan, Hocini Mélèze, Hsu Li-Fern, Shah Dipen C, Scavée Christophe, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Garrigue Stéphane, Clémenty Jacques, Jaïs Pierre. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004 Jun 22;109 (24):3007–13. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 45.Atienza Felipe, Almendral Jesús, Ormaetxe José Miguel, Moya Angel, Martínez-Alday Jesús Daniel, Hernández-Madrid Antonio, Castellanos Eduardo, Arribas Fernando, Arias Miguel Ángel, Tercedor Luis, Peinado Rafael, Arcocha Maria Fe, Ortiz Mercedes, Martínez-Alzamora Nieves, Arenal Angel, Fernández-Avilés Francisco, Jalife José. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J. Am. Coll. Cardiol. 2014 Dec 16;64 (23):2455–67. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 46.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narayan Sanjiv M, Baykaner Tina, Clopton Paul, Schricker Amir, Lalani Gautam G, Krummen David E, Shivkumar Kalyanam, Miller John M. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J. Am. Coll. Cardiol. 2014 May 6;63 (17):1761–8. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narayan Sanjiv M, Krummen David E, Clopton Paul, Shivkumar Kalyanam, Miller John M. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J. Am. Coll. Cardiol. 2013 Jul 9;62 (2):138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillem Maria S, Climent Andreu M, Castells Francisco, Husser Daniela, Millet Jose, Arya Arash, Piorkowski Christopher, Bollmann Andreas. Noninvasive mapping of human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 May;20 (5):507–13. doi: 10.1111/j.1540-8167.2008.01356.x. [DOI] [PubMed] [Google Scholar]
- 50.Ramanathan Charulatha, Ghanem Raja N, Jia Ping, Ryu Kyungmoo, Rudy Yoram. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat. Med. 2004 Apr;10 (4):422–8. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuculich Phillip S, Wang Yong, Lindsay Bruce D, Faddis Mitchell N, Schuessler Richard B, Damiano Ralph J, Li Li, Rudy Yoram. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010 Oct 5;122 (14):1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahadevan Jayakumar, Ryu Kyungmoo, Peltz Leora, Khrestian Celeen M, Stewart Robert W, Markowitz Alan H, Waldo Albert L. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004 Nov 23;110 (21):3293–9. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 53.Cochet Hubert, Dubois Rémi, Sacher Frédéric, Derval Nicolas, Sermesant Maxime, Hocini Mélèze, Montaudon Michel, Haïssaguerre Michel, Laurent François, Jaïs Pierre. Cardiac arrythmias: multimodal assessment integrating body surface ECG mapping into cardiac imaging. Radiology. 2014 Apr;271 (1):239–47. doi: 10.1148/radiol.13131331. [DOI] [PubMed] [Google Scholar]
- 54.Haissaguerre Michel, Hocini Meleze, Denis Arnaud, Shah Ashok J, Komatsu Yuki, Yamashita Seigo, Daly Matthew, Amraoui Sana, Zellerhoff Stephan, Picat Marie-Quitterie, Quotb Adam, Jesel Laurence, Lim Han, Ploux Sylvain, Bordachar Pierre, Attuel Guillaume, Meillet Valentin, Ritter Philippe, Derval Nicolas, Sacher Frederic, Bernus Olivier, Cochet Hubert, Jais Pierre, Dubois Remi. Driver domains in persistent atrial fibrillation. Circulation. 2014 Aug 12;130 (7):530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 55.Berte Benjamin, Relan Jatin, Sacher Frederic, Pillois Xavier, Appetiti Anthony, Yamashita Seigo, Mahida Saagar, Casassus Frederic, Hooks Darren, Sellal Jean-Marc, Amraoui Sana, Denis Arnaud, Derval Nicolas, Cochet Hubert, Hocini Mélèze, Haïssaguerre Michel, Weerasooriya Rukshen, Jaïs Pierre. Impact of electrode type on mapping of scar-related VT. J. Cardiovasc. Electrophysiol. 2015 Jul 22; () doi: 10.1111/jce.12761. [DOI] [PubMed] [Google Scholar]
- 56.Chang Shih-Lin, Lin Yenn-Jiang, Tai Ching-Tai, Lo Li-Wei, Tuan Ta-Chuan, Udyavar Ameya R, Hu Yu-Feng, Chiang Shuo-Ju, Wongcharoen Wanwarang, Tsao Hsuan-Ming, Ueng Kwo-Chang, Higa Satoshi, Lee Pi-Chang, Chen Shih-Ann. Induced atrial tachycardia after circumferential pulmonary vein isolation of paroxysmal atrial fibrillation: electrophysiological characteristics and impact of catheter ablation on the follow-up results. J. Cardiovasc. Electrophysiol. 2009 Apr;20 (4):388–94. doi: 10.1111/j.1540-8167.2008.01358.x. [DOI] [PubMed] [Google Scholar]
- 57.Gerstenfeld Edward P, Marchlinski Francis E. Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm. 2007 Mar;4 (3 Suppl):S65–72. doi: 10.1016/j.hrthm.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa Hiroshi, Ikeda Atsushi, Sharma Tushar, Lazzara Ralph, Jackman Warren M. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circ Arrhythm Electrophysiol. 2012 Apr;5 (2):417–24. doi: 10.1161/CIRCEP.111.968602. [DOI] [PubMed] [Google Scholar]
- 59.Hooks Darren A, Yamashita Seigo, Capellino Stefano, Cochet Hubert, Jais Pierre, Sacher Frederic. Ultra-Rapid Epicardial Activation Mapping During Ventricular Tachycardia Using Continuous Sampling from a High-Density Basket (Orion(TM) ) Catheter. J. Cardiovasc. Electrophysiol. 2015 Oct;26 (10):1153–4. doi: 10.1111/jce.12685. [DOI] [PubMed] [Google Scholar]
- 60.Bollmann Andreas, Hilbert Sebastian, John Silke, Kosiuk Jedrzej, Hindricks Gerhard. Insights from preclinical ultra high-density electroanatomical sinus node mapping. Europace. 2015 Mar;17 (3):489–94. doi: 10.1093/europace/euu276. [DOI] [PubMed] [Google Scholar]
- 61.Hilbert Sebastian, Kosiuk Jedrzej, John Silke, Hindricks Gerhard, Bollmann Andreas. A guide to the porcine anatomy for the interventional electrophysiologist. Fluoroscopy and high density electroanatomical mapping. J Cardiovasc Transl Res. 2015 Feb;8 (1):67–75. doi: 10.1007/s12265-015-9610-z. [DOI] [PubMed] [Google Scholar]
- 62.Thajudeen Anees, Jackman Warren M, Stewart Brian, Cokic Ivan, Nakagawa Hiroshi, Shehata Michael, Amorn Allen M, Kali Avinash, Liu Ezh, Harlev Doron, Bennett Nathan, Dharmakumar Rohan, Chugh Sumeet S, Wang Xunzhang. Correlation of scar in cardiac MRI and high-resolution contact mapping of left ventricle in a chronic infarct model. Pacing Clin Electrophysiol. 2015 Jun;38 (6):663–74. doi: 10.1111/pace.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hilbert Sebastian, Kosiuk Jedrzej, John Silke, Hindricks Gerhard, Bollmann Andreas. First Case of Automatic His Potential Detection With a Novel Ultra High-density Electroanatomical Mapping System for AV Nodal Ablation. Indian Pacing Electrophysiol J. 2015 Apr 9;15 (1):79–81. doi: 10.1016/S0972-6292(16)30848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hilbert Sebastian, Kosiuk Jedrzej, John Silke, Hindricks Gerhard, Bollmann Andreas. An integrative approach to slow pathway modulation in AVNRT using a novel ultra high-density electroanatomical mapping system. Clin Res Cardiol. 2015 Aug;104 (8):697–9. doi: 10.1007/s00392-015-0847-y. [DOI] [PubMed] [Google Scholar]
- 65.Bollmann Andreas, Hilbert Sebastian, John Silke, Kosiuk Jedrzej, Hindricks Gerhard. Initial Experience With Ultra High-Density Mapping of Human Right Atria. J. Cardiovasc. Electrophysiol. 2016 Feb;27 (2):154–60. doi: 10.1111/jce.12852. [DOI] [PubMed] [Google Scholar]
- 66.Bollmann Andreas, Kosiuk Jedrzej, Hilbert Sebastian, John Silke, Hindricks Gerhard. Percutaneous transapical access for pulmonary vein mapping and ablation in a porcine model with a new high-density electroanatomical mapping system. Int J Clin Exp Med. 2015;8 (8):12631–6. [PMC free article] [PubMed] [Google Scholar]
- 67.Anter Elad, Tschabrunn Cory M, Contreras-Valdes Fernando M, Li Jianqing, Josephson Mark E. Pulmonary vein isolation using the Rhythmia mapping system: Verification of intracardiac signals using the Orion mini-basket catheter. Heart Rhythm. 2015 Sep;12 (9):1927–34. doi: 10.1016/j.hrthm.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Verma Atul, Sanders Prashanthan, Macle Laurent, Deisenhofer Isabel, Morillo Carlos A, Chen Jian, Jiang Chen-yang, Ernst Sabine, Mantovan Roberto. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial-Part II (STAR AF II): design and rationale. Am. Heart J. 2012 Jul;164 (1):1–6.e6. doi: 10.1016/j.ahj.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Piorkowski Christopher, Grothoff Matthias, Gaspar Thomas, Eitel Charlotte, Sommer Philipp, Huo Yan, John Silke, Gutberlet Matthias, Hindricks Gerhard. Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2013 Feb;6 (1):e7–10. doi: 10.1161/CIRCEP.112.973719. [DOI] [PubMed] [Google Scholar]
- 70.Eitel Charlotte, Piorkowski Christopher, Hindricks Gerhard, Gutberlet Matthias. Electrophysiology study guided by real-time magnetic resonance imaging. Eur. Heart J. 2012 Aug;33 (15) doi: 10.1093/eurheartj/ehr414. [DOI] [PubMed] [Google Scholar]
- 71.Hilbert Sebastian, Sommer Philipp, Gutberlet Matthias, Gaspar Thomas, Foldyna Borek, Piorkowski Christopher, Weiss Steffen, Lloyd Thomas, Schnackenburg Bernhard, Krueger Sascha, Fleiter Christian, Paetsch Ingo, Jahnke Cosima, Hindricks Gerhard, Grothoff Matthias. Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace. 2016 Apr;18 (4):572–7. doi: 10.1093/europace/euv249. [DOI] [PubMed] [Google Scholar]


