Abstract
Idiopathic Ventricular Premature Contraction (VPC) is currently more routinely referred for electrophysiology evaluation. Usually it carries a good prognosis but, when symptomatic or suspected to produce ventricular dysfunction, will require treatment. Nowadays, RF ablation has great advantages over antiarrhythmic drugs. Classically the outflow tract (right or left), with the typical inferior axis with left (eventually right) bundle brunch block like ECG morphology, is considered the most frequent site of origin for idiopathic VPC, but with the widespread of EP procedures and advancement of technology making possible to map and ablate difficult locations, it is possible to see a growing and changing population referred for idiopathic VPC ablation, displaying that, almost any region of the heart may be source of this kind of arrhythmia that can be successfully treated. A well-planned procedure, with the presumed region of origin settled and employing the current technology and knowledge (tips), will have a high chance of cure.
Keywords: Idiopathic, Ventricular Premature Contraction, Ablation, Outflow tract
Introduction
Ventricular premature contraction (VPC) in a apparently healthy young adult with no previous history is not so uncommon, and may cause emotional stress until the accurate diagnosis is done. Described as “idiopathic ventricular arrhythmia”, it is defined as the arrhythmia not related with any detectable structural cardiac disease. It accounts for about 20% of all patients referred for evaluation of ventricular arrhythmias. Most patients are symptomatic and will need treatment; other are asymptomatic but have a high burden of the arrhythmia and may be at risk of developing a reversible form of left ventricular dysfunction (tachycardia-induced dilated cardiomyopathy)[1] and will need treatment as well.[2] Pharmacologic treatment of these arrhythmias, usually with beta-blockers, calcium channel blockers and even amiodarone, has only modest efficacy (around 25 to 50%). Most young patients do not desire long-term medical therapy or are not drug tolerant. Some patients can even feel a worsening of symptoms after medications due to drug induced bradycardia or to an increase in VPC burden. Considering that usually the source of the arrhythmia is unifocal, ablation has undoubtedly some advantages over medical treatment, since it has a high chance of cure.[3] Therefore, broadly speaking, the key decision is if the patient needs or not treatment, if so, the best option is usually the RF ablation.
Distribution of FOCI
Ventricular premature contraction in structural normal heart may arise from any region but the outflow tract VPC / VT is the most common form, known almost as synonymous of idiopathic ventricular arrhythmia; one concern, is to differentiate from initial form of arrhythmogenic right ventricular cardiomyopathy/dysplasia. The reason these arrhythmias come most frequently from outflow is still not clear. The typical electrocardiogram features is the inferior axis and left branch block morphology. For a long time it has been taught that the right ventricular outflow tract (RVOT) accounts for the majority (about 80%) of idiopathic VPCs, however, taken into account the great growing of referred population, the foci location has changed significantly in the last decade. Maybe due to the ability of mapping complex or difficult structures and the widespread of ablation procedures, the kind of patient that is referred for electrophysiology evaluation nowadays has been show a shift to other regions, outside of RVOT. Recently Penela et al.[4] studied 117 patients with outflow tract arrhythmias and found almost the same distribution of foci from left and right side (left: 51%; Right: 49%). A newly and big study from Latchamsetty et al.[5]give a compelling evidence for distribution of foci in current VPC ablations based on 8 international centers, comprising 1,185 patients between 2004 and 2013. They found and described the origin as follow: RVOT: 45%; Aortic Cusps: 15%; Papillary Muscles: 5%; Epicardium: 11%; Other (not specified) origin: 24%. Therefore any area of the heart may be source of idiopathic ectopy.
Outflow Tract is still the most common site and is classified as one group apart. This area include the following structures: right ventricular outflow tract (RVOT), left ventricular outflow tract (LVOT), the aortic sinuses of Valsalva, the area of aortomitral continuity, the superior basal septum near the His bundle, the pulmonary artery, and the epicardial surface of the outflow tracts. Although some clinical differences[4] may exist, these arrhythmias have similar characteristics and share the same behavior. Actually the right and left ventricular outflow tracts are in close relationship and this explains the difficult of differentiating them by ECG criterion and the phenomenon of shifting, when 2 discrete morphologies are seeing changing during the catheter ablation.
Tricuspid Annulus was the source of origin of 8% of idiopathic VPC/VT as described by Tada et al.;[6] they show that the majority (74%) of them originated from the septum portion. Yamada et al[7] presented a case report where they did ablation of tricuspid annulus VPC using a halo-type catheter to help mapping.
Mitral annulus ventricular arrhythmias have been described as 5% of idiopathic arrhythmias in a large series;[8] the authors showed that in all patients an S-wave was present in lead V6.
Pappilary Muscles has a rich network of Purkinje fibers, [9] these structures are accountable for 4,2% of idiopathic VT/PVC in one series;[10] they may arise in normal hearts but, when compared with fascicular and mitral annular PVC, it seems that these arrhythmias occur in older patients and are often related with the presence of coronary artery disease and left ventricular dysfunction;[11] they also showed that papillary ectopy has a wider QRS duration than fascicular arrhythmias.
The Fascicles of the left bundle branch are classically knowing as source of a typical idiopathic VT, but it may arise ectopy as well; it is ready identified by a narrow QRS and the characteristic ECG morphology of the fascicle of origin: left posterior fascicle with a right bundle branch block and left axis deviation; left anterior fascicle with morphology of right bundle branch block and right axis deviation; high septal fascicle with relatively narrow QRS complex and normal axis.
Epicardial and/or Peri-Vascular are other source of idiopathic ectopy. [12] Scanavacca et al suggest that these arrhythmias may be accessed by the venous system or by subxiphoid epicardial mapping, but do not recommend an epicardial approach on the first procedure for outflow tract arrhythmias;[13] one concern is the presence of the coronary arteries and phrenic nerves. A delay in the initial time of the QRS complex indicated a epicardial origin as suggest by the Maximum Deflection Index (MDI). [14]
One important aspect in counseling patients with idiopathic arrhythmias, essentially outflow tract, is the challenge of predicting the site of origin, therefore discussion about treatments options and intervention planning. There are several ECG criteria that try to locate the focus ([Figure 1]), some of them are based on complex process analysis or formulas. The inspection of QRS morphology of the VPC compared with the normal sinus rhythm (“the V2 transition ratio”)[15] is practical and helpful, [Figure 2].
Figure 1. Scheme of the main ECG morphologies of the basal PVCs. There is a progressive modification of the QRS from negative to positive in lead V1 according the origin of the PVC from the anterior RVOT to the epicardium of the posterior left ventricle.
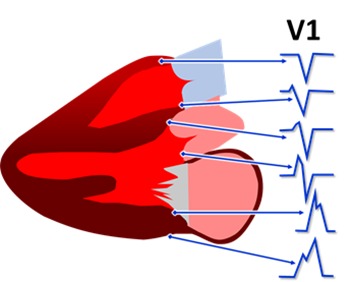
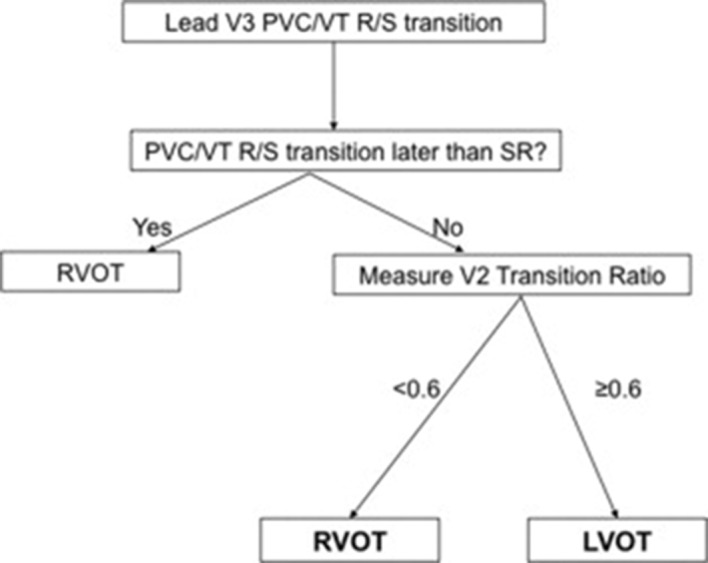
Figure 2. Algorithm developed by Betensky BP et al.15 for OTA ECG localization. If the PVC transition to an R>S occurs later than the SR transition then the PVC origin is the RVOT (100% specificity). If the PVC transition occurs at or earlier than the SR transition (i.e., SR transition lead V3 or later), then the V2 transition ratio is measured. If the transition ratio is <0.6, then RVOT origin is likely. If the transition ratio is ≥0.6, then LVOT origin is likely (sensitivity 95%, specificity 100%).
Beyond morphologic criteria, others ECG characteristics and clinical aspects may be valuable in arrhythmia discrimination. Recently Bradfield et al.[16] demonstrated that arrhythmias that arise from sinus of Valsalva and great cardiac vein have a highly variable coupling interval with the preceding normal QRS, probably due to a lack of electronic coupling with the surrounding myocardial. PVC originated from RV or LV myocardial have a relative fixe coupling interval compare with PVC from sinus of Valsalva and great cardiac vein; a pronounced variability in couple interval (Delta > 60ms) helps discriminate the origin of PVC. Another interesting study from Penela et al.[4] evaluated clinical characteristics in patients with outflow tract arrhythmias; they showed that the presence of hypertension, male gender and age > 50 years were independent predictive of LV outflow tract origin and a score with these three variables was proposed.
Tips of Ablation
Different from reentry ventricular tachycardias, which can be usually reproduced with programmed ventricular stimulation techniques, ventricular premature beats has the disadvantage of being less easily reproduced. All antiarrhythmic medications should be discontinued for at least five half-lives before the ablation. Not uncommonly, even in patients with very frequent clinical arrhythmia, it may be necessary to perform the procedure in the absence of ectopic beats. It is helpful to see in the Holter if the arrhythmia is more frequent during the sleep or vigil. In the last case a deep sedation must be avoided.
One of the key point of ablation of idiopathic VPC is to have the arrhythmia (spontaneously) in the moment of the procedure or to identify a protocol that induce the arrhythmia; It is really important for mapping and for having an end point during the procedure. There is no unique maneuver or protocol that works satisfactorily for ever case.
Despite being unpredictable, it is usually possible to trigger the ectopies appearance, so we always try to perform the procedure on the day that the patient was scheduled, but many researchers suspend the procedure if there is no spontaneous arrhythmia.
At the beginning, in the case there are no arrhythmias, with the patient fully monitored and awaked, some ventilation maneuvers (depth breath or Valsalva) may provoke VPC allowing to get recordings (that must be saved) for pace mapping. After sedation, ventilation changes induced by the anesthesiologist, like short hypoxia periods, may also reproduce the arrhythmia. After placing the catheters, there is a good chance that some atrial or ventricular stimulation protocols with different cycle length or extra-stimuli, may reproduce the arrhythmia during the stimulation or after stopping, during the pause. Ventricular stimulation must be attempted with programmed stimulation or burst. Some people try isoproterenol (despite epinephrine seems to be better), but in our experience it may be trick; some times with the sinus rate increasing it may eliminate any sporadic ectopy that could be present; also, with the vigorous myocardial contraction due to isoproterenol, the manipulation of the catheter (and pace mapping) is more difficult or even risky. It seems that isoproterenol works better when it is given as small bolus than continuously (progressive), because there is a better chance that the arrhythmia is induced after the peak effect of the drug, when the sinus rate is slowing down.
Vagal Stimulation
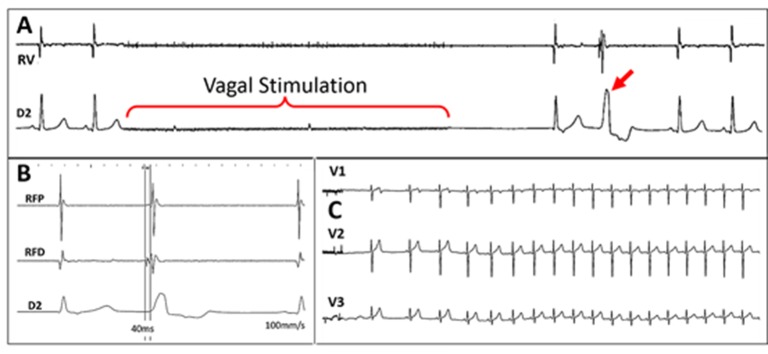
Another very interesting issue is autonomic stimulation to promote cardiac reflex for triggering some arrhythmias.[17,18] This can be important to achieve the morphology in 12 lead ECG of a PVC that eventually becomes completely absent at the time of the procedure in order to proceed with the pace mapping. We are studying the potentiality of vagal stimulation performed by placing a catheter inside the internal jugular, up to the superior wisdom tooth level by using even the RF catheter temporarily detached from the RF generator and connected to a neurostimulator, [Figure 3]. In this place, it is usually easy to get an intense vagal stimulation that causes transient asystole, [Figure 4-A]. Soon after the vagal effect, a reflex sympathetic response occurs usually causing the appearance of PVC, [Figure 4-B]. Depending on the case, that may be better than the isoproterenol infusion because it triggers the sympathetic response by natural paths. Paradoxically, by causing an important heart rate increase, the isoproterenol may prevent the PVC appearance just because of the diastolic extent reduction.
Figure 3. Scheme of the methodology for vagal stimulation during EP studies developed by authors. The RF catheter, temporarily detached from the RF generator and connected to a neurostimulator, is advanced to the internal jugular vein. This position usually has a great proximity with the vagus nerve allowing its stimulation. This maneuver must be only accomplished after being the patient anesthetized.
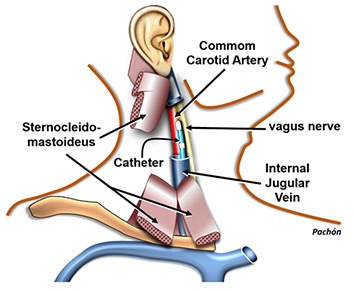
Figure 4. Case of a patient who was symptomatic due to a very frequent monomorphic VPC. However, in the EP laboratory the arrhythmia was completely absent. A: during vagal stimulation there is an immediate asystole followed by a junctional beat and one PVC (red arrow). This response was typically reproducible. All other attempts to reproduce the PVC were unsuccessful. B: The PVC triggered by the vagal stimulation was mapped and a good precocity was obtained allowing the ablation; C: After ablation, vagal stimulations were unable to reinduce new VPCs. Currently, this patient is asymptomatic and has no more arrhythmia.
Pace Mapping
It is a great aid technique pacing point-to-point areas suspected from the ECG PVC morphology. The main objective is to reproduce the morphology of spontaneous PVC in 12-lead ECG through electrical stimulation of a certain point. This step can be accomplished by direct subjective comparison of the operator or aided with automated computerized systems, [Figure 5]. A major advantage of this method is that it can be performed in the absence of the arrhythmia since the PVC morphology in a 12-lead ECG technically identical to the ablation ECG, can be compared. An important drawback is that the pace mapping identifies the starting point of the PVC appearance in the myocardium and not necessarily the actual focal origin.[19] Despite being not totally necessary, the use of three-dimensional electroanatomical mapping system and, recently, the rotational angiography may be of great value for mapping assistance.[20]
Figure 5. Computerized aid for pace mapping. Pace mapping is extremely useful in the PVC ablation however, it has some disadvantages like the time consuming and a huge dependency of the subjective interpretation of the operator. This software, developed by the authors, as other products on the market, has promoted great agility and efficiency in the pace mapping technique. In A and B there are two examples of pace mapping inappropriate for ablation, however in C the mapping is excellent that is, a place with a high probability of ablation success (all the flags are green).
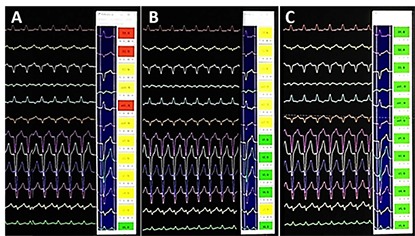
Computer Aided Pace Mapping
Other area of great interest is to improve the performance and the quality of the pace mapping technique, making it faster and less de- pendent of the interpretation of the operator. Considering that sometimes we do not have the PVC frequency as desired, pace mapping is the main approach being the technique that allows getting close of the site of origin and save time. There are some reports dealing with automatic template for computerized interpretation of pace mapping.[21]
Ablation of Valsalva Sinus PVCs
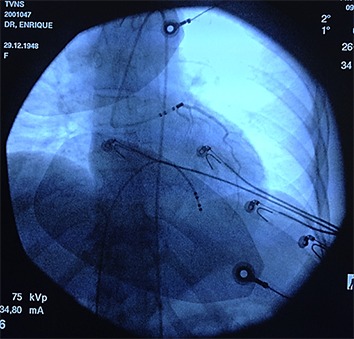
A very delicate situation is when PVC is located in the sinus of Valsalva because of the risk of coronary lesion. Only the non-coronary sinus does not originate PVCs as it relates only with the fibrous skeleton of the heart. That means, whenever there are PVCs of this location they are surely originated from the left or right coronary sinus, whose ablations should deal with the risk of coronary injury, [Figure 6]. One fundamental tip for ablation in the right or left Valsalva coronary sinus is to perform the coronariography immediately before the RF release. A simple and practical way we have proposed is to place the contrast dye in the irrigation system of the ablation catheter. After positioning the RF catheter in the most appropriate mapping place, radiographic contrast product is directly injected through the irrigation system. This should be repeated immediately before each new ablation case the catheter position has been changed. If the contrast shows that we are far from coronary ostium we can ablate without risk. Conversely, if it is found that we are very close to the coronary ostium the ablation must be suspended ([Figure 7]).
Figure 6. Scheme of the cardiac fibrous skeleton and its relationship with Valsalva sinuses. The non-coronary sinus is related with fibrous tissue only and is the solely one that does not gives rise to PVCs. The coronary sinuses are relatively frequent source of PVCs and the ablation in these places must have special care for avoiding coronary injury.
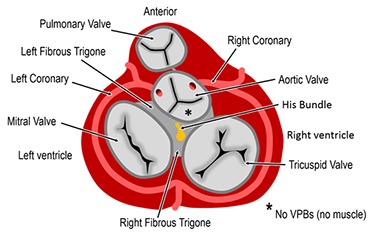
Figure 7. Forbidden PVC ablation. Method proposed and routinely employed by the authors using the irrigation system of the ablation catheter, placed in the best mapping position, by injecting X-ray dye for verifying if it is in a risk position, associated with a coronary ostium. In this example, a young woman had a ventricular tachycardia originated from the left coronary sinus. The best place for ablation was located in the left coronary ostium. The ablation catheter was relocated and the ablation was finally successful but performed outside the ostium, despite being a suboptimal position.
Activation Mapping
It can be performed directly with the RF catheter or through the electroanatomic mapping system. It is certainly the key element to the success of ablation. Generally, the potential that indicate the position of the arrhythmogenic focus with great chance of success precede the QRS surface of the PVC onset in 10 to 60ms,[22] Figure 4-B. The morphology of these potentials is also of great value especially the QS pattern in unipolar recording of the distal pole[23] or the reverse potentials comparing two distal dipoles of the ablation catheter.[24] When myocardium mapping is not quite appropriate in the myocardial the outflow tracts myocardium it is essential to make an inspection on arterial insertions, above the pulmonary and aortic valves,[25,16] as well on the mitral annulus,[8] as these places are often the real origin of these arrhythmias, [Figure 1].
Disclosures
None.
References
- 1.Dukes Jonathan W, Dewland Thomas A, Vittinghoff Eric, Mandyam Mala C, Heckbert Susan R, Siscovick David S, Stein Phyllis K, Psaty Bruce M, Sotoodehnia Nona, Gottdiener John S, Marcus Gregory M. Ventricular Ectopy as a Predictor of Heart Failure and Death. J. Am. Coll. Cardiol. 2015 Jul 14;66 (2):101–9. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasdemir Can, Ulucan Cem, Yavuzgil Oguz, Yuksel Alper, Kartal Yildirim, Simsek Evrim, Musayev Oktay, Kayikcioglu Meral, Payzin Serdar, Kultursay Hakan, Aydin Mehmet, Can Levent H. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J. Cardiovasc. Electrophysiol. 2011 Jun;22 (6):663–8. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 3.Tondo C, Carbucicchio C, Russo A D. Idiopathic Ventricular Tachycardia: Transcatheter Ablation Or Antiarrhythmic Drugs? Jornal of Atrial Fibrillation. 2015;0:26–29. doi: 10.4022/jafib.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penela Diego, De Riva Marta, Herczku Csaba, Catto Valentina, Pala Salvatore, Fernández-Armenta Juan, Acosta Juan, Cipolletta Laura, Andreu David, Borras Roger, Rios Jose, Mont Lluis, Brugada Josep, Carbucicchio Corrado, Zeppenfeld Katja, Berruezo Antonio. An easy-to-use, operator-independent, clinical model to predict the left vs. right ventricular outflow tract origin of ventricular arrhythmias. Europace. 2015 Jul;17 (7):1122–8. doi: 10.1093/europace/euu373. [DOI] [PubMed] [Google Scholar]
- 5.Latchamsetty R, Yokokawa M, Morady F. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. J Am Coll Cardiol EP. 2015;1:116–23. [Google Scholar]
- 6.Tada Hiroshi, Tadokoro Kazuyoshi, Ito Sachiko, Naito Shigeto, Hashimoto Tohru, Kaseno Kenichi, Miyaji Kohei, Sugiyasu Aiko, Tsuchiya Taketsugu, Kutsumi Yasunori, Nogami Akihiko, Oshima Shigeru, Taniguchi Koichi. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: Prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm. 2007 Jan;4 (1):7–16. doi: 10.1016/j.hrthm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Takumi, Allison Jeffery Scott, McElderry Hugh Thomas, Doppalapudi Harish, Epstein Andrew E, Plumb Vance J, Kay George Neal. Successful catheter ablation of premature ventricular contractions originating from the tricuspid annulus using a Halo-type catheter. Europace. 2008 Oct;10 (10):1228–9. doi: 10.1093/europace/eun184. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai Koji, Yamauchi Yasuteru, Takahashi Atsushi, Yokoyama Yasuhiro, Sekiguchi Yukio, Watanabe Jun, Iesaka Yoshito, Shirato Kunio, Aonuma Kazutaka. Idiopathic left ventricular tachycardia originating from the mitral annulus. J. Cardiovasc. Electrophysiol. 2005 Oct;16 (10):1029–36. doi: 10.1111/j.1540-8167.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 9.Rawling D A, Joyner R W, Overholt E D. Variations in the functional electrical coupling between the subendocardial Purkinje and ventricular layers of the canine left ventricle. Circ. Res. 1985 Aug;57 (2):252–61. doi: 10.1161/01.res.57.2.252. [DOI] [PubMed] [Google Scholar]
- 10.Ban Ji-Eun, Lee Hyun-Soo, Lee Dae-In, Park Hwan-Cheol, Park Jae-Seok, Nagamoto Yasutsugu, Choi Jong-Il, Lim Hong-Euy, Park Sang-Weon, Kim Young-Hoon. Electrophysiological characteristics related to outcome after catheter ablation of idiopathic ventricular arrhythmia originating from the papillary muscle in the left ventricle. Korean Circ J. 2013 Dec;43 (12):811–8. doi: 10.4070/kcj.2013.43.12.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al'Aref Subhi J, Ip James E, Markowitz Steven M, Liu Christopher F, Thomas George, Frenkel Daniel, Panda Nikhil C, Weinsaft Jonathan W, Lerman Bruce B, Cheung Jim W. Differentiation of papillary muscle from fascicular and mitral annular ventricular arrhythmias in patients with and without structural heart disease. Circ Arrhythm Electrophysiol. 2015 Jun;8 (3):616–24. doi: 10.1161/CIRCEP.114.002619. [DOI] [PubMed] [Google Scholar]
- 12.Daniels David V, Lu Yen-Yu, Morton Joseph B, Santucci Peter A, Akar Joseph G, Green Alex, Wilber David J. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006 Apr 4;113 (13):1659–66. doi: 10.1161/CIRCULATIONAHA.105.611640. [DOI] [PubMed] [Google Scholar]
- 13.Scanavacca M, Lara S, Hardy C, Pisani C F. How To Identify and Treat Epicardial Origin Of Outflow Tract Tachycardias. Journal of Atrial Fibrillation. 0;0:48–52. doi: 10.4022/jafib.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels David V, Lu Yen-Yu, Morton Joseph B, Santucci Peter A, Akar Joseph G, Green Alex, Wilber David J. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006 Apr 4;113 (13):1659–66. doi: 10.1161/CIRCULATIONAHA.105.611640. [DOI] [PubMed] [Google Scholar]
- 15.Betensky Brian P, Park Robert E, Marchlinski Francis E, Hutchinson Matthew D, Garcia Fermin C, Dixit Sanjay, Callans David J, Cooper Joshua M, Bala Rupa, Lin David, Riley Michael P, Gerstenfeld Edward P. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J. Am. Coll. Cardiol. 2011 May 31;57 (22):2255–62. doi: 10.1016/j.jacc.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Bradfield Jason S, Homsi Mohamed, Shivkumar Kalyanam, Miller John M. Coupling interval variability differentiates ventricular ectopic complexes arising in the aortic sinus of valsalva and great cardiac vein from other sources: mechanistic and arrhythmic risk implications. J. Am. Coll. Cardiol. 2014 May 27;63 (20):2151–8. doi: 10.1016/j.jacc.2014.02.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pachon MJC, Pachon EI, Santillana PTG, Lobo TJ, Pachon CTC, Pachon M Juan, Albornoz VRN, Zerpa AJC. Simplified Method for Vagal Effect Evaluation in Cardiac Ablation and Electrophysiological Procedures. In Press; doi:10.1016/j.jacep.2015.06.008. J Am Coll Cardiol EP. 2015;0:0–0. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Hasdemir Can, Alp Alpay, Aydin Mehmet, Can Levent H. Human model simulating right ventricular outflow tract tachycardia by high-frequency stimulation in the left pulmonary artery: autonomics and idiopathic ventricular arrhythmias. J. Cardiovasc. Electrophysiol. 2009 Jul;20 (7):759–63. doi: 10.1111/j.1540-8167.2009.01442.x. [DOI] [PubMed] [Google Scholar]
- 19.Bogun Frank, Taj Majid, Ting Michael, Kim Hyungjin Myra, Reich Stephen, Good Eric, Jongnarangsin Krit, Chugh Aman, Pelosi Frank, Oral Hakan, Morady Fred. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008 Mar;5 (3):339–44. doi: 10.1016/j.hrthm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Orlov Michael V, Ansari Mohammad M, Akrivakis Spyridon T, Jadidi Amir, Nijhof Niels, Natan Shaw R, Wylie John V, Hicks Amy, Armstrong James, Jais Pierre. First experience with rotational angiography of the right ventricle to guide ventricular tachycardia ablation. Heart Rhythm. 2011 Feb;8 (2):207–11. doi: 10.1016/j.hrthm.2010.09.087. [DOI] [PubMed] [Google Scholar]
- 21.Kuteszko Rafal, Pytkowski Mariusz, Farkowski Michal M, Maciag Aleksander, Sterlinski Maciej, Jankowska Agnieszka, Kowalik Ilona, Zajac Dariusz, Firek Bohdan, Demkow Marcin, Szwed Hanna. Utility of automated template matching for the interpretation of pace mapping in patients ablated due to outflow tract ventricular arrhythmias. Europace. 2015 Sep;17 (9):1428–34. doi: 10.1093/europace/euu392. [DOI] [PubMed] [Google Scholar]
- 22.Bogun Frank, Taj Majid, Ting Michael, Kim Hyungjin Myra, Reich Stephen, Good Eric, Jongnarangsin Krit, Chugh Aman, Pelosi Frank, Oral Hakan, Morady Fred. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008 Mar;5 (3):339–44. doi: 10.1016/j.hrthm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Man K C, Daoud E G, Knight B P, Bahu M, Weiss R, Zivin A, Souza S J, Goyal R, Strickberger S A, Morady F. Accuracy of the unipolar electrogram for identification of the site of origin of ventricular activation. J. Cardiovasc. Electrophysiol. 1997 Sep;8 (9):974–9. doi: 10.1111/j.1540-8167.1997.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 24.van Huls van Taxis Carine F B, Wijnmaalen Adrianus P, den Uijl Dennis W, Gawrysiak Marcin, Putter Hein, Schalij Martin J, Zeppenfeld K. Reversed polarity of bipolar electrograms for predicting a successful ablation site in focal idiopathic right ventricular outflow tract arrhythmias. Heart Rhythm. 2011 May;8 (5):665–71. doi: 10.1016/j.hrthm.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Timmermans Carl, Rodriguez Luz-Maria, Crijns Harry J G M, Moorman Antoon F M, Wellens Hein J J. Idiopathic left bundle-branch block-shaped ventricular tachycardia may originate above the pulmonary valve. Circulation. 2003 Oct 21;108 (16):1960–7. doi: 10.1161/01.CIR.0000095026.19339.BB. [DOI] [PubMed] [Google Scholar]