Abstract
Introduction:
Cryoballoon ablation (CB) has proven effective for treating patients with paroxysmal atrial fibrillation (PAF). We analyzed our seven year follow-up of patients, treated for PAF with first (CB1) and second generation (CB2), with demonstration of LA-PV disconnection with bidirectional block (BB) after adenosine (AD).
Methods:
Since November 2008 to May 2015, 128 patients, 97 male (58±7 years), without heart disease, highly symptomatic, refractory to antiarrhythmic drugs (AAD) were treated, and follow-up (1411 ±727 days). Left atrial size: 37±6 mm.
Results:
A total of 439 PV were successfully isolated (91.9%). Acute reconduction: 44 PV (9%): 16 after CB; 16 unmasked by AD; 12 extrapulmonary muscular connections (EMC). Main complication was phrenic nerve palsy (PNP): 9 (7 %). On follow-up, 114 patients (89%) remain asymptomatic in sinus rhythm (SR), free of medication. Fourteen patients (11%) had arrhythmia recurrence: 12 male (52±8 years). Early recurrences occurred in 9 male. Late recurrences presented 3 male at 24, 27 and 60 months, and 2 female at 7 and 40 months respectively. All recurrence patients were Redo, and remain in SR without medication during follow-up.
Conclusions:
CB alone is very effective and safe for the definitive treatment of patients suffering PAF with 72.6% success rate, increasing up to 89.1% when this protocol is applied in a single procedure. After Redo, all population group (100%), remain in sinus rhythm, freedom of arrhythmia, without AAD, in this very long term follow-up. Checking for BB, AD protocol, and ruling out EMC allowed-us to identified 14.8% of patients with underlying substrate for potential arrhythmia recurrence. CB2 applications entail a highest risk of PNP. Patients with a rough estimated profile of low ALARMEc score (≤ 1) have an excellent long term outcome, being this series the largest follow-up described so far, for patients treated for PAF with CB.
Keywords: Cryoballoon, Paroxysmal Atrial Fibrillation, Ablation
Introduction
Complete electrical isolation of pulmonary veins (PVI) from the left atrium (LA) is crucial to cure patients (pts) with Atrial Fibrillation (AF).[1-4]
The Cryoballoon catheter ablation technique (CB) has proven effective to achieve this electrical disconnection of pulmonary veins (PV) from LA, resulting in a demonstrated effectiveness to treat pts suffering from PAF.[5-11]
However, some observations12 have shown, at least when first generation CB was used that cryoenergy CB application doesn’t produce a homogeneous circumferential lesion in all PV, which is related to their anatomical shape, thickness and size with a non-uniform distribution of the atrial muscle around them.
The more elliptic rather than circular variable form at the PV-LA junction level where cryoenergy is delivered can result in a nonuniform and persistent cellular lesion which, as is generally accepted, is the principal cause of PV reconnection after CB ablation.[13] A better quantification of the Cryoablation and the anatomical extent of PV have been better clarify recently.[14] Incomplete lesions with dormant tissue despite a “perfect” occlusion can occur leading to a residual conduction (RC) gaps causing, or responsible for PV reconduction which is the main underlying anatomical substrate for clinical arrhythmia recurrence.[15,16]
Adenosine has been used to “unmask” RC in apparently isolated PV with RF[17] and the routine use of AD after acute CB-PVI allows to identify incomplete lesions with dormant tissue not evident in basal conditions [18-20] and focal RF applications[21] or freeze “touch-up”[18-20] eliminate such RC.
The only no evidence of PV/ electrical activity on the circularmapping- catheter at the LA-PV junction level after CB-PVI is not enough to assure complete PV-LA electrical disconnection and checking for entry and exit block is mandatory to confirm it.[22-24]
We analyzed the seven year follow-up experience of our pts, initially treated with CB for PAF, with a prospective protocol with demonstration of complete BB electrical PV-LA-PV block postcryo and after AD as the main target end point to achieve in all cases.
Methods
Since November 2008 to November 2015, a total cohort of 128 pts (mean age 56±13 years), highly symptomatic, suffering from recurrent PAF, refractory to medical treatment ([Table 1]), were treated with the “CB” and followed-up.
Table 1. Demographic and clinical pts/features.
| 128 pts (mean age 53±13) | |
|---|---|
| Male/female | 97 (75.8%) / 31 (24.2%) |
| Mean age (male/female) | 58±7 / 61±10 years |
| Mean years/ suffering PAF | 5±5 years (1-5) |
| Mean number/ episodes PAF/ year | 54±67 (2-200) |
| Hypertension | 36 (28%) |
| Diabetes | 6 (4.7%) |
| Structural heart disease | NONE |
Prior to CB, all pts were previously treated with membrane active antiarrhythmic drugs: Class IC (88.2%); Class III (2.3%); Beta Blockers (BtB) (84.3%) and BtB +1C: 76.5%.
None with structural heart disease. Morphological and structural data can be showed on [Table 2]:
Table 2. Morphological and structural LA/PV/LV data.
LA: Left Atrium. PV: Pulmonary Vein. LCT: Left Common Trunk. RCT: Right Common Trunk. LVEF: Left Ventricular Ejection Fraction. AP: Antero-Posterior (parasternal long axis). TR: Transversal. SI: Supero-Inferior.
| DIAMETERS (mm) | LA | PV (483) | LCT (26) | RCT (3) | Mean LVEF 67±5% (59-79) |
|---|---|---|---|---|---|
| AP | 37±6 (21-50) | 18±5 (8-32) | 26±6 (18-35) | 28±1 (27-29) | |
| SI | 53±8 (40-75) | 20±4 (10-28) | 26±5 (17-31) | 28±5 (23-33) | |
| TR | 46±7 (35-61) | Mean LA/AREA (cm2) 22±4 (11-32) |
Exclusion Criteria:
- Prior Stroke, TIA or thromboembolism.
- Cryoglobulinemia and hematological or coagulation disorders.
- Presence of intracavitary thrombi as well as clinically- significant associated comorbidity
Previous Studies And Anatomical Approach:
2D-Transtoracic echocardiogram (TTE) as well as, same day, transesophageal echocardiogram was performed in all cases, to assess cardiac anatomy and to rule out intramural thrombi.
3D/ high resolution/64- slice Multidetector CT scan (Toshiba Aquilion 64, TSX-101A, Tokyo, Japan), and in some alternative cases, RMN (1.5T/ Magnetom Symphony, Siemens, Germany) were used for typification and better definition of cardiac anatomy, morphology, number, caliber and size of PV in addition to internal endoluminal navigation analysis to assess the thickness of the interpulmonary ridge and the morphological shape and size of PV ostium to choose the optimal CB size and the best orientation to address the balloon wedging at the LA-PV junction in an attempt to induce the biggest cryo lesion at the most proximal antral location including the interpulmonary ridge at the carina level in a sort of different morphological anatomical variants, as showed in [Figure 1].
Figure 1: Endoluminal and CT Scan reconstruction anatomical approach, to assess diameter/ shape and sizes of PV/LA-PV junction level and interpulmonary ridge, in relation to the size of CB to be used and the orientation for better PV-LA wedging.

Procedure:
All pts provided informed consent prior to the procedure. The procedure was approved by hospital’s clinical ethics committee. Prior to the procedure, all antiarrhythmic drugs (AAD) were discontinued at least 5 times their half-life; 48 hours for beta blockers and at least 10 days for Amiodarone.
All procedures were performed under general anesthesia with orotracheal intubation under propofol for anesthesia induction, cisatracurium for neuromuscular relaxation (only at the time of intubation), continuous perfusion of remifentanil for analgesia and mechanical ventilation maintained with Sevoflurane gas.
Transeptal Approach:
Seldinger technique was used for all vascular access. A decapolar 6 French electrocatheter through an antecubital vein was positioned into the coronary sinus (CS) for pacing and anatomical reference purposes. Cuatripolar/6French catheter was positioned at the A-V-nodal-his bundle junction through left femoral vein, for the same anatomical reference purpose, being moved later to superior vena cava (SVC) for pacing during CB applications at the right sided PV.
Through right femoral vein, an introducer and fast-cath 8.5 French sheet SLO, (Saint Jude medical, Minnesota, USA), was advanced over a 0.32 mm J typed shape guide wire to the SVC. Then, the guide wire is withdrawal, and a modified Brockenbrough needle (BRKO 71 cm beveled cut 30º/ Saint Jude Medical, MN, USA) is advance through the SLO sheet, and descending the whole transeptal assembly to embed fossa ovalis.
After gaining left atrial access, a bolus of 10.000 IU of sodium heparin was administered, followed by continuous perfusion as needed to maintain the activation clotting time ≥300 sec, as previously described.[25] At the end of the procedure, anticoagulation is reversed with protamine and 1grm. of lysine acetylsalicylate given i.v, along with low molecular weight heparine depending on patient’s body surface (1 mgr/Kg body weight) given subcutaneously, in addition to 100 mg of flecainide given intravenously in 10 minutes. Continuous intravenous perfusion of sodium heparine adjusted to patient’s body weight is started 4 hours later after removing all catheters from the vascular bed. Twenty-four hours later, oral anticoagulation with Vitamin K antagonist dicumarol is started targeting an international normalized ratio (INR) in the range of 2.0 to 3.0, plus additional platelet inhibition with 100 mg of ASA.
PV/Cartography/ Mapping:
Once in the LA chamber, the long 0.32mm guide-wire is advanced into the left superior PV (LSPV) and selective PV angiogram is performed, and in the same manner for the remaining veins, Left Inferior (LIPV), Right Superior (RSPV) and Right Inferior (RIPV).
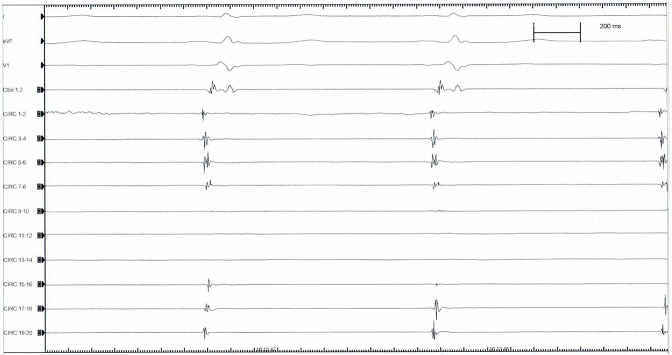
After removing the entire transeptal assembly, keeping the guidewire in the LSPV, a steerable 15F over-the wire sheath (Flex Cath, Cryocath, Medtronic,USA) is advanced and positioned in the LA. Then basal electrical cartography of the veins is obtained ([Figure 2A]) with a circular duodecapolar mapping catheter with adjustable diameter ( Reflexion spiral, Saint Jude Medical, MN, USA) positioned at the PV-LA junction antrum level, starting on LSPV and followed by LIPV, RSPV and RIPV respectively. We used a 20 pole circular mapping catheter to achieve sharper signals and better recognition between PV potentials and far-field atrial activity. This variable catheter adjustable in diameter is more useful when varying PV size or common ostium encountered, and also, allows for better contact and stability at the ostium of the PV when the circular catheter is fully expanded, leading to relative oversizing. Although, when fully expanded electrobipoles overlap and could cause contact signal artifact and repetition of recorded signals.
Figure 2A: Atrial far-field and synchronous PV electrical activity as recorded with the circular catheter mapping at the PV-LA junction antral level.
After recording the LA- PV junction electrical activity we pace CS to separate atrial far-field electrograms from PV electrical activity [Figure 2B], as in sinus rhythm it is difficult to distinguish because they are activated synchronously. After 30 minutes of CB applications all PVs were mapping again to assess electrical PV-LA isolation.
Figure 2B: Asynchronous atrial far-field (Af) and PV electrical potential (PVP) as recorded on circular catheter mapping by pacing CS.
Cryoballoon:
After withdrawing the circular catheter mapping, a 28 or 23 mm double walled CB catheter (Artic Front, Medtronic, USA) is advanced over the wire up to the LA, inflated and positioned in the PV ostium of each vein and gently pushed against the PV-LA antrum to get a perfect occlusion achieved when selective contrast medium injected (50% ratio with 0.9% saline solution) is full retained into the vein with no evidence of contrast leakage back to the atrium (grade IV) according to the degree of occlusion classification proposed and used by Neumann et al[8] to grade I with poor occlusion leading to an immediate rapid outflow contrast medium back to the LA. Until the second generation CB (CB2) was commercially available (April, 2013) patients were treated with the first generation CB (CB1).
Bidirectional LA-PV-LA Block Protocol
Exit Block: By pacing PV from all 20 poles of the circular catheter mapping at high amplitude voltage (20 mA) with consistent 1:1 PV capture and no evidence whatsoever of any atrial response.
Entry Block: By pacing LA from the CS-Catheter at three different cycle lengths (600, 500, 400 ms) with consistent 1:1 LA capture and no evidence whatsoever of any PV electrical activity in any of the 20 poles of circular-catheter mapping positioned at the LA-PV junction antral level.
AD Protocol:
Included bolus i.v administration of increasing doses (12-18-24… mgrs.), and pacing PV/LA when A-V nodal conduction block occurred.
Extrapulmonary Muscular Connections (Emc)/ Rule-Out Protocol:
Included pacing distal vein from the circular catheter mapping after complete BB demonstrated at the LA-PV junction antral level[(Figure 4.A)] and the demonstration of 1:1 PV-LA conduction resumed. [(Figure 4.B)]
Figure 4: A.Upper panel: Left side: pacing proximal antrum (circular 13-14) showing exit block (right side). B. Lower panel: Left side (same patient): pacing distal vein (circular 13-14), 1:1 PV/LA conduction resumed (right side).

RF Protocol:
Focal RF applications were used for eliminating RC gaps when evident after single CB application or after checking for BB Block, post -AD, or when EMC was demonstrated. ([Figure 5]). Sixty second “touch-up” of focal RF was used to eliminate all residual gaps only when evident in no more than 2 pairs of the circular catheter mapping. Otherwise, when more a repeated new CB application was performed.
Figure 5: Same patient as figure 4 A, B. Upper panel right side: pacing gap (red circle) distal vein with RF catheter (left side) with: 1:1 PV/LA conduction (third and fourth paced beat) demonstration at the right side recording. Lower panel (left side): pacing gap RF catheter, 1:1 PV/LA conduction is evident (three paced beat). After stop pacing, RC gap is evident (red circle), followed by RF application. After focal RF, exit block is demonstrated (right side).

Figure 3: Diagram flow showing the type of balloon used for different group of pts, occlusion degree, and temperature reached.

Phrenic Nerve Physiology Control:
Phrenic nerve physiology was monitored in all cases during right-sided PV/CB applications, by placing the cuatripolar electrocatheter in SVC and pacing at 2.000 ms cycle length, checking the intensity of diaphragm contractions by intermittent fluoroscopy and tactile feedback placing the operator’s hand on the patient´s abdomen, and immediately stop freezing when intensity of the diaphragm contraction weakens or is suddenly stopped.
Follow-Up Protocol:
Before discharge the hospital, TTE was performed in all cases to rule out pericardial effusion and chest-X-ray taked in a deep breath, upright position, to confirm normal phrenic nerve physiology.
The immediate follow-up included holter monitoring at 7, 15,30,45,60 and 90 days respectively, and thorax CT-Scan at 30 and 90 days. All pts received AAD, mostly Class IC+ BtB, and oral anticoagulation with vitamin K antagonist dicumarol is started 1 day after PVI, targeting an INR within 2.0 to 3.0 range for at least three months, along with additional platelet inhibition agent (ASA, 100 mgrs/daily).
After a three-month blanking period on medication, all AAD were discontinued, and follow-up started to count. All pts were monitored by continuous daily trans telephonic information in case of symptoms, and monthly ECG holter monitoring was routinely done over 1411±727 days (46.6±24.2 months) of follow-up.
Results
Acute PVI And LA-PV Reconduction
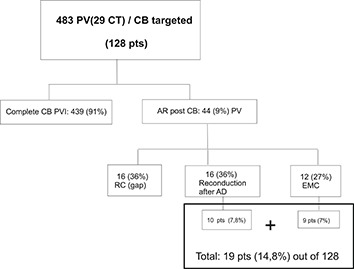
A total of 483 PV including 29 CT (26 Left/3Right), were treated with CB and complete PVI demonstrated in 439 (91.9%). Acute reconduction post CB showed 44 PV (9%) 16 PV out of 483 (3.3%) after single CB. In 16 PV out of 483 (3.3%), RC was unmasked after AD, in 10 patients. In 12 PV out of 483 (2.7%), EMC could be demonstrated in 9 pts. In six out of 16 of the acute reconnected PVs, RC appear after incomplete CB occlusion (degree III), [Figure 7] and in the same proportional rate after AD, [Figure 8 A,B], all eliminated by focal RF applications [Figure 8C]. Interestingly, all acute PV-LA RC (44 PV) occurred only with CB1.
Figure 7: A, Residual conduction gap (red circle) evident after incomplete CB occlusion (B, C) (degree III) with contrast leakage evident (arrow) as compare with PV/LA electrical activity recording in the same patient before the incomplete CB application (D).
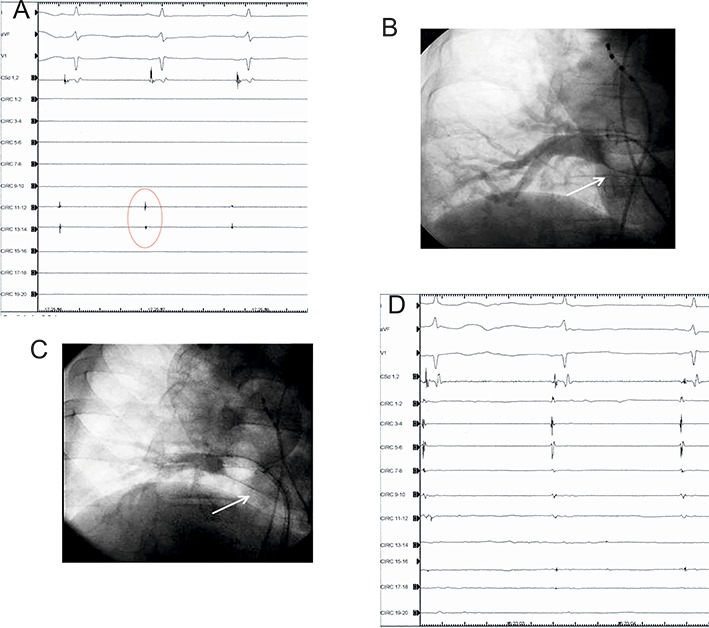
Figure 8A: Upper panel: showing PV electrical activity recorded at the 10 bipoles of the circular catheter mapping (left side) placed at the PV-LA junction antral level (right side).Lower panel: after CB application occlusion degree IV (left side), PV electrical activity is no more recording at circular catheter mapping (right side).

Figure 8C: Same patient as in [Figure 8A] and [8B]B. By placing RF catheter (lower right side figure) on PV dormant tissue location unmasked by AD, and pacing gap from RF catheter (upper side recording) 1:1 PV-LA conduction showed on first and second (left side) paced beats during AD effect, as evident with completed A-V conduction block, followed by RF application.

Follow-Up:
Follow-up of 1411±727 days started to count after three month blanking-period when AAD was discontinued.
Arrhythmia Recurrences:
On follow-up, 14 pts (10.9%) out of 128 experienced clinical recurrence of the arrhythmia: 12 male (52±8) and 2 female (63±13) years respectively. Early recurrence occurred immediately at the early stage of follow-up in 9 males (mean age 50±7 years), when medication was stopped after the three-month blanking-period. Late recurrences occurred in 3 males (mean age 55±9 years) at 24, 27 and 60 months, and 2 female (mean age 63±13 years) at 7 and 40 months respectively.
All 14 recurrence pts allow for a second procedure (Redo).
In a Redo follow-up of 41±16 months, all 14 pts remain in sinus rhythm without medication.
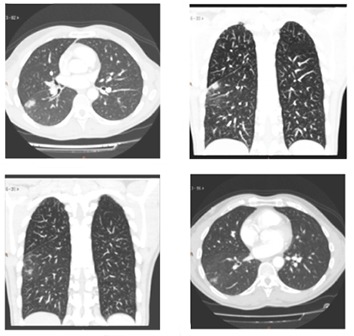
The remaining 114 pts (89.1%) followed-up 1411±727 days, are asymptomatic, free of drugs, in sinus rhythm. Seven pts (5.4%) had aphonia. Transient PNP: 7 (5.4%). Permanent PNP: 2 (1.5%), Pulmonary infiltrates ([Figure 9]) : 5 (3.9%).Mild dyspepsia: 2 (1.5%), Severe intraprocedural bronchospasm: 2 (1.5%), Discrete hemoptoic sputum: 2 (1.5%).
Figure 9: CT Scan slides showing pulmonary infiltrate (red circles).
No major side effects or complications occurred in our pts treated with CB, with no mortality, none atrioesophageal fistula, and none pulmonary vein stenosis.
Side Effects And Complications Follow-Up:
Aphonia: lasting ≤ 72 hours. Transient PNP: full complete recovery during the procedure. Permanent PNP: still evident on follow-up (1-3 years). Pulmonary infiltrates: In asymptomatic pts were shown at first month’s CTScan control performed, there having been no evidence 2 months later on another CT-Scan routinely performed. Mild dyspepsia: quick complete resolution ≤ 72 hours on omeprazole and protective gastric diet. Discrete hemoptoic sputum: lasting ≤ 72 hours. Severe intraprocedural Bronchospasm: requiring 48 hours of treatment in the ICU.
Discussion
Over the last few years, CB ablation technology has emerged into the arrhythmia arena as an useful and safe tool to treat pts suffering from atrial fibrillation by achieving through its applications an acute electrical disconnection of the PV from the LA in a range of (90-100 %) in the majority of the series already published,[26] which is the main key to cure this arrhythmia.
Figure 8B: Same patient as in Figure A. Upper panel: (left side): pacing (circular 1-2) at PV-LA junction antral level (right side), demonstrated exit block. Lower panel: dormant tissue unmasked by AD (red circle), at the time of complete A-V conduction block, and 1:1 PV-LA conduction demonstrated (second and third paced beats), by pacing gap.

Although in the majority of the clinical and randomized studies published, the results of the CB technique do not significantly differ in the short, medium and long-term outcome from those using RF as an energy source,[27-30] this “point to point” technique, can be more tedious, and time-consuming , most likely requiring better operator skill and involving an inherent clinically-significant risk of major complications, sometimes difficult to manage and treat, such as reentry left atrial tachyarrhythmia, thromboembolic events, pericardial effusion, PV stenosis or atrioesophageal fistula[31-34] which can be avoided or minimizing their incident by a “single shot” CB technique.
Since the first human experience published by Van Belle et al 5 treating pts suffering PAF with CB ablation, the technique has become widely-used as useful and safe tool to face the definitive treatment of this disturbing arrhythmia, by achieving ≥ 95% of acute electrical PVI in the majority of the series already published.[26]
Side Effects And Complications:
Aphonia: We cannot say for certain that this complication was strictly CB-related, first of all, because as far as we know, it has not been previously described, which is hardly surprising, especially after the findings described in the largest survey published so far,[35] focusing this complications topic in 500 consecutive pts. Although we can argue the possibility that this complication has been associated with endotracheal intubation maneuvers during general anesthesia, as orotracheal intubation, when difficult, can cause some laryngeal or vocal chord trauma, this however was not the case regarding our 7 patients affected in whom the orotracheal intubation, was smoothly performed and non-traumatic. Hence the most logical explanation for this symptom does not seem to have likely had anything to do with this orotracheal maneuvers. We raised the question, of a possible transient injury of the left recurrent laryngeal nerve, as has been warned by Cabrera and colleagues[36] in an unpublished abstract presentation at Hearth Rhythm 2011meeting, especially when CB applications take place deeper into the LSPV, along with the structural displacement towards the anatomical left recurrent laryngeal nerve bed, by strongly wedging the balloon in the venous ostium for a better occlusion.
Table 3. Side effects and complications.
| TYPE | Pts |
|---|---|
| Aphonia | 7 (5.4)% |
| Transient Phrenic nerve palsy | 7 (5.4)% |
| Phrenic nerve paralysis | 2 (1.5)% |
| Pulmonary infiltrates | 5 (3.9)% |
| Dyspepsia | 2 (1.5)% |
| Bronchospam | 2 (1.5)% |
| Hemoptoic sputum | 2 (1.5)% |
Moreover, all 7 pts who experienced aphonia were treated with the CB2 which had been designed with a new technological implementation resulting in a more homogeneous intake and distribution of the refrigerant flow around the balloon sphere, increasing the surface contact cooling that might induce deeper lesions with greater likelihood of affecting more extracardiac structures.[35,39]
PNP occurred in 9 pts (7 %). Only 2 are still permanent after 1 and 3 years follow-up respectively, with not clinical compromise, doing a normal life and completely asymptomatic. This incidental complication rate is consistent with the majority of the largest CB series already published: 4.7%,[10-37] 7%,[5] 7.2%,[35] 7.5%,[8] with some discrete higher incidence 11.1%[9] and 11.2%.[11]
In our series, the common characteristic of this complication was the lower nadir temperature level reached, of ≤ 60º C in the majority of cases (78%) [Table 4]. As the majority of pts were treated with the CB1 (74 out of 128), the PNP balloon-related rate was: 5.4% CB1 vs 9.2% CB2 (5 pts out of 54). This higher PNP rate occurring with CB2 as compared to CB1 has been showed by others.[38,39] In 340 consecutive pts treated by Aryana et al [38] with CB1 (140) and 200 with CB2, PNP occurred with CB1 in 12.1% pts vs 16.2% when CB2 was used. In a similar difference percentage rates Fürnkranz et al[39] reported 8.7 % of PNP with CB2 vs 5.7% when CB1 was employed.
Table 4. Ocurrence of PNP related to the CB used, time of application and nadir temperature reached.
TRANSIENT PHRENIC NERVE PALSY
| TºC | Seconds | CB mm | CB Generation | 78% Mean TºC≥ -60ºC | |
|---|---|---|---|---|---|
| 1 | -68 | 122 | 28 | FIRST | |
| 2 | -73 | 222 | 28 | FIRST | |
| 3 | -55 | 89 | 28 | SECOND | First Gen CB: 55.5% |
| 4 | -56 | 165 | 23 | SECOND | |
| 5 | -60 | 115 | 28 | SECOND | |
| 6 | -68 | 100 | 28 | SECOND | |
| 7 | -65 | 190 | 28 | SECOND | Second Gen CB: 45.5% |
| PERMANENT PHRENIC NERVE PARALYSIS | |||||
| 1 | -70 | 100 | 28 | FIRST | |
| 2 | -68 | 156 | 28 | FIRST |
Important to remark in our study that the 2 pts with persistent PNP after 1 and 3 years of follow-up, PNP occurred suddenly at 100 and 156 seconds of CB1 applications, when the lowest temperatures of -70 and -68ºC were respectively reached. In the other 7 pts with transient PNP, the CB applications were immediately stopped as soon as weakness of the diaphragm intensity contraction was adverted.
The highest level of PNP (19%) reached with the CB2 observed by Cherchia et al 40 related with the lower CB temperature reached, has move to this group to stop CB applications when -60 º C nadir temperature level is reached, in addition to limiting the freeze application time to 180 seconds, in an attempt to avoid major complications.[41,42,35] At the same time, these authors have proposed a modification technique to prevent PNP, consisting after tight wedging of the inflated CB inside the RSPV ostium, to withdraw it until a small leak of contrast is observed, since the CB volume increases slightly at the onset of CB application. This technical maneuvers described by Casado-Arroyo et al[43] from the same Brussel’s group, offers the advantage of a more proximal CB application and it has been suggested to use by others.[44] Martins et al suggest the use of Casado-Arroyo technique, particularly when the vertical projection of the PN reaches the distal part of the CB (Zone B1 in their study) with a 98% of negative predictive value, and Ströker et al 45 had recently emphasized the need to perform a preprocedural anatomic assessment, in order to evaluate the risk of PN injury, such PV orientation, larger PV dimensions, shorter distance to SVC, the presence of early branches originating from the main ostium, and right –sided long CT; anatomical variations which were associated with PN injury.
Pulmonary Infiltrates: Of unknown origin, mostly showed on the right side in distal pleural location, found in 5 asymptomatic pts (3.9%) on CT-Scan control routinely performed 1 month after the procedure, which were no longer evident at the 3-month control CT-Scan performed, at follow-up. Those pulmonary infiltrates, radiologicaly in appearance of inflammatory aspect, producing no clinical impact on pts, strongly suggest a probably origin related with the transmission of cold into the lung parenchyma during CB application, as it has been experimentally demonstrated in dogs[46] as small subtle foci of ablated –related superficial pleural fibrosis.[47]
Bronchospasm:
Severe intraprocedural bronchospasm occurred in two pts who had a past medical history of mild chronic bronchitis whom required medical treatment for ≤ 48 hours in the ICU. This complication might have a difficult explanation and could have been due to a combination of several factors working together, such as prior bronchial damage in pts with chronic bronchitis, the possibility of major injury due to ice formation inside the bronchial lumen[48] as well as the possible trigger effect of AD which, although anecdotic, has been described.[49]
Hemoptoic Sputum: Two pts presented discrete hemoptoic sputum on the immediate post- procedure, being otherwise on oral anticoagulation treatment regime and completely asymptomatic. This type of complication might also be due to several factors working together or may even have a different origin. Firstly, as it has been experimentally demonstrated,[50,51] the expansion of ice within the fragile microvasculature leads to the interruption of vascular integrity, which is the reason for the intramyocardial hemorrhage, as well as the hemoptysis associate with cryo injury to the lung tissues, and secondly, the possibility of bronchial erosion as has been demonstrated[52] as a cause of hemoptysis.
Dyspepsia: Two pts complain of mild dyspepsia. As no esophagogastroduodenal endoscopy study was performed, we cannot assure it was related or not, with some reversible esophageal ulceration.[53]
Epicardial PV-LA muscular connections: Electrically functioning EMC with PV-LA 1:1 conduction [Figure 4 A], [Figure 4 B] demonstrated, was found in our pts using this protocol in 12 PV (2.5% of total 483 PV), totaling 27.2% of the all post CB- PV reconducted (44 PV), in 9 pts (7%).
Since the first human demonstration of the presence of electrical conduction between PV was made,[54] other investigators had demonstrated the incidence of the interpulmonary vein electrical connections as being responsible of the maintenance of the arrhythmia in a single pt with PAF,[55,56] and Takahashi et al demonstrated in 49 consecutive pts, the presence of electrical connections between contiguous PV in 14% of the pts underwent atrial RF catheter ablation to treat their drug-resistant AF.[57]
Perez- Castellano et al[58] using RF catheter ablation for ostial PVI in 100 consecutive pts with drug-refractory atrial fibrillation, found in 3% of the veins, venoatrial epicardial connections inserted at distance from the venous ostium, and 10% with epicardial connections between the ipsilateral PV in 20% of pts resistant to atrial ablation, suggesting a different disconnection approach for PV showing those extrapulmonary epicardial connections associated with an increased rate of early recurrence of conduction.
The morphological evidence of these muscular connections between contiguous veins has been demonstrated by Cabrera et al,[59] confirming the anatomical underlying substrate of such electricallyfunctioning connections. More recently, Squara et al[60] have demonstrated the prevalence of those electrical connections between ipsilateral pulmonary veins and their implications for ablation and AD testing in 30 pts submitted to RF catheter ablation. They found a high presence of ipsilateral PV connections after antral PVI in up to 65.6% of total PV sets without carina ablation, lowered to 17.7% when the carina ablation was performed, emphasizing the need for carina ablation. Squara et al 60 also described acute reconnection of at least 1 of the PV to the LA, in 18% of the PV sets.
In our work 44 PV (9.1%) of the total PV faced (483) showed acute reconduction after single CB [Figure 6].As we have not conducted any previous study to assess the prevalence of these possible direct connections between ipsilateral pulmonary veins, and quantifying the incidence rate that the electrical impulse originating from 1 PV would propagate to the adjacent ipsilateral vein, as well as the indirect connections LA muscular sleeves, the only way to demonstrated some EMC in a practical clinical setting is the demonstration of complete BB at the PV-LA junction antral level by pacing from all 10 pairs of poles of the duodecapolar circular catheter mapping, following by demonstration that 1:1 PV-LA conduction resumed by pacing distal vein [Figure 4 A], [Figure 4 B] no further than 5-10mm from the endocardium of the interpulmonary isthmus, as Cabrera et al[59] have demonstrated as the limit of distance where the insertion of the muscular connections, can be founded.
Figure 6: Diagram flow showing total acute reconnected PV and number of patients. AR: acute reconnection.
By doing so, we found electrically -functioning EMC in 12 (2.5%) of the total PV-LA reconnected after CB-PVI, totaling 27% of the all early- reconducted veins, which is consistent with the figures published by others,[58] having carried out the same protocol, to ruleout EMC, by pacing distal vein after PVI-atrial isolation. Another interesting aspect to be taken in account is that, in the majority of CB-applications the interpulmonary ridge of the PV isthmus at the carina level is, affected, regardless of PV anatomy, (except for long CT) affected by cryo lesion at the endocardial superior and inferior aspects during CB applications at the superior and inferior PV.
Interestingly, those 12 EMC were demonstrated after acute BB was achieved in 9 pts, totaling a 7% of the total population of pts treated.
AD Protocol And Acute Early PV-LA Reconnection:
A total of 483 PV including 29 CT were treated with CB and complete CBPVI demonstrated in 439 (90.9%). Acute reconduction post CB was PV; 36% of the reconducted ones), show acute reconnection due to incomplete lesion with “dormant tissue” unmasked by AD,[17,18] by inducing hyperpolarization to restore excitability by activating ADsensitive potassium channels restoring conduction of dormant PV as it has been better clarified and demonstrated by Datino et al[61]
Arrhythmia Recurrence And Reconduction:
For analysis of the location of conduction gaps, the ipsilateral LA-PV junction was divided into four segments in a clockwise sense, starting at 10 o’clock (Superior, inferior, anterior, and posterior, for left PVs, and superior, inferior, septal, and lateral, for right PVs ) [Figure 10].
Figure 10: Diagram representation for the different number of RC found in the different segment location.

Forteen pts (10.9%) had clinical recurrence of the arrhythmia and allow for a Redo. Fifty-four PV including 2 CT were newly CB treated in a Redo with CB2. Reconduction was encountered in 29 PV (53.7%) in different segment locations [Figure 10_A] revealing the almost even distribution of RC in the superior segments, with greater number of reconductions shown at the inferior aspect of the LSPV. Also the PV showing the largest number of reconduction was the LSPV (37.9%) [Table 5].
Figure 10A: Segment distribution appearance of RC gaps.
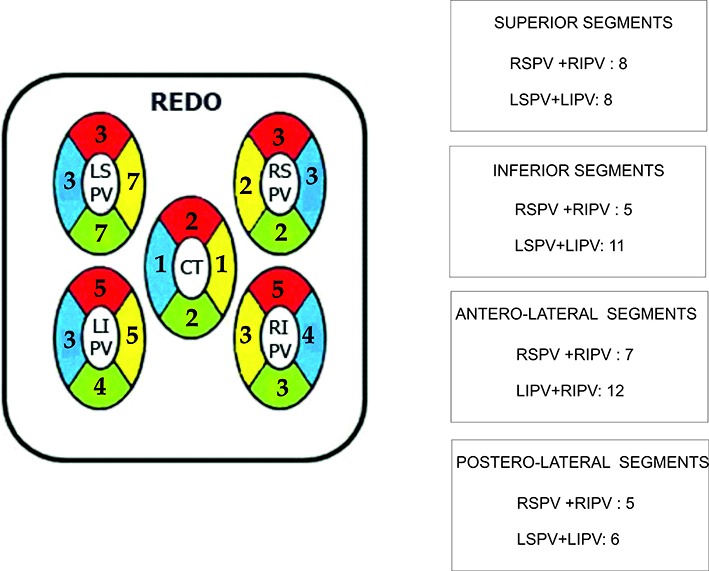
Table 5. Number of PV showing reconduction and their percentages of the total PV reconnected.
14 pts: 54 PV (2CT)
29 PV reconnected (53.7%)
| PV | nº | % |
|---|---|---|
| CT | 2 | 6,8% |
| LSPV | 11 | 37,9% |
| LIPV | 7 | 24,1% |
| RSPV | 4 | 13,7% |
| RIPV | 5 | 17,2% |
Finally, the RC segment distribution on Redo cases was random, being unrelated to those shown at first procedure [Figure 10_B].
Figure 10B: Reconduction was shown at first procedure in 6 pts with 23 PV including 1 TC. Left side upper figure showed number of gaps after CB, and unmasked by AD (lower left figure), as compared to number and distribution of gaps showing at Redo (right figure) on the same pts.

Fürnkranz et al 15 find the inferior segments of the LA-PV junction most often affected by reconduction in addition to the LSPV having shown the high rate of reconduction (63%), suggesting that the superior ridge may have contributed to this relatively high rate of reconduction in this LSPV.
In addition to the above, Fürnkranz et al hypothesized in their aforementioned study that the high incidence of inferior conduction gaps might be due to different causes, related to the difficulty sometimes involves in deflecting the sheath/balloon system in order to reach the inferior aspect of PV, resulting in incomplete balloontime contact. Conversely when approaching superior PV, both sheaths and balloon can be used to create a strong push onto the PV ostium to occlude the blood flow, achieving better occlusion and more permanent tissue cryolesion.
In the original study conducted by Chierchia et al,[19] enrolling 39 pts treated for PAF with CB1, AD testing after CB induced a LAPV reconnection only in 7 (4.6%) PV which often occurred in the inferior aspect of the lower veins, especially of the right inferior. All these RC gaps being eliminated by further CB applications or focal cryo “Touch-up”. Chierchia et al,[16] have also shown a 2.8% early spontaneous reconnection after 30 minutes of CB applications in a cohort of 26 pts treated for PAF with CB1.More recentlyin a study conducted by Ciconte et al 20 in 50 consecutive pts treated for PAF or early persistent AF ≤ 6 months, with CB2, spontaneous (4 veins) and AD-induced (4 veins) PV reconnections occurred in the 4% of initially isolated veins (8 veins) in 6 pts (12%).
Our results are consistent with the aforementioned studies, entailing 36% totally reconnected PV showing spontaneous reconduction, totaling 3.3% of the total 483PV-CB treated. Beside the highest reconduction gaps on the inferior aspect of the LSPV, conversely to the segment location reconduction showed by Fürnkranz et al[15] in 26 pts refered for RF PV ablation after CB first procedure failed, inferior segments showed gaps in 85% and 77% at the lateral and septal location respectively and 42% and 31% respectively at the lateral and septal aspect of the superior segments, our segment reconduction locations showed a most uniform distribution.
One possible underlying explanation for this, perhaps being the fact that in our pts all procedures were performed by the same operator (JMP) and were evaluated individually case by case, based not only the size of balloon to be used, but also on the orientation to be applied[15] according to PV anatomy, morphology, and angle direction, previously assessed with CT-PV slide reconstructions, in conjunction with the aforementioned endoluminal anatomical approach [Figure 1]. All of these factors combined might play an important role toward achieving better occlusions and more uniform lesions adding to minimize possibilities of PV-LA reconduction, which is the principal cause of clinical arrhythmia recurrences.
We have not done any protocol to rule out non PV-Foci, as a potential cause of arrhythmia recurrence,62 given that all recurrences were Redo, and PV-LA reconduction was evidenced in all cases.
Patient’s Risk Profile Of Recurrent Arrhythmia
We have not calculated the individual risk of recurrence of the arrhythmia based on the clinical patient data profile to assess the ALARMEc score, proposed by Neumann’s group,[63,64] but rather a rough estimate for our entire pt population treated, without atrial enlargement, suffering PAF, having normal renal function, with glomerular filtration rate ≥ 68 mL/min, none with structural heart disease, and only 33% of them with some metabolic disorder. We can approximate and estimate an ALARMEc score ≤ 1, according to the most favorable outcome of these patients, in whom the arrhythmia substrate mostly underlying on PV triggers.
Duration Of The Procedure
We experience a rapidly important decrease on the total duration time of the procedure in relation with the learning curve.[65] For the first 10 cases performed the mean time duration of procedure (since the transeptal approach to withrawal LA catheters) was 361 minutes and the mean fluoroscopy time: 86 minutes. For the last 20 pts treated, the mean duration time was 150 ±39 minutes, and fluoroscopy time 35±10 minutes.
Statistical Analysis
Continuous variables are expressed as mean ± SD. Categorical variables are expressed as percentages.
Study Limitations
This study has some limitations. First, this is a single center study. Second, because of the large interval of time (monthly) between holter monitoring recording on follow up, to detect arrhythmia events, along with patients whom eventually do not feeling symptoms, the success rate may have been overestimated, as none of the patient population included in the study were monitored with and implantable loop recorded.
Conclusions
The results and follow-up of our series of 128 pts treated for PAF over 7 years, allow us to conclude: 1. Cryoenergy PV applications doesn’t induce a homogeneous circumferential lesion in all PV which dependents on the PV anatomy, shape, size and thickness as well as the uniform distribution of cold and temperature reached at the PV-LA junction level which is the main cause of spontaneous reconnection or incomplete lesions with dormant tissue. 2. Routine use of AD after acute CB-PVI allowed-us to identify incomplete lesions with dormant tissue in 7.8% of pts. 3. Electrically functioning EMC might be identified in 7% of pts by pacing distal vein after complete antral PVI. 4. In summary, checking for BB, AD protocol and, pacing distal vein after PVI to rule out EMC allowed-us to identified 14.8% of pts with underlying tissue substrate for potential arrhythmia recurrence. 5. All residual gaps can be eliminated by further CB shots or focal RF applications. 6. All RC gaps occurred only with CB1 applications. 7. CB2 applications by inducing a wider and deeper lesion, minimize the RC gap appearance wich entail a lower arrhythmia recurrence rate, but involving a higher risk of damage extracardiac structures, such us PNP (9.2% CB2 vs 5.4% CB1). 8. Single CB technique is highly effective and safe for the definitive treatment of pts suffering from PAF with a 72.6% success rate, increasing up to 89.1% when this protocol is applied in a single procedure. After a second procedure performed in recurrences pts, the entire pt population group (100%), remain in sinus rhythm, free of arrhythmia, without AAD, in this very long term follow-up. 9. However, late recurrences, generates some concern about greater increased number of pts with recurrent arrhythmia over a longer course of time, especially for pts whom don’t feel symptoms of the arrhythmia. 10. Patients with an estimated low ALARMEc score (≤ 1) have an excellent long term outcome. 11. Finally, to the best of our knowledge, this series includes the largest follow-up described to date, in pts treated for PAF with CB technique.
Acknowledgements
We gratefully thank to Dr. Francisco Ivorra Miralles, president of HLA-ASISA group for his continuous support and economical resources in favor of clinical patient care and research.
Disclosures
None.
References
- 1.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997 Feb 4;95 (3):572–6. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Shah D C, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102 (20):2463–5. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 4.Oral Hakan, Knight Bradley P, Tada Hiroshi, Ozaydin Mehmet, Chugh Aman, Hassan Sohail, Scharf Christoph, Lai Steve W K, Greenstein Radmira, Pelosi Frank, Strickberger S Adam, Morady Fred. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002 Mar 5;105 (9):1077–81. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 5.Van Belle Yves, Janse Petter, Rivero-Ayerza Maximo J, Thornton Andrew S, Jessurun Emile R, Theuns Dominic, Jordaens Luc. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur. Heart J. 2007 Sep;28 (18):2231–7. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 6.Van Belle Yves, Janse Petter, Theuns Dominic, Szili-Torok Tamas, Jordaens Luc. One year follow-up after cryoballoon isolation of the pulmonary veins in patients with paroxysmal atrial fibrillation. Europace. 2008 Nov;10 (11):1271–6. doi: 10.1093/europace/eun218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein Gunnar, Oswald Hanno, Gardiwal Ajmal, Lüsebrink Ulrich, Lissel Christoph, Yu Hong, Drexler Helmut. Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008 Jun;5 (6):802–6. doi: 10.1016/j.hrthm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Neumann Thomas, Vogt Jürgen, Schumacher Burghard, Dorszewski Anja, Kuniss Malte, Neuser Hans, Kurzidim Klaus, Berkowitsch Alexander, Koller Marcus, Heintze Johannes, Scholz Ursula, Wetzel Ulrike, Schneider Michael A E, Horstkotte Dieter, Hamm Christian W, Pitschner Heinz-Friedrich. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J. Am. Coll. Cardiol. 2008 Jul 22;52 (4):273–8. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Chun Kyoung-Ryul Julian, Schmidt Boris, Metzner Andreas, Tilz Roland, Zerm Thomas, Köster Ilka, Fürnkranz Alexander, Koektuerk Buelent, Konstantinidou Melanie, Antz Matthias, Ouyang Feifan, Kuck Karl Heinz. The 'single big cryoballoon' technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 2009 Mar;30 (6):699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero-de Loma-Osorio Angel, Izquierdo-de Francisco Maite, Martínez-Brotons Angel, Sánchez-Gómez Juan M, Mascarell-Gregori Beatriz, Ruiz-Ros Vicente, Cuenca-Romero Isabel, García-Civera Roberto, Chorro-Gascó Francisco J, Ruiz-Granell Ricardo. Medium-term results of cryoballoon ablation of the pulmonary veins in patients with paroxysmal and persistent atrial fibrillation. First experience of a Spanish center. J Interv Card Electrophysiol. 2013 Aug;37 (2):189–96. doi: 10.1007/s10840-013-9797-3. [DOI] [PubMed] [Google Scholar]
- 11.Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 12.Paylos JM, Ferrero C, Ramírez R, Molezun R, Quiñones MA. Gadolinium Delayed-Enhancement MRI to assess the extension of residual cryo-balloon catheter induced lesion at the left-atrium-PV junction level in patients treated for paroxysmal atrial fibrillation. Heart Rhythm. 2010;0:0–0. [Google Scholar]
- 13.Liu Christopher F. Pulmonary vein reconnection after cryoballoon ablation: back to the drawing board. Heart Rhythm. 2010;7 (2):191–2. doi: 10.1016/j.hrthm.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Kenigsberg David N, Martin Natalia, Lim Hae W, Kowalski Marcin, Ellenbogen Kenneth A. Quantification of the cryoablation zone demarcated by pre- and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28-mm second-generation cryoballoon. Heart Rhythm. 2015 Feb;12 (2):283–90. doi: 10.1016/j.hrthm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Fürnkranz Alexander, Chun K R Julian, Nuyens Dieter, Metzner Andreas, Köster Ilka, Schmidt Boris, Ouyang Feifan, Kuck Karl-Heinz. Characterization of conduction recovery after pulmonary vein isolation using the "single big cryoballoon" technique. Heart Rhythm. 2010;7 (2):184–90. doi: 10.1016/j.hrthm.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Chierchia Gian Battista, de Asmundis Carlo, Müller-Burri Stephan-Andreas, Sarkozy Andrea, Capulzini Lucio, Paparella Gaetano, Chierchia Sergio, Roos Markus, Brugada Pedro. Early recovery of pulmonary vein conduction after cryoballoon ablation for paroxysmal atrial fibrillation: a prospective study. Europace. 2009 Apr;11 (4):445–9. doi: 10.1093/europace/eun352. [DOI] [PubMed] [Google Scholar]
- 17.Arentz Thomas, Macle Laurent, Kalusche Dietrich, Hocini Mélèze, Jais Pierre, Shah Dipen, Haissaguerre Michel. "Dormant" pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1041–7. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 18.Tritto Massimo, De Ponti Roberto, Salerno-Uriarte Jorge A, Spadacini Giammario, Marazzi Raffaella, Moretti Paolo, Lanzotti Marcelo. Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. Eur. Heart J. 2004 Dec;25 (23):2155–63. doi: 10.1016/j.ehj.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Chierchia Gian Battista, Yazaki Yoshinao, Sorgente Antonio, Capulzini Lucio, de Asmundis Carlo, Sarkozy Andrea, Duytschaever Matthias, De Ponti Roberto, Brugada Pedro. Transient atriovenous reconnection induced by adenosine after successful pulmonary vein isolation with the cryothermal energy balloon. Europace. 2009 Dec;11 (12):1606–11. doi: 10.1093/europace/eup339. [DOI] [PubMed] [Google Scholar]
- 20.Ciconte Giuseppe, Chierchia Gian-Battista, DE Asmundis Carlo, Sieira Juan, Conte Giulio, Juliá Justo, DI Giovanni Giacomo, Wauters Kristel, Baltogiannis Giannis, Saitoh Yukio, Mugnai Giacomo, Catanzariti Domenico, Tondo Claudio, Brugada Pedro. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):845–51. doi: 10.1111/jce.12421. [DOI] [PubMed] [Google Scholar]
- 21.Paylos JM, Ferrero C, Delgado M, Ramírez R, Quiñones MA. Complementary value of adenosine and radiofrequency in the cryo-balloon isolation of pulmonary veins for paroxysmal atrial fibrillation. Heart Rhythm. 2011;0:0–0. [Google Scholar]
- 22.Haïssaguerre M, Jaïs P, Shah D C, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng J T, Roudaut R, Clémenty J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000 Mar 28;101 (12):1409–17. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 23.Natale Andrea, Raviele Antonio, Arentz Thomas, Calkins Hugh, Chen Shih-Ann, Haïssaguerre Michel, Hindricks Gerhard, Ho Yen, Kuck Karl Heinz, Marchlinski Francis, Napolitano Carlo, Packer Douglas, Pappone Carlo, Prystowsky Eric N, Schilling Richard, Shah Dipen, Themistoclakis Sakis, Verma Atul. Venice Chart international consensus document on atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2007 May;18 (5):560–80. doi: 10.1111/j.1540-8167.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 24.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 25.Paylos Jesús M, Hoyt Robert H, Ferrero Clara, Berrio Carmen, Rey Arancha, Delgado Isabel, Veiga Jesús J, Moreno José L. Complete pulmonary vein isolation using balloon cryoablation in patients with paroxysmal atrial fibrillation. Rev Esp Cardiol. 2009 Nov;62 (11):1326–31. doi: 10.1016/s1885-5857(09)73361-4. [DOI] [PubMed] [Google Scholar]
- 26.Andrade Jason G, Khairy Paul, Guerra Peter G, Deyell Marc W, Rivard Lena, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Dubuc Marc. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011 Sep;8 (9):1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Kühne Michael, Suter Yves, Altmann David, Ammann Peter, Schaer Beat, Osswald Stefan, Sticherling Christian. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010 Dec;7 (12):1770–6. doi: 10.1016/j.hrthm.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Siddoway D, Friehling M, Voigt A, Saba S, Jain S. Improved resource utilization with similar efficacy during early adoption of cryoballoon pulmonary vein isolation as compared to radiofrequency ablation for paroxysmal atrial fibrillation. JAFIB. JAFIB. 2015;0:15–19. doi: 10.4022/jafib.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luik Armin, Radzewitz Andrea, Kieser Meinhard, Walter Marlene, Bramlage Peter, Hörmann Patrick, Schmidt Kerstin, Horn Nicolas, Brinkmeier-Theofanopoulou Maria, Kunzmann Kevin, Riexinger Tobias, Schymik Gerhard, Merkel Matthias, Schmitt Claus. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study. Circulation. 2015 Oct 6;132 (14):1311–9. doi: 10.1161/CIRCULATIONAHA.115.016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt Martin, Dorwarth Uwe, Andresen Dietrich, Brachmann Johannes, Kuck Karlheinz, Kuniss Malte, Willems Stephan, Deneke Thomas, Tebbenjohanns Jürgen, Gerds-Li Jin-Hong, Spitzer Stefan, Senges Jochen, Hochadel Matthias, Hoffmann Ellen. German ablation registry: Cryoballoon vs. radiofrequency ablation in paroxysmal atrial fibrillation--One-year outcome data. Heart Rhythm. 2016 Apr;13 (4):836–44. doi: 10.1016/j.hrthm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Saad Eduardo B, Rossillo Antonio, Saad Cynthia P, Martin David O, Bhargava Mandeep, Erciyes Demet, Bash Dianna, Williams-Andrews Michelle, Beheiry Salwa, Marrouche Nassir F, Adams James, Pisanò Ennio, Fanelli Raffaele, Potenza Domenico, Raviele Antonio, Bonso Aldo, Themistoclakis Sakis, Brachmann Joannes, Saliba Walid I, Schweikert Robert A, Natale Andrea. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation. 2003 Dec 23;108 (25):3102–7. doi: 10.1161/01.CIR.0000104569.96907.7F. [DOI] [PubMed] [Google Scholar]
- 32.Dagres Nikolaos, Hindricks Gerhard, Kottkamp Hans, Sommer Philipp, Gaspar Thomas, Bode Kerstin, Arya Arash, Husser Daniela, Rallidis Loukianos S, Kremastinos Dimitrios Th, Piorkowski Christopher. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J. Cardiovasc. Electrophysiol. 2009 Sep;20 (9):1014–9. doi: 10.1111/j.1540-8167.2009.01493.x. [DOI] [PubMed] [Google Scholar]
- 33.Pappone Carlo, Oral Hakan, Santinelli Vincenzo, Vicedomini Gabriele, Lang Christopher C, Manguso Francesco, Torracca Lucia, Benussi Stefano, Alfieri Ottavio, Hong Robert, Lau William, Hirata Kirk, Shikuma Neil, Hall Burr, Morady Fred. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004 Jun 8;109 (22):2724–6. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 34.Jaïs Pierre, Sanders Prashanthan, Hsu Li-Fern, Hocini Mélèze, Sacher Frederic, Takahashi Yoshihide, Rotter Martin, Rostock Thomas, Bordachar Pierre, Reuter Sylvain, Laborderie Julien, Clémenty Jacques, Haïssaguerre Michel. Flutter localized to the anterior left atrium after catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Mar;17 (3):279–85. doi: 10.1111/j.1540-8167.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 35.Mugnai Giacomo, de Asmundis Carlo, Ciconte Giuseppe, Irfan Ghazala, Saitoh Yukio, Velagic Vedran, Ströker Erwin, Wauters Kristel, Hünük Burak, Brugada Pedro, Chierchia Gian-Battista. Incidence and characteristics of complications in the setting of second-generation cryoballoon ablation: A large single-center study of 500 consecutive patients. Heart Rhythm. 2015 Jul;12 (7):1476–82. doi: 10.1016/j.hrthm.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera JA, Murillo M, Climent V, Pizarro G, González-Caballero E, Fuertes B, Bayona S, Sánchez-Quintana D. Relationship between the left recurrent laynged nerve and left atrium: a study in patients with and without atril fibrilation. Heart Rhythm. 2011;0:0–0. [Google Scholar]
- 37.Fürnkranz Alexander, Bordignon Stefano, Dugo Daniela, Perotta Laura, Gunawardene Melanie, Schulte-Hahn Britta, Nowak Bernd, Schmidt Boris, Chun Julian K R. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):840–4. doi: 10.1111/jce.12417. [DOI] [PubMed] [Google Scholar]
- 38.Aryana Arash, Morkoch Shemsa, Bailey Sean, Lim Hae W, Sara Rahmani, d'Avila André, O'Neill P Gearoid. Acute procedural and cryoballoon characteristics from cryoablation of atrial fibrillation using the first- and second-generation cryoballoon: a retrospective comparative study with follow-up outcomes. J Interv Card Electrophysiol. 2014 Nov;41 (2):177–86. doi: 10.1007/s10840-014-9942-7. [DOI] [PubMed] [Google Scholar]
- 39.Fürnkranz Alexander, Bordignon Stefano, Schmidt Boris, Perrotta Laura, Dugo Daniela, De Lazzari Manuel, Schulte-Hahn Britta, Nowak Bernd, Chun Julian K R. Incidence and characteristics of phrenic nerve palsy following pulmonary vein isolation with the second-generation as compared with the first-generation cryoballoon in 360 consecutive patients. Europace. 2015 Apr;17 (4):574–8. doi: 10.1093/europace/euu320. [DOI] [PubMed] [Google Scholar]
- 40.Chierchia Gian-Battista, Di Giovanni Giacomo, Ciconte Giuseppe, de Asmundis Carlo, Conte Giulio, Sieira-Moret Juan, Rodriguez-Mañero Moises, Casado Ruben, Baltogiannis Giannis, Namdar Mehdi, Saitoh Yukio, Paparella Gaetano, Mugnai Giacomo, Brugada Pedro. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: 1-year follow-up. Europace. 2014 May;16 (5):639–44. doi: 10.1093/europace/eut417. [DOI] [PubMed] [Google Scholar]
- 41.Chierchia Gian-Battista, Di Giovanni Giacomo, Sieira-Moret Juan, de Asmundis Carlo, Conte Giulio, Rodriguez-Mañero Moises, Casado-Arroyo Ruben, Baltogiannis Giannis, Paparella Gaetano, Ciconte Giuseppe, Sarkozy Andrea, Brugada Pedro. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Interv Card Electrophysiol. 2014 Mar;39 (2):145–51. doi: 10.1007/s10840-013-9855-x. [DOI] [PubMed] [Google Scholar]
- 42.Ciconte Giuseppe, de Asmundis Carlo, Sieira Juan, Conte Giulio, Di Giovanni Giacomo, Mugnai Giacomo, Saitoh Yukio, Baltogiannis Giannis, Irfan Ghazala, Coutiño-Moreno Hugo Enrique, Hunuk Burak, Velagić Vedran, Brugada Pedro, Chierchia Gian-Battista. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015 Apr;12 (4):673–80. doi: 10.1016/j.hrthm.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 43.Casado-Arroyo Ruben, Chierchia Gian-Battista, Conte Giulio, Levinstein Moisés, Sieira Juan, Rodriguez-Mañero Moises, di Giovanni Giacomo, Baltogiannis Yannis, Wauters Kristel, de Asmundis Carlo, Sarkozy Andrea, Brugada Pedro. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm. 2013 Sep;10 (9):1318–24. doi: 10.1016/j.hrthm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Martins Raphaël P, Hamon David, Césari Olivier, Behaghel Albin, Behar Nathalie, Sellal Jean-Marc, Daubert Jean-Claude, Mabo Philippe, Pavin Dominique. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2014 Mar;11 (3):386–93. doi: 10.1016/j.hrthm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Ströker Erwin, de Asmundis Carlo, Saitoh Yukio, Velagić Vedran, Mugnai Giacomo, Irfan Ghazala, Hünük Burak, Tanaka Kaoru, Belsack Dries, Buyl Ronald, Brugada Pedro, Chierchia Gian-Battista. Anatomic predictors of phrenic nerve injury in the setting of pulmonary vein isolation using the 28-mm second-generation cryoballoon. Heart Rhythm. 2016 Feb;13 (2):342–51. doi: 10.1016/j.hrthm.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Sarabanda Alvaro V, Bunch T Jared, Johnson Susan B, Mahapatra Srijoy, Milton Mark A, Leite Luiz R, Bruce G Keith, Packer Douglas L. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J. Am. Coll. Cardiol. 2005 Nov 15;46 (10):1902–12. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 47.Andrade Jason G, Dubuc Marc, Guerra Peter G, Landry Evelyn, Coulombe Nicolas, Leduc Hugues, Rivard Léna, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Pulmonary vein isolation using a second-generation cryoballoon catheter: a randomized comparison of ablation duration and method of deflation. J. Cardiovasc. Electrophysiol. 2013 Jun;24 (6):692–8. doi: 10.1111/jce.12114. [DOI] [PubMed] [Google Scholar]
- 48.Verma Nishant, Gillespie Colin T, Lin Albert C, Knight Bradley P. Ice formation in the left mainstem bronchus during cryoballoon ablation for the treatment of atrial fibrillation. Heart Rhythm. 2016 Mar;13 (3):814–5. doi: 10.1016/j.hrthm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Drake I, Routledge P A, Richards R. Bronchospasm induced by intravenous adenosine. Hum Exp Toxicol. 1994 Apr;13 (4):263–5. doi: 10.1177/096032719401300407. [DOI] [PubMed] [Google Scholar]
- 50.Avitall Boaz, Urboniene Dalia, Rozmus Grzegorz, Lafontaine Dan, Helms Ray, Urbonas Arvydas. New cryotechnology for electrical isolation of the pulmonary veins. J. Cardiovasc. Electrophysiol. 2003 Mar;14 (3):281–6. doi: 10.1046/j.1540-8167.2003.02357.x. [DOI] [PubMed] [Google Scholar]
- 51.Avitall Boaz, Lafontaine Daniel, Rozmus Grzegorz, Adoni Naveed, Le Khoi M, Dehnee Abdelkader, Urbonas Arvydas. The safety and efficacy of multiple consecutive cryo lesions in canine pulmonary veins-left atrial junction. Heart Rhythm. 2004 Jul;1 (2):203–9. doi: 10.1016/j.hrthm.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 52.van Opstal J M, Timmermans C, Blaauw Y, Pison L. Bronchial erosion and hemoptysis after pulmonary vein isolation by cryoballoon ablation. Heart Rhythm. 2011 Sep;8 (9) doi: 10.1016/j.hrthm.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed Humera, Neuzil Petr, d'Avila Andre, Cha Yong-Mei, Laragy Margaret, Mares Karel, Brugge William R, Forcione David G, Ruskin Jeremy N, Packer Douglas L, Reddy Vivek Y. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009 Jul;6 (7):962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 54.Tritto M, De Ponti R, Zardini M, Spadacini G, Oliveira M, Salerno-Uriarte J A. Electrical connections between pulmonary veins in humans: evidence after radiofrequency ablation of the venoatrial junction. Circulation. 2001 Aug 14;104 (7):E30–1. doi: 10.1161/hc3201.094105. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi Yoshihide, Iesaka Yoshito, Takahashi Atsushi, Hiraoka Masayasu. Electrical connection between left superior and inferior pulmonary veins in a patient with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2002 May;13 (5):490–2. doi: 10.1046/j.1540-8167.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 56.Matsuo Seiichiro, Jaïs Pierre, Wright Matthew, Lim Kang-Teng, Knecht Sébastien, Haïssaguerre Michel. Maintenance of atrial fibrillation by pulmonary vein tachycardia with ostial conduction block: evidence of an interpulmonary vein electrical connection. J. Cardiovasc. Electrophysiol. 2008 Oct;19 (10):1101–4. doi: 10.1111/j.1540-8167.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi Atsushi, Iesaka Yoshito, Takahashi Yoshihide, Takahashi Ryoko, Kobayashi Kenzaburo, Takagi Katsumasa, Kuboyama Osamu, Nishimori Takeo, Takei Hidenobu, Amemiya Hiroshi, Fujiwara Hideomi, Hiraoka Masayasu. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002 Jun 25;105 (25):2998–3003. doi: 10.1161/01.cir.0000019585.91146.ab. [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Castellano Nicasio, Villacastín Julián, Salinas Jorge, Vega Mercedes, Moreno Javier, Doblado Manuel, Ruiz Eduardo, Macaya Carlos. Epicardial connections between the pulmonary veins and left atrium: relevance for atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2011 Feb;22 (2):149–59. doi: 10.1111/j.1540-8167.2010.01873.x. [DOI] [PubMed] [Google Scholar]
- 59.Cabrera José Angel, Ho Siew Yen, Climent Vicente, Fuertes Beatriz, Murillo Margarita, Sánchez-Quintana Damián. Morphological evidence of muscular connections between contiguous pulmonary venous orifices: relevance of the interpulmonary isthmus for catheter ablation in atrial fibrillation. Heart Rhythm. 2009 Aug;6 (8):1192–8. doi: 10.1016/j.hrthm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Squara Fabien, Liuba Ioan, Chik William, Santangeli Pasquale, Maeda Shingo, Zado Erica S, Callans David, Marchlinski Francis E. Electrical connection between ipsilateral pulmonary veins: prevalence and implications for ablation and adenosine testing. Heart Rhythm. 2015 Feb;12 (2):275–82. doi: 10.1016/j.hrthm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Datino Tomás, Macle Laurent, Qi Xiao-Yan, Maguy Ange, Comtois Philippe, Chartier Denis, Guerra Peter G, Arenal Angel, Fernández-Avilés Francisco, Nattel Stanley. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010 Mar 2;121 (8):963–72. doi: 10.1161/CIRCULATIONAHA.109.893107. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi Kentaro, An Yoshimori, Nagashima Michio, Hiroshima Kenichi, Ohe Masatsugu, Makihara Yu, Yamashita Kennosuke, Yamazato Schoichiro, Fukunaga Masato, Sonoda Koichiro, Ando Kenji, Goya Masahiko. Importance of nonpulmonary vein foci in catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2015 Sep;12 (9):1918–24. doi: 10.1016/j.hrthm.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Berkowitsch Alexander, Kuniss Malte, Greiss Harald, Wójcik Maciej, Zaltsberg Sergey, Lehinant Stefan, Erkapic Damir, Pajitnev Dimitri, Pitschner Heinz-Friedrich, Hamm Christian W, Neumann Thomas. Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long term follow-up. Pacing Clin Electrophysiol. 2012 May;35 (5):532–43. doi: 10.1111/j.1540-8159.2012.03350.x. [DOI] [PubMed] [Google Scholar]
- 64.Wójcik Maciej, Berkowitsch Alexander, Zaltsberg Sergey, Hamm Christian W, Pitschner Heinz F, Kuniss Malte, Neumann Thomas. Cryoballoon ablation of atrial fibrillation: How important is the proper selection of patients? Cardiol J. 2015;22 (2):194–200. doi: 10.5603/CJ.a2014.0100. [DOI] [PubMed] [Google Scholar]
- 65.Wójcik Maciej, Berkowitsch Alexander, Greis Harald, Zaltsberg Sergey, Hamm Christian W, Pitschner Heinz F, Kuniss Malte, Neumann Thomas. Learning curve in cryoballoon ablation of atrial fibrillation: eight-year experience. Circ. J. 2014;78 (7):1612–8. doi: 10.1253/circj.cj-13-1253. [DOI] [PubMed] [Google Scholar]