Abstract
After the success story of implantable cardioverter/defibrillator systems, prevention of sudden cardiac death (SCD) remains one of the main duties in cardiology. For patients with unkown or transient risk profile for SCD, a wearable cardioverter/defibrillator (WCD) has been established for temporary and effective prevention of sudden arrhythmic death. Several studies have shown safety and efficacy of the WCD, even though randomized studies proving a mortality benefit are still lacking. This review provides an overview of actual WCD data and usage, special indications and possible risks and complications. WCD use is effective and adequate for temporary prevention of SCD in chosen populations. In particular, it provides secured time for sophisticated risk stratification to identify patients at persistent risk for SCD. Nevertheless, prospective randomized trials seem mandatory to prove a prognostic relevance and the economic value of this device.
Keywords: Sudden Cardiac Death, Ventricular Arrhythmia, Wearable Cardioverter/Defibrillator
Introduction
Sudden cardiac arrest (SCA) due to tachyarrhythmias remains a major cause of death in western countries.[1,2] The implantable cardioverter/defibrillator (ICD) has been used for more than 30 years and is considered one cornerstone for primary and secondary prevention of sudden cardiac death (SCD) in high-risk patients.[3] Decades have passed since the milestone trials in ICD therapy. The MADIT, MADIT-II and SCD-HeFT trial enrolled patients between 1990 and 2001.[4-6] Since then, interventional and drug therapies for these patients have evolved tremendously and concomitantly may have affected risks for SCD. Additionally, ICD therapy may be accompanied by several device-related problems, especially lead failure.[7] Recently, van der Heijden et al. described an overall-incidence for device related adverse events of 20% inappropriate shocks, device infections of 6% and 18% lead failures within 12 years of follow-up.[8] ICD therapy has been shown to be cost-effective.[9,10] However, a relevant proportion of patients getting implanted do not meet evidence-based criteria for implantation.[11] Therefore, careful but however secured risk assessment before ICD implantation may be even more cost-effective.
Risk Stratification
A large number of possible risk markers like microvolt T-wave alternans, tests for autonomic dysfunction or signal averaged ECG have been proposed. Though, they did not find their way into clinical routine and are actually not supported by the guidelines.[12] The only evidence-based risk stratifying marker today remains left ventricular ejection fraction, thereby reading “left ventricular ejection fraction (LVEF)”.[12]
Having said this, in a recent study, Sjöblom et al. investigated evolution of LVEF in 91 patients after myocardial infarction (MI).[13] 45% of the patients met the ICD criteria of LVEF ≤35% 40 days after MI. However, the authors found further significant improvement in LVEF in 6 more patients at 3 months follow-up (p=0.01), meaning that these patients no longer met the criteria for ICD implantation. These findings show that there is further improvement in LVEF beyond the initial 40 days post MI. Furthermore, 10% of the patients presented with life-threatening ventricular arrhythmias within the first 9 weeks post MI, emphasizing the arrhythmic risk and the need for antiarrhythmic prophylaxis in this early phase. Accordingly, in patients with recent onset non-ischemic cardiomyopathy, the IMAC study showed no benefit from early ICD implantation.[14]
Wearable Cardioverter/Defibrillator
For patients with unkown or transient risk profile for SCD, a wearable cardioverter/defibrillator (WCD) (LifeVest®, ZOLL, Pittsburgh, PA, USA) has been established for temporary but however effective prevention of sudden arrhythmic death ([Figure 1]). The WCD continuously analyzes the heart rhythm using 4 non-adhesive electrodes incorporated in a light garment. The ECG is registered via 2 non-standard leads (front-back and side-side). When a life-threatening arrhythmia is detected, the WCD runs an alarm cascade including audible, visual and tactile alarms. If the patient is conscious, he can withhold any therapy by pressing two response buttons on the monitor. In case of unconsciousness and consequently release of the response button, the WCD will deliver a biphasic shock after having deployed contact gel through the contact electrodes. Programming of detection rates can include a ventricular tachycardia (VT) and a ventricular fibrillation (VF) zone, programmable from 120 to 250 beats per minute. The shock energy can be chosen from 75 to 150 J. All ECG with detected arrhythmias are stored in the device and regularly transferred to a webserver where the attending physician can review all episodes as well as the patient’s compliance. Since the arrhythmia detection of the WCD is performed via surface nonadhesive electrodes, there is a considerable risk for motion-related noise artifacts.
Figure 1. Wearable Cardioverter Defibrillator (WCD).

The WCD is used in patients at undefined or temporary risk for SCD as well as in patients at known persistent high risk but with transient contraindications for implantation of an ICD. Common indications for WCD wearing are shown in [table 1].
Table 1. Common indications for wearable cardioverter/defibrillators[49].
| Acute myocardial infarction with/without PCI and a LVEF ≤35% |
| Coronary revascularization (PCI or CABG) and a LVEF ≤35% |
| Non-ischemic cardiomyopathy, (acute) myocarditis, stress (Takotsubo) cardiomyopathy, peripartum cardiomyopathy, LVEF ≤35% |
| Waiting for heart transplantation |
| ICD explantation until re-implantation) |
| Post VT ablation in patients with only moderately reduced LVEF. |
WCD often gets misclassified as an alternative to permanent ICD or a “bridge to ICD”. This does not give sufficient consideration to the capabilities and the concept of WCD usage. This review provides an overview of actual WCD data and usage, special indications and possible risks and complications.
Clinical Data
In 1998, Auricchio et al. reported the first 10 patients with successful termination of ventricular arrhythmia by the WCD.[15] Subsequently, WCD was shown to be safe and effective in detection and termination of VF.[16-18]
[Table 2] summarizes available data on WCD. Despite the manifest gaps in evidence, based on these registries and case reports, the actual ESC guidelines on prevention for SCD give a Class IIb level of evidence C indication for the WCD “for adult patients with poor LV systolic function who are at risk for sudden arrhythmic death for a limited period, but are not candidates for an implantable defibrillator (e.g. bridge to transplant, bridge to transvenous implant, peripartum cardiomyopathy, active myocarditis and arrhythmias in the early post-myocardial infarction phase)”.[12]
Table 2. Registries on WCD (MI: myocardial infarction, PPCM: Peripartum cardiomyopathy, NICM: non-ischemic cardiomyopathy, CHD: congenital heart disease, IA: inherited arrhythmias).
| Publication | Year | Patients (n) | Etiology | Prospective/ retrospective | Appropriate shocks | Compliance (h/d) | Cumulative wearing time | Unnecessary shocks |
|---|---|---|---|---|---|---|---|---|
| WEARIT/ BIROAD[18] | 2004 | 289 | Miscellaneous | Prospective | 6 | n/a | Mean 3.1 months | 6 (2%) |
| Klein[50] | 2009 | 354 | Miscellaneous | Retrospective | 27 (27.6%) | Mean 21.3 | Mean 106 days | 0 |
| Saltzberg[38] | 2009 | 258 | PPCM/ NICM | Retrospective | 0/2 | 18.3/17.0 | Mean 75±81/56±54 days | 0/0 |
| Dillon[17] | 2010 | 2105 | Miscellaneous | Retrospective | 54 | Median 21.3 | Median 36 days (3-365) | 34 |
| Chung[47] | 2004 | 3569 | Miscellaneous | Retrospective | 76 (2.1%) | Mean 19.9±4.7 | Mean 52.6±69.9 days | 67 (1.9%) |
| Rao[32] | 2011 | 162 | CHD, IA | Retrospective | 0/2% | Mean 19 | Mean 27/29 days | 0/3% |
| Epstein[19] | 2013 | 8453 | Post MI | Retrospective | 146 shocks in 133 (1.6%) patients | Median 21.8 | Mean 69±61 (median 57) days | 114 in 99 patients |
| Zishiri[51] | 2013 | 4958 | Miscellaneous | Retrospective observational cohort study | 18 shocks in 11 patients | n/a | n/a | n/a |
| Tanawuttiwat[40] | 2014 | 97 | Explantation of ICD | Retrospective | 4 | Median 20 | Median 21 days | 2 |
| Sasaki[52] | 2014 | 9 | Retrospective | 1 | Mean 23.7 | Mean 21 days | 0 | |
| Duncker[39] | 2014 | 9 | PPCM | Prospective | 4 | Mean 22.0±2.4 | Mean 133±103 days | 0 |
| Opreanu[34] | 2015 | 122 | Candidates for cardiac transplant | Retrospective | 7 | Mean 17±7 (median 20) | Mean 127±392 (median 39) days | 2 |
| WEARIT-II[24] | 2015 | 2000 | ICM, NICM, CHD | Prospective | 120 in 41 patients | Median 22.5 | Median 90 days | 10 patients |
Epstein et al. analyzed 8453 patients wearing the WCD early post MI [19] and found 133 patients (1.6%) receiving 309 appropriate shocks. Beyond that, 114 inappropriate shocks occurred in 99 patients. Mean time from MI to WCD prescription was 9±9 days and mean time from prescription to first shock delivery was 22±32 days. This shows that commonly accepted waiting time between MI and ICD implantation of 40 days and 3 months respectively nevertheless may remain a period at risk for life-threatening arrhythmias. These findings are consistent with those of the VALIANT study which found the highest mortality in the first 30 days after MI.[20] The DINAMIT[21] and IRIS[22] study suggested no survival benefit from ICD implantation early post MI, even though in both studies, the rate of SCD was halved in the ICD group. Unfortunately, this reduction of SCD was negated by an increased number of non-sudden cardiovascular deaths.[23] The reason for this difference has not been clarified sufficiently. Furthermore, relevant differences are obvious comparing the populations of these two studies with a population of a recent heart failure trial, e.g. in terms of optimized heart failure medication. Given the great improvement in heart failure therapy and interventional development for the treatment of acute MI in the last years, the amount of non-sudden cardiac deaths may therefore be relevantly reduced, leaving the amount of preventable arrhythmic death at the disposal of antiarrhythmic devices.
Despite the great amount of descriptive or cohort studies on WCD and the presumed self-evident benefit of the WCD, most of these studies lack a control group, not to mention a randomization. Therefore, the net benefit of the WCD still remains to be proven. The only randomized controlled trial on patients after MI is the multicenter VEST trial with a total of 1900 randomized patients (www.clinicaltrials.gov, NCT01446965), which is about to complete enrollment. The primary endpoint is sudden death mortality.
The recently published WEARIT-II prospective registry[24] enrolled 2000 patients receiving WCD for a median of 90 days. 40% of the patients had ischemic cardiomyopathy, 46% had non-ischemic cardiomyopathy and 14% had congenital heart disease. Authors reported 120 ventricular tachyarrhythmias in 41 patients (2%) and only 10 patients (0.5%) receiving an inappropriate shock. Even though the WEARIT-II registry represents the greatest prospective database on WCD published to date, it still does not answer the relevant unsettled questions on hard endpoints in WCD use.
Special Indications
Especially for possibly transient circumstances which may temporarily elevate the risk for life-threatening arrhythmias, the WCD offers protected time for any further diagnostics, risk stratification, re-evaluation or simply letting any sort of therapy work without letting the patient at risk during this period.
Rare Cardiomyopathies
Successful use of the WCD has been described in acute or suspected myocarditis,[25,26] stress cardiomyopathy, [27,28] noncompaction cardiomyopathy,[29] alcohol toxic cardiomyopathy,[30] congenital heart disease[24,31,32] as well as in children.[33] Candidates for cardiac transplantation can likewise be provided a WCD until transplantation.[34] However, since these days waiting for a donor organ may take several years, implanting an ICD has to be seriously considered.
ICD therapy in inherited arrhythmia syndromes like long QT syndrome, Brugada syndrome or arrhythmogenic right ventricular dysplasia is challenging due to the rather young patient age, limited available data and elevated complication rate.[35] WCD can facilitate and cover time to diagnosis and risk stratification in these patients. In the study by Rao et al., among 119 patients with inherited arrhythmias receiving a WCD, the predominant indication was pending genetic testing.[32] Again, the WCD should not be considered as a bridge to ICD in these patients, but rather as a tool for serving protected time to exclude a diagnosis or to stratify the patient as low-risk and thus omitting ICD implantation.
Peripartum cardiomyopathy
The concept of temporary prophylaxis for SCD of the WCD is particularly attractive for transient pathologies leading to a temporarily elevated risk for SCD. Peripartum cardiomyopathy (PPCM) represents a rare idiopathic cardiomyopathy leading to heart failure LV dysfunction towards the end of pregnancy or in the months following delivery.[36] Even if initial LVEF often is severely affected at the time of diagnosis, there is a high potential for LVEF recovery after starting an optimal heart failure medication regimen.37 In a large cohort of 107 patients with PPCM wearing a WCD, Saltzberg et al. did not report any arrhythmic event (though 3 patients died after WCD use).[38] This may eventually be due to the retrospective character of their analysis. On the contrary, we recently reported 12 consecutively admitted patients with first diagnosis of PPCM.[39] 7 patients presented with a LVEF of ≤35% and received a WCD for 3 to 6 months. Among these 7 patients, we observed 4 events of VF in 3 of the patients. Patients significantly recovered in LVEF during follow-up. Our data strongly suggest an elevated risk for life-threatening arrhythmias in these young mothers early after diagnosis of PPCM and warrant an uninterrupted use of the WCD in all patients in the early phase of PPCM during recovery.
ICD explantation due to infection
Due to increasing numbers of ICD implantations and subsequent generator exchanges, numbers of device infections with need of system explantation are increasing, too. Especially for patients with secondary prophylactic indications for an ICD, continuous monitoring after explantation seems mandatory. Besides inpatient monitoring for the period of antibiotic therapy, outpatient management using a WCD for this period seems reasonable. Tannawuttiwat et al. presented a retrospective analysis of 97 patients wearing a WCD after ICD removal.[40] 2 patients received 4 shocks, 1 patient received 2 unnecessary shocks. 8 patients (8.2%) died (5 patients in hospital, 3 patients at home), no one was wearing the WCD at the time of death. In a cost-effectiveness model, the WCD was shown to be cost-effective in comparison to inpatient strategy until re-implantation.[41]
Malignancies
WCD can successfully be used in cancer patients who often present a contraindication for ICD implantation.[42] Special considerations can be raised on patients with planned radiotherapy adjacent to an implanted ICD. Depending on the local findings and the planned radiation protocol, an explantation of the ICD with temporary use of a WCD for the radiation period and and subsequent re-implantation may be a favorable strategy, as reported by Bowers et al.[43]
Renal disease
Patients who are in end-stage renal disease are known to have a high risk for ventricular arrhythmias, but are as well known to show reduced benefit from primary prophylactic ICD therapy due to competing risks.[44] Nevertheless, Wan et al. reported 84 SCA episodes in 75 patients on hemodialysis showing the important arrhythmic burden in these patients.[45] Not all of these episodes were tachyarrhythmias, but 18 episodes were described as asystoles not treated by the WCD.
The actually enrolling WED-HED study (www.clinicaltrials. gov, NCT02481206) is a multi-center, prospective, randomized controlled clinical trial with 1:1 assignment of treatment and control. It will evaluate the impact of WCD use on sudden cardiac death in incident hemodialysis patients.
Risks and Complications of WCD Wearing
The WEARIT/BIROAD registry reported two patients with unsuccessful defibrillation due to incorrectly placed therapy electrodes.[18] Accurate and thorough training and instruction of the patients therefore seems mandatory. The effectiveness and reliability of the WCD mainly depends on the patient’s compliance.
In the WEARIT/BIROAD registry 65 out of 289 patients discontinued WCD use before prematurely, 30% did so due to comfort or lifestyle issues.[18]
LaPage et al. reported one case of missensing a fatal ventricular arrhythmia in a patient with unipolar pacemaker stimulation.[46] Unipolar stimulation should be avoided in patients wearing the WCD.
Sudden cardiac arrest may be due to asystole in some patients. Chung et al. reported 23 patients showing asystole events, 17 of which died.[47] Asystole is a relevant cause of SCD in patients with reduced LV function[48] and to date, there are no data about the relevance of backup pacing on mortality benefit in ICD patients.
Since the WCD is not able to provide pacing for bradyarrhythmia, asystole may lead to SCD even though the patient is wearing the WCD. Nevertheless, an asystole event will trigger the alarm cascade and may call possible bystander’s attention to the patient.
Inappropriate therapies are another concern during WCD use. They are reported occurring in 0.5-3% of the patients.[24,32] Like in implanted devices, there is the possibility of T wave oversensing. If the patient fails to press the response buttons, inadequate shock will be delivered ([Figure 2]). Since the WCD is programmed to deliver a “synchronized” shock in case of regular VT, a “synchronized” shock triggered by an oversensed T wave may happen with a high risk of induction of VF. This underlines again the need for a dedicated training of the patient in handling the WCD.
Figure 2. ECG shows normal sinus rhythm with T wave oversensing. The patient did not press the response buttons and therefore received an inappropriate therapy. Fortunately, the shock was triggered to the true R-wave.
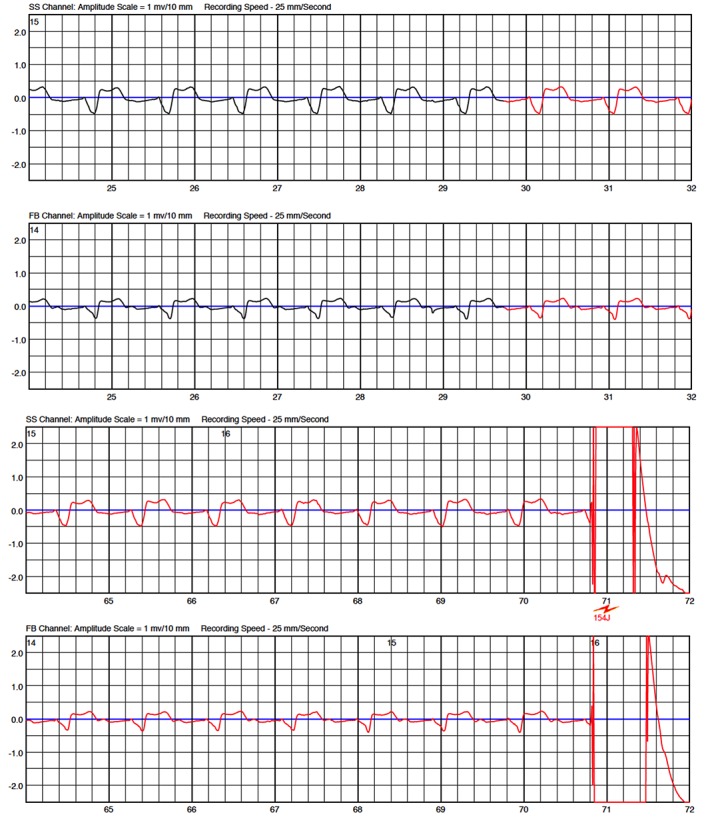
One of our patients presented a hemodynamic instable VT and fell unconscious. VT was detected and shocked by the WCD. Immediately after WCD shock, the patient developed irregular ventricular rhythm. This rhythm was inappropriately classified as a ventricular tachyarrhythmia by the WCD. Since the patient was still not fully conscious, he failed to press the response buttons and therefore received a second shock, which was inappropriate ([Figure 3]). Besides the psychic and painful consequences, inadequate therapies bear the risk for proarrhythmogenity by triggering malignant arrhythmias. The concept of pressing the response buttons gives relevant safety, but however there are possible scenarios in which the patient may not be capable to withhold therapies, as shown in our case.
Figure 3. Episode of a patient with dilative cardiomyopathy. Patient fell on the floor and remained unconscious. ECG shows ventricular tachycardia that was adequately detected and WCD shock was delivered. After WCD shock, the patient remained unconscious and developed irregular ventricular rhythm going along with some noising leading to oversensing and delivery of another – inappropriate – WCD shock.
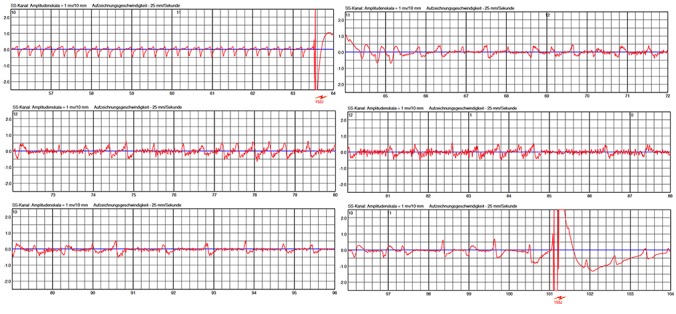
Undersensing due to low amplitudes during VF is a major concern in ICD. Low amplitudes in VF may even more occur in surface ECG. In one patient, we noticed VF undersensing due to very low amplitudes during VF ([Figure 4]).
Figure 4. Spontaneous ventricular fibrillation (VF) occurred in this patient with peripartum cardiomyopathy. Amplitude of VF rapidly decreased followed by myopotentials (black arrow) which were possibly linked to hypoxia-related seizures. Due to this high-frequency lowamplitude signals, VF criteria were no longer satisfied and detection was cancelled (white arrows). VF was redetected several more times. Probably due to some increase in VF amplitude, VF was finally redetected and the life-saving shock was successfully applied 80 seconds after onset of the tachycardia.

Workflow
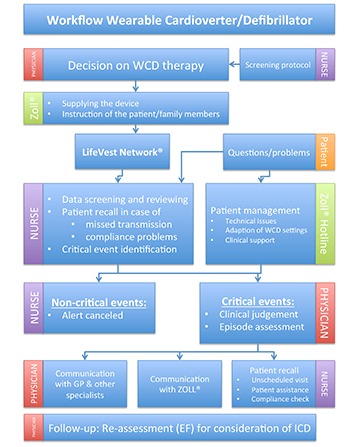
The LifeVest Network® (Zoll, Pittsburgh, PA, USA) permits surveillance of WCD patients via remote monitoring. As a result of increasing patient numbers being considered for WCD wearing, the process of screening, prescription, training and remote monitoring of the patients becomes more and more time consuming. Increasing patient numbers require structured and optimized patient management strategies to assure both, reliable logistics and adequate handling of critical events. In our department, we have established a workflow determining responsibilities and sequences in the course of a WCD wearing ([Figure 5]). Screening possible patients for WCD wearing is performed by a trained nurse using a screening protocol considering the indications listed in [table 1]. The decision for WCD wearing is then taken by a physician taking into account device-related issues (efficacy and safety), patient-related factors (compliance, aptitude, acceptance), and disease-related aspects (indications and survival benefit). Cautious instruction of the patient (and, if feasible, family members) is performed by the manufacturer when supplying the device. WCD data (arrhythmia events, compliance, etc.) is available through the telemonitoring system. Any event reported in LifeVest-Network® is verified by the responsible nurse and submitted for further review to the physician, if classified as “critical event”. “Critical events” were defined as: (1) any therapy delivery, (2) any sustained or non-sustained tachycardia, (3) any abnormality in the ECG not convincingly attributed to noise. In order to identify patients at nontransient, enduring risk for SCD, a careful follow-up for re-evaluation of LVEF is scheduled after 3 months. We use this standardized workflow to facilitate and optimize patient management in clinical routine.
Figure 5. Workflow of the Wearable Cardioverter/Defibrillator (WCD) program at Hannover Medical School (GP: general practitioner, EF: left ventricular ejection fraction).
Perspective
The WCD also offers new diagnostic options that may be used in future versions. The exceptional chance of this device consists in a continuous ECG monitor for 3 to 6 months. It already detects asystole events (without giving any therapeutic options), but it could just as well detect other arrhythmias. By detecting asymptomatic atrial fibrillation, the WCD could enable stroke prevention at an early stage. Continuous analysis of the ECG during this long period in high-risk or assumingly high-risk patients has never been done before. Additionally, the device can offer supplemental parameters, such as heart failure indices or tests, which may be relevant in some patients. Technicians and developer of the manufacturer should yield this hoard to discover new unprecedented insights in rhythmic and arrhythmic evolution in these patients. This tool offers completely new options for future ECG risk stratification.
Conclusions
WCD use is effective and adequate for temporary prevention of sudden arrhythmic death in chosen populations. In particular, it provides secured time for sophisticated risk stratification to identify patients at persistent risk for SCD. Nevertheless, prospective randomized trials seem mandatory to prove a prognostic effect/ relevance and the economic value of this device.
Disclosures
None.
References
- 1.Goldberger Jeffrey J, Basu Anirban, Boineau Robin, Buxton Alfred E, Cain Michael E, Canty John M, Chen Peng-Sheng, Chugh Sumeet S, Costantini Otto, Exner Derek V, Kadish Alan H, Lee Byron, Lloyd-Jones Donald, Moss Arthur J, Myerburg Robert J, Olgin Jeffrey E, Passman Rod, Stevenson William G, Tomaselli Gordon F, Zareba Wojciech, Zipes Douglas P, Zoloth Laurie. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014 Jan 28;129 (4):516–26. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 2.Martens Eimo, Sinner Moritz F, Siebermair Johannes, Raufhake Carsten, Beckmann Britt M, Veith Stefan, Düvel Dieter, Steinbeck Gerhard, Kääb Stefan. Incidence of sudden cardiac death in Germany: results from an emergency medical service registry in Lower Saxony. Europace. 2014 Dec;16 (12):1752–8. doi: 10.1093/europace/euu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuzner J, Gradaus R. [ICD therapy in the primary prevention of sudden cardiac death: Risk stratification and patient selection]. Herzschrittmacherther Elektrophysiol. 2015 Jun;26 (2):75–81. doi: 10.1007/s00399-015-0371-9. [DOI] [PubMed] [Google Scholar]
- 4.Moss A J, Hall W J, Cannom D S, Daubert J P, Higgins S L, Klein H, Levine J H, Saksena S, Waldo A L, Wilber D, Brown M W, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N. Engl. J. Med. 1996 Dec 26;335 (26):1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 5.Moss Arthur J, Zareba Wojciech, Hall W Jackson, Klein Helmut, Wilber David J, Cannom David S, Daubert James P, Higgins Steven L, Brown Mary W, Andrews Mark L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002 Mar 21;346 (12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy Gust H, Lee Kerry L, Mark Daniel B, Poole Jeanne E, Packer Douglas L, Boineau Robin, Domanski Michael, Troutman Charles, Anderson Jill, Johnson George, McNulty Steven E, Clapp-Channing Nancy, Davidson-Ray Linda D, Fraulo Elizabeth S, Fishbein Daniel P, Luceri Richard M, Ip John H. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005 Jan 20;352 (3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Kleemann Thomas, Becker Torsten, Doenges Klaus, Vater Margit, Senges Jochen, Schneider Steffen, Saggau Werner, Weisse Udo, Seidl Karlheinz. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007 May 15;115 (19):2474–80. doi: 10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijden Aafke C, Borleffs C Jan Willem, Buiten Maurits S, Thijssen Joep, van Rees Johannes B, Cannegieter Suzanne C, Schalij Martin J, van Erven Lieselot. The clinical course of patients with implantable cardioverter-defibrillators: Extended experience on clinical outcome, device replacements, and device-related complications. Heart Rhythm. 2015 Jun;12 (6):1169–76. doi: 10.1016/j.hrthm.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Thijssen Joep, van den Akker van Marle M Elske, Borleffs C Jan Willem, van Rees Johannes B, de Bie Mihály K, van der Velde Enno T, van Erven Lieselot, Schalij Martin J. Cost-effectiveness of primary prevention implantable cardioverter defibrillator treatment: data from a large clinical registry. Pacing Clin Electrophysiol. 2014 Jan;37 (1):25–34. doi: 10.1111/pace.12238. [DOI] [PubMed] [Google Scholar]
- 10.Smith Tim, Jordaens Luc, Theuns Dominic A M J, van Dessel Pascal F, Wilde Arthur A, Hunink M G Myriam. The cost-effectiveness of primary prophylactic implantable defibrillator therapy in patients with ischaemic or non-ischaemic heart disease: a European analysis. Eur. Heart J. 2013 Jan;34 (3):211–9. doi: 10.1093/eurheartj/ehs090. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib Sana M, Hellkamp Anne, Curtis Jeptha, Mark Daniel, Peterson Eric, Sanders Gillian D, Heidenreich Paul A, Hernandez Adrian F, Curtis Lesley H, Hammill Stephen. Non-evidence-based ICD implantations in the United States. JAMA. 2011 Jan 5;305 (1):43–9. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priori Silvia G, Blomström-Lundqvist Carina, Mazzanti Andrea, Blom Nico, Borggrefe Martin, Camm John, Elliott Perry Mark, Fitzsimons Donna, Hatala Robert, Hindricks Gerhard, Kirchhof Paulus, Kjeldsen Keld, Kuck Karl-Heinz, Hernandez-Madrid Antonio, Nikolaou Nikolaos, Norekvål Tone M, Spaulding Christian, Van Veldhuisen Dirk J. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015 Nov 1;36 (41):2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 13.Sjöblom Johanna, Muhrbeck Josephine, Witt Nils, Alam Mahbubul, Frykman-Kull Viveka. Evolution of left ventricular ejection fraction after acute myocardial infarction: implications for implantable cardioverter-defibrillator eligibility. Circulation. 2014 Aug 26;130 (9):743–8. doi: 10.1161/CIRCULATIONAHA.114.009924. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard Richard, Mather Paul J, Alexis Jeffrey D, Starling Randall C, Boehmer John P, Thohan Vinay, Pauly Daniel F, Markham David W, Zucker Mark, Kip Kevin E, McNamara Dennis M. Implantable cardiac defibrillators and sudden death in recent onset nonischemic cardiomyopathy: results from IMAC2. J. Card. Fail. 2012 Sep;18 (9):675–81. doi: 10.1016/j.cardfail.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auricchio A, Klein H, Geller C J, Reek S, Heilman M S, Szymkiewicz S J. Clinical efficacy of the wearable cardioverter-defibrillator in acutely terminating episodes of ventricular fibrillation. Am. J. Cardiol. 1998 May 15;81 (10):1253–6. doi: 10.1016/s0002-9149(98)00120-9. [DOI] [PubMed] [Google Scholar]
- 16.Reek Sven, Geller J Christoph, Meltendorf Ulf, Wollbrueck Anke, Szymkiewicz Steven J, Klein Helmut U. Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol. 2003 Oct;26 (10):2016–22. doi: 10.1046/j.1460-9592.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 17.Dillon Katie A, Szymkiewicz Steven J, Kaib Thomas E. Evaluation of the effectiveness of a wearable cardioverter defibrillator detection algorithm. J Electrocardiol. 2009 Jul 3;43 (1):63–7. doi: 10.1016/j.jelectrocard.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Feldman Arthur M, Klein Helmut, Tchou Patrick, Murali Srinivas, Hall W Jackson, Mancini Donna, Boehmer John, Harvey Mark, Heilman M Stephen, Szymkiewicz Steven J, Moss Arthur J. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004 Jan;27 (1):4–9. doi: 10.1111/j.1540-8159.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 19.Epstein Andrew E, Abraham William T, Bianco Nicole R, Kern Karl B, Mirro Michael, Rao Sunil V, Rhee Edward K, Solomon Scott D, Szymkiewicz Steven J. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J. Am. Coll. Cardiol. 2013 Nov 19;62 (21):2000–7. doi: 10.1016/j.jacc.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 20.Solomon Scott D, Zelenkofske Steve, McMurray John J V, Finn Peter V, Velazquez Eric, Ertl George, Harsanyi Adam, Rouleau Jean L, Maggioni Aldo, Kober Lars, White Harvey, Van de Werf Frans, Pieper Karen, Califf Robert M, Pfeffer Marc A. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005 Jun 23;352 (25):2581–8. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 21.Hohnloser Stefan H, Kuck Karl Heinz, Dorian Paul, Roberts Robin S, Hampton John R, Hatala Robert, Fain Eric, Gent Michael, Connolly Stuart J. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004 Dec 9;351 (24):2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 22.Steinbeck Gerhard, Andresen Dietrich, Seidl Karlheinz, Brachmann Johannes, Hoffmann Ellen, Wojciechowski Dariusz, Kornacewicz-Jach Zdzisława, Sredniawa Beata, Lupkovics Géza, Hofgärtner Franz, Lubinski Andrzej, Rosenqvist Mårten, Habets Alphonsus, Wegscheider Karl, Senges Jochen. Defibrillator implantation early after myocardial infarction. N. Engl. J. Med. 2009 Oct 8;361 (15):1427–36. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 23.Dorian Paul, Hohnloser Stefan H, Thorpe Kevin E, Roberts Robin S, Kuck Karl-Heinz, Gent Michael, Connolly Stuart J. Mechanisms underlying the lack of effect of implantable cardioverter-defibrillator therapy on mortality in high-risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation. 2010 Dec 21;122 (25):2645–52. doi: 10.1161/CIRCULATIONAHA.109.924225. [DOI] [PubMed] [Google Scholar]
- 24.Kutyifa Valentina, Moss Arthur J, Klein Helmut, Biton Yitschak, McNitt Scott, MacKecknie Bonnie, Zareba Wojciech, Goldenberg Ilan. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation. 2015 Oct 27;132 (17):1613–9. doi: 10.1161/CIRCULATIONAHA.115.015677. [DOI] [PubMed] [Google Scholar]
- 25.Prochnau Dirk, Surber Ralf, Kuehnert Helmut, Heinke Matthias, Klein Helmut U, Figulla Hans R. Successful use of a wearable cardioverter-defibrillator in myocarditis with normal ejection fraction. Clin Res Cardiol. 2010 Feb;99 (2):129–31. doi: 10.1007/s00392-009-0093-2. [DOI] [PubMed] [Google Scholar]
- 26.Strauss Margit, Kouraki Kleopatra, Skarlos Alexandros, Zahn Ralf, Kleemann Thomas. A patient with severely reduced LV function and electrical storm saved by wearable cardioverter-defibrillator: a case report. Herzschrittmacherther Elektrophysiol. 2013 Jun;24 (2):136–8. doi: 10.1007/s00399-013-0264-8. [DOI] [PubMed] [Google Scholar]
- 27.Nascimento Francisco O, Krishna Rama K, Hrachian Hakop, Santana Orlando. Wearable cardioverter defibrillator in stress cardiomyopathy and cardiac arrest. BMJ Case Rep. 2013;2013 () doi: 10.1136/bcr-2013-009789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters S, Klein H U. WCD LifeVest: risk stratification in a case of Tako-Tsubo cardiomyopathy with QT interval prolongation. Herz. 2012 Mar;37 (2):219–21. doi: 10.1007/s00059-011-3440-9. [DOI] [PubMed] [Google Scholar]
- 29.Stöllberger C, Finsterer J. Wearable Cardioverter‐Defibrillator in a Patient with Left Ventricular Noncompaction/Hypertrabeculation, Coronary Artery Disease, and Polyneuropathy. Annals of Noninvasive. 2015;0:0–0. doi: 10.1111/anec.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knops R E, Kooiman K M, Ten Sande J N, de Groot J R, Wilde A A M. First experience with the wearable cardioverter defibrillator in the Netherlands. Neth Heart J. 2012 Feb;20 (2):77–81. doi: 10.1007/s12471-011-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Chingping, Oren Jess W, Szymkiewicz Steven J. Successful use of wearable cardioverter defibrillator in a patient with dextrocardia and persistent left superior vena cava. Ann Noninvasive Electrocardiol. 2013 Sep;18 (5):487–90. doi: 10.1111/anec.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao Mohan, Goldenberg Ilan, Moss Arthur J, Klein Helmut, Huang David T, Bianco Nicole R, Szymkiewicz Steven J, Zareba Wojciech, Brenyo Andrew, Buber Jonathan, Barsheshet Alon. Wearable defibrillator in congenital structural heart disease and inherited arrhythmias. Am. J. Cardiol. 2011 Dec 1;108 (11):1632–8. doi: 10.1016/j.amjcard.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Everitt Melanie D, Saarel Elizabeth V. Use of the wearable external cardiac defibrillator in children. Pacing Clin Electrophysiol. 2010 Jun 1;33 (6):742–6. doi: 10.1111/j.1540-8159.2010.02702.x. [DOI] [PubMed] [Google Scholar]
- 34.Opreanu Madalina, Wan Chingping, Singh Vini, Salehi Negar, Ahmad Jaffri, Szymkiewicz Steven J, Thakur Ranjan K. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: A national database analysis. J. Heart Lung Transplant. 2015 Oct;34 (10):1305–9. doi: 10.1016/j.healun.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Duncker D, König T, Hohmann S, Veltmann C. [Primary and secondary prophylactic ICD therapy in congenital electrical and structural cardiomyopathies]. Herzschrittmacherther Elektrophysiol. 2015 Jun;26 (2):82–93. doi: 10.1007/s00399-015-0372-8. [DOI] [PubMed] [Google Scholar]
- 36.Sliwa Karen, Hilfiker-Kleiner Denise, Petrie Mark C, Mebazaa Alexandre, Pieske Burkert, Buchmann Eckhart, Regitz-Zagrosek Vera, Schaufelberger Maria, Tavazzi Luigi, van Veldhuisen Dirk J, Watkins Hugh, Shah Ajay J, Seferovic Petar M, Elkayam Uri, Pankuweit Sabine, Papp Zoltan, Mouquet Frederic, McMurray John J V. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010 Aug;12 (8):767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 37.Blauwet Lori A, Libhaber Elena, Forster Olaf, Tibazarwa Kemi, Mebazaa Alex, Hilfiker-Kleiner Denise, Sliwa Karen. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart. 2013 Mar;99 (5):308–13. doi: 10.1136/heartjnl-2012-302760. [DOI] [PubMed] [Google Scholar]
- 38.Saltzberg Mitchell T, Szymkiewicz Steven, Bianco Nicole R. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J. Card. Fail. 2012 Jan;18 (1):21–7. doi: 10.1016/j.cardfail.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Duncker David, Haghikia Arash, König Thorben, Hohmann Stephan, Gutleben Klaus-Jürgen, Westenfeld Ralf, Oswald Hanno, Klein Helmut, Bauersachs Johann, Hilfiker-Kleiner Denise, Veltmann Christian. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur. J. Heart Fail. 2014 Dec;16 (12):1331–6. doi: 10.1002/ejhf.188. [DOI] [PubMed] [Google Scholar]
- 40.Tanawuttiwat Tanyanan, Garisto Juan D, Salow Arturo, Glad Joann M, Szymkiewicz Steve, Saltzman Heath E, Kutalek Steven P, Carrillo Roger G. Protection from outpatient sudden cardiac death following ICD removal using a wearable cardioverter defibrillator. Pacing Clin Electrophysiol. 2014 May;37 (5):562–8. doi: 10.1111/pace.12319. [DOI] [PubMed] [Google Scholar]
- 41.Healy Christopher A, Carrillo Roger G. Wearable cardioverter-defibrillator for prevention of sudden cardiac death after infected implantable cardioverter-defibrillator removal: A cost-effectiveness evaluation. Heart Rhythm. 2015 Jul;12 (7):1565–73. doi: 10.1016/j.hrthm.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 42.Everitt MD, Verma A, Saarel EV. The wearable external cardiac defibrillator for cancer patients at risk for sudden cardiac death. Community Oncology. 2011;0:400–403. [Google Scholar]
- 43.Bowers Robert W, Scott Paul A, Roberts Paul R. Use of external defibrillator jacket to facilitate safe delivery of radiotherapy for lung cancer - a report of two cases. Indian Heart J. 2014 Mar 4;66 (1):111–4. doi: 10.1016/j.ihj.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldenberg Ilan, Vyas Anant K, Hall W Jackson, Moss Arthur J, Wang Hongyue, He Hua, Zareba Wojciech, McNitt Scott, Andrews Mark L. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2008 Jan 22;51 (3):288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Wan Chingping, Herzog Charles A, Zareba Wojciech, Szymkiewicz Steven J. Sudden cardiac arrest in hemodialysis patients with wearable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2014 May;19 (3):247–57. doi: 10.1111/anec.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaPage Martin J, Canter Charles E, Rhee Edward K. A fatal device-device interaction between a wearable automated defibrillator and a unipolar ventricular pacemaker. Pacing Clin Electrophysiol. 2008 Jul;31 (7):912–5. doi: 10.1111/j.1540-8159.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 47.Chung Mina K, Szymkiewicz Steven J, Shao Mingyuan, Zishiri Edwin, Niebauer Mark J, Lindsay Bruce D, Tchou Patrick J. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J. Am. Coll. Cardiol. 2010 Jul 13;56 (3):194–203. doi: 10.1016/j.jacc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gang Uffe Jakob Ortved, Jøns Christian, Jørgensen Rikke Mørch, Abildstrøm Steen Zabell, Haarbo Jens, Messier Marc D, Huikuri Heikki V, Thomsen Poul Erik Bloch. Heart rhythm at the time of death documented by an implantable loop recorder. Europace. 2010 Feb;12 (2):254–60. doi: 10.1093/europace/eup383. [DOI] [PubMed] [Google Scholar]
- 49.Klein Helmut U, Goldenberg Ilan, Moss Arthur J. Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur. Heart J. 2013 Aug;34 (29):2230–42. doi: 10.1093/eurheartj/eht167. [DOI] [PubMed] [Google Scholar]
- 50.Klein Helmut U, Meltendorf Ulf, Reek Sven, Smid Jan, Kuss Sebastian, Cygankiewicz Iwona, Jons Christian, Szymkiewicz Steven, Buhtz Frank, Wollbrueck Anke, Zareba Wojciech, Moss Arthur J. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defibrillator (WCD). Pacing Clin Electrophysiol. 2010 Mar;33 (3):353–67. doi: 10.1111/j.1540-8159.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 51.Zishiri Edwin T, Williams Sarah, Cronin Edmond M, Blackstone Eugene H, Ellis Stephen G, Roselli Eric E, Smedira Nicholas G, Gillinov A Marc, Glad Jo Ann, Tchou Patrick J, Szymkiewicz Steven J, Chung Mina K. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013 Feb;6 (1):117–28. doi: 10.1161/CIRCEP.112.973552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki Shingo, Tomita Hirofumi, Shibutani Shuji, Izumiyama Kei, Higuma Takumi, Itoh Taihei, Sasaki Kenichi, Horiuchi Daisuke, Kimura Masaomi, Okumura Ken. Usefulness of the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circ. J. 2014;78 (12):2987–9. doi: 10.1253/circj.cj-14-1098. [DOI] [PubMed] [Google Scholar]


