Abstract
The availability of intravenous (IV) Sotalol has equalized the treatment options since both amiodarone and sotalol are available in both IV and oral formulations. A review of the efficacy of sotalol as compared to amiodarone both for conversion of atrial fibrillation (AF) and maintenance of normal sinus rhythm (NSR) following cardiac surgery was undertaken. Standard methods of meta-analysis were employed. Full text publications of clinical trials written in English that compared the efficacy of sotalol to amiodarone were included in the analysis. For the conversion of AF to NSR, five studies were found eligible for the analysis. Two studies clinically compared sotalol to amiodarone for the maintenance of NSR after cardiac surgery. The common relative success of sotalol was 0.947 (95Cl: 0.837 to 1.071, P = 0.385), revealing essentially no differences in efficacy for conversion between amiodarone and sotalol. The average conversion rate was 47% with sotalol and 52% with amiodarone. The conversion rates were lower for persistent AF (sotalol 22% and amiodarone 27%), while greatest for recent onset AF (88% sotalol and 77% for amiodarone). The risk of developing post-operative atrial fibrillation was practically the same in both regimes, relative risk = 1.214 (95% CI: 0.815-1.808, p=0.339). In summary, sotalol and amiodarone are equally effective in AF conversion and maintenance of NSR post-cardiac surgery.
Keywords: Sotalol, Atrial Fibrillation, Treatment, Meta-Analysis
Introduction
Atrial fibrillation (AF) is the most common arrhythmia with an estimated prevalence of between 2.7 million and 6.1 million patients in the United States.[1] Atrial fibrillation results in significant morbidity including thromboembolic events, stroke, heart failure and increased risk of mortality.[2] Currently there are two management strategies for AF, a “rate control” strategy which aims to control the rate of ventricular response, and a “rhythm control” strategy which aims to restore and maintain normal sinus rhythm.[2] The restoration of sinus rhythm either with electrical cardioversion or antiarrhythmic drugs and successful maintenance of sinus rhythm has been recently reported to yield improvements in symptoms and quality of life.[2,4] However, neither strategy offers a mortality benefit.[2,3] The role of sotalol is well established for maintenance of sinus rhythm after successful restoration of normal sinus rhythm (NSR).[2] With the introduction of intravenous (IV) Sotalol, we thought it useful to compare the efficacy of sotalol to amiodarone, both for maintenance of sinus rhythm and the conversion of AF. Sotalol’s role in pharmacologic conversion of AF is controversial. Prior meta-analyses have reviewed the role of sotalol in maintenance of sinus rhythm and prevention of AF following cardiac surgery,[5,6] but its efficacy in AF conversion as compared to amiodarone has not been reviewed.
Methods
A systematic review of the published literature was undertaken and meta-analyses were performed to assess the efficacy and safety of sotalol in the pharmacologic conversion of AF and the maintenance of sinus rhythm following cardiac surgery.
Publications of clinical trials on pharmacologic conversion of AF that evaluated the efficacy of sotalol in comparison to amiodarone were collected for inclusion in this report. Studies could employ either IV or oral route of administration for sotalol or amiodarone. Publications were limited to full text papers written in English. Selected publications must have had sufficient information on patient selection, study methods, and primary outcome(s) to be included. Studies that used electrophysiologic drug testing during induced AF were not included.
The following databases were searched from the earliest date possible to June 30 2015: PubMed, SCOPUS, CINAHL, Cochran Database of Systematic Reviews. The strategy and the results of the search of PubMed are shown in [Figure 1]. The keywords “sotalol” and “amiodarone” resulted in 700 publications. Using the combination of “sotalol”, “amiodarone” and “atrial fibrillation” reduced the number of the publications to 319. When the search was limited to publications written in English with human subjects, the number of publications was reduced to 245. Further limiting the search to reviews and clinical trials resulted in 160 publications, of these, 54 were original reports and 106 reviews. For the review on AF conversion, of the 54 original reports, 49 did not meet the inclusion criteria, either being not relevant (most often the topic was maintenance of sinus rhythm without data on pharmacologic AF conversion), or the studies were case series without a comparator. As a result, 5 publications met inclusion criteria and were analyzed for AF conversion in this study. The search of SCOPUS, CINAHL, and Cochran Database of Systematic Reviews did not result in additional eligible publications. A summary tabulation of the 5 published studies is enumerated in ([Table 1]).
Figure 1. Flow Chart of Study Selection This flow chart shows the search strategy for studies on the comparative efficacy of amiodarone and sotalol in AF conversion employed with PubMed . The systematic review resulted in 5 eligible studies .
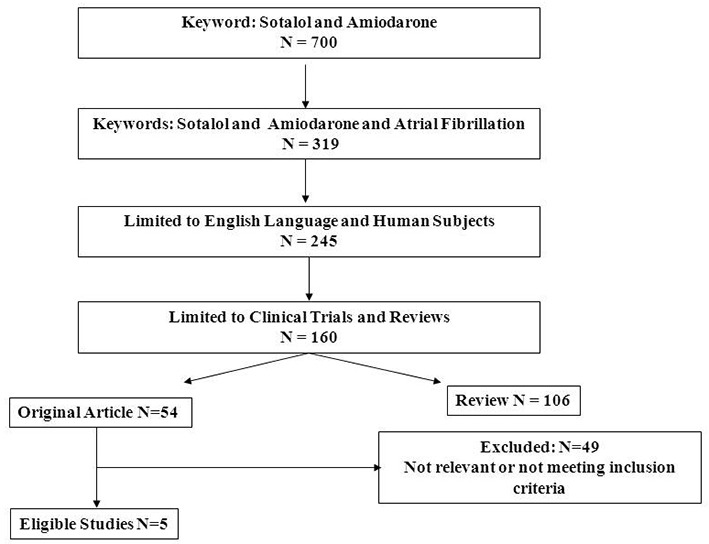
Table 1. Summary Tabulation of the Clinical Trials .
AF; Atrial Fibrillation, AFL; Atrial Flutter, h; hour, IV; intravenous, MI; myocardial infarction. Note: Wong et al.[15] did not provide dosing regimens
| Authors | Study Type | Drug Regimen | AF Duration Before Treatment | Follow up for Efficacy | Patient Conditions/ Characteristics |
|---|---|---|---|---|---|
| Joseph et al.[7] | Randomized | 1. IV sotalol 1.5 mg/kg over 30 min | <24 h | 48 h. | Emergency |
| 2. IV amiodarone 5 mg/kg over 30 min | symptomatic | ||||
| then 3x400 mg oral/day for 2 days | AF | ||||
| Singh et al.[8] | Randomized | 1. Oral sotalol 2x80 mg a day | >72 h. | 28 days | Persistent AF |
| Double blind | for 1 week, 2x160 mg thereafter | to years | eligible for | ||
| 2. Oral amiodarone 800 mg a day | cardioversion | ||||
| for 2 weeks then 600 mg/day for 2 weeks, | |||||
| Thomas et al.[9] | Randomized | 1. IV sotalol 1.5 mg/kg over 10 min | <48 h. | 12 h. | Emergency |
| Open Label | then 2x80 mg oral a day | in 80% | admission for | ||
| 2. IV amiodarone 10 mg/kg over 30 min | of the patients | symptomatic | |||
| Then 2x200 mg oral a day | recent onset | ||||
| AF |
For maintenance of sinus rhythm following cardiac surgery, using the key word “sotalol” resulted in 2,683 publications, searching for “sotalol” and “Atrial Fibrillation” reduced the articles to 495, those in English and on human subjects were 370, 242 were original reports and only 2 publications directly compared sotalol to amiodarone.[6]
The studies were grouped according to the treatment employed, i.e. sotalol versus amiodarone, as well as the goal of therapy: AF conversion, or maintenance of sinus rhythm.
For each group, a meta-analysis was performed to obtain the common relative success of the primary endpoint (AF conversion). Additionally, data were extracted for adverse events. The statistical analysis was performed by using the Comprehensive Meta-Analysis software (BiostatTM, Englewood, NJ, USA). Heterogeneity of the studies was assessed for each outcome in each group by using Q statistics and I2 statistics. Those studies that were homogeneous for an outcome were analyzed by the fixed effect model, while those studies that were heterogeneous for an outcome were analyzed by the random effect model to determine the common relative success (relative risk of successful AF conversion) and the relative risk of developing AF post cardiac surgery. The relative success is the ratio of the proportion of patients who had successful AF conversion in the sotalol versus the amiodarone group. The common relative success is the weighted estimate of the success ratios across the studies. The relative risk was computed by a similar approach resulting in the weighted estimate of the relative risks of developing AF. A two-sided alpha error of less than 0.05 was considered to be statistically significant (p<0.05). Existence of publication bias was evaluated by using a funnel plot and Egger’s regression intercept.
Results
Sotalol vs. Amiodarone for AF Conversion
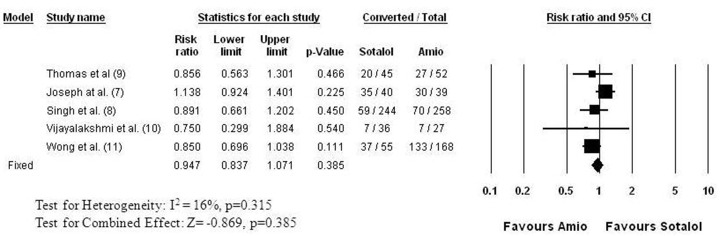
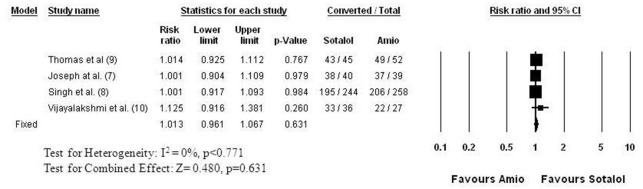
Five studies[7,8,9,10,11] evaluated the efficacy of amiodarone in comparison to sotalol for AF conversion ([Figure 2]). This comparison has the largest patient population, with 420 patients receiving sotalol and 544 receiving amiodarone. While the studies differed in the duration of AF before an attempt at cardioversion, the follow up time to evaluate success, as well as in the dosing regimens, the studies were homogenous for the primary outcome, AF conversion. The common relative success of sotalol was 0.947 (95% CI: 0.837 to 1.071, p=0.387), indicating no difference compared to amiodarone in pharmacologic conversion of AF. The conversion rate ranged between 19 and 88% in the sotalol and between 26 and 79% in the amiodarone groups, with an average conversion rate of 49% with sotalol and 52% with amiodarone. The conversion rates were the lowest in persistent AF studies[8,10] ranging between 19 and 24% for sotalol and 26 and 27% for amiodarone, while in recent onset AF of less than 24 hour duration (paroxysmal AF) the success rate was 88% for sotalol and 77% for amiodarone. Four of these five studies[7,8,9,10] evaluated the combined success rate of pharmacologic and electrical AF conversion ([Figure 3]). Those patients, who did not convert by their assigned drug treatment received direct current (DC) cardioversion. None of the studies found significant difference in the success of pharmacologic and DC conversion between the amiodarone and sotalol groups ([Figure 3]). The meta-analysis indicates a practically identical efficacy with a common relative success of 1.013 (95% CI: 0.961 to 1.067, p=0.631). The success rate ranged between 80 and 96% in the sotalol groups and between 80 and 95% in the amiodarone groups.
Figure 2. Relative Success of AF Conversion to Normal Sinus Rhythm: Sotalol versus Amiodarone Each row shows the name of the first author of the publication followed by the reference number in parenthesis, the relative success (Risk ratio: “risk” of successful AF conversion) with 95% confidence interval (95% CI), and the significance (p) of the difference in success. The number of patients of whom AF converted and the total number of the patients (Event/Total) in the sotalol and the amiodarone arms are also shown for each study. The graphic presentations of the results are shown on the right (Forrest Plot). The boxes represent the relative success and the lines represent the 95% CI for individual studies. The size of boxes and the thickness of lines reflect the weight of a study in the analysis. The result of the meta-analysis is shown in the last row (bottom) numerically, as well as graphically (the diamond in the Forest Plot). Test for heterogeneity: I2 is the percentage of total variation in study estimates that is due to heterogeneity (16%.). The studies effect sizes were homogenous, thus the fixed effect model was employed for meta-analysis. The combined relative success is 0.947 (95% CI: 0.837 to 1.071, p=0.385), indicating no difference between the two drugs in pharmacologic conversion of AF .
Figure 3. Relative Success of AF Conversion to Normal Sinus Rhythm: Sotalol versus Amiodarone Followed by DC Shock in Non Converters Those patients, who did not convert by the drug they were randomized to receive (amiodarone or sotalol) underwent direct current (DC) electrical cardioversion. The figure shows the combined success of pharmacological and DC cardioversion. The studies and data elements are organized as in Figure 2. The studies were homogenous (I2 = 0%), thus the fixed effect model was employed for meta-analysis. None of the studies found a significant difference between the amiodarone and sotalol groups. The meta-analysis indicates practically identical efficacy with a common relative success of 1.013 (95% CI: 0.961 to 1.067, p=0.631).
Three studies reported data about the suppression of AF after successful conversion of AF.[8,9,10] One study with 12 hour follow up reported early recurrence,[9] one study reported AF recurrence during 6 weeks and 6 months follow up,[10] and one study reported long term AF suppression during 12 months follow up.[8] The results are shown in [Figure 4]. [Figure 4] shows that with longer follow up time the risk of AF recurrence increases more in patients who received sotalol compared to those who received amiodarone. The relative risk of AF recurrence on sotalol became significant at 6 months (p=0.027) and became more significant during 1 year follow up (p<0.001). The meta-analysis ([Figure 4]) indicates a significantly higher common relative risk of AF recurrence for sotalol (relative risk: 1,462, 95% CI: 1.260 to 1.697, p<0.001). Overall, these results translate to less effective long term AF suppression with oral sotalol therapy than with amiodarone.
Figure 4. Recurrence of AF among Patients with AF Conversion: Sotalol versus Amiodarone The studies and data elements are organized as in Figure 2. The studies were homogeneous (I2 = 2%), thus the fixed effect model was employed for meta-analysis. The combined (weighted mean) relative risk of AF recurrence is 1,462 (95% CI: 1.260 to 1.697, p<0.001) indicating a significantly higher common relative risk of AF recurrence for sotalol. The figure also indicates that with longer follow up time the risk of AF recurrence increases more in patients who received sotalol comped to those who received amiodarone. The relative risk of AF recurrence on sotalol became significant at 6 months follow up (p=0.027) and became more significant during 1 year follow up (p<0.001).
Two studies reported adverse events affecting the cardiovascular system.[7,9] Combining these 2 studies, there were 2 cases of symptomatic bradycardia and 2 cases of hypotension among patients who received sotalol. There were more cases of adverse events among patients who received amiodarone including 2 cases of bradycardia, 5 cases of hypotension, and 4 cases of left ventricular failure. Discontinuation of therapy was similar for amiodarone and sotalol with a relative risk of 1.194 (95% CI: 0.311 to 4.587, p=0.796).
Sotalol vs. Amiodarone for Maintenance of NSR Post Cardiac Surgery
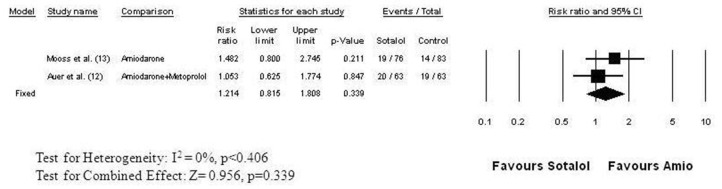
Two clinical trials directly compared the efficacy of sotalol with amiodarone for the prevention of atrial fibrillation after cardiac surgery.[12,13] Both were randomized double-blind trials ([Figure 5]). In the study of Mooss and colleagues,[13] atrial fibrillation occurred in 17% of the 83 patients taking amiodarone and in 25% of the 76 patients taking sotalol (RR=1.482, 95% CI: 0.80 to 2.745, p = 0.211), a nonsignificant difference. In the study of Auer and coworkers,[12] patients were randomized to receive placebo, sotalol, metoprolol, or amiodarone plus metoprolol. Atrial fibrillation occurred in 20 of the 63 patients (32%) randomized to sotalol and in 19 of the 63 patients (30%) randomized to amiodarone plus metoprolol. The risk of developing postoperative atrial fibrillation was practically the same with both regimens (RR = 1.05, 95% CI: 0.625 to 1.774, p = 0.847).[6] The combined relative risk of developing postoperative atrial fibrillation on sotalol therapy was 1.214 (95% CI: 0.815 to 1.808, p = 0.339) ([Figure 5]). Discontinuation of therapy due to adverse events (hypotension, bradycardia, AV block) were similar for amiodarone (8.9%) and sotalol (13.7%) in the post cardiac surgery setting with a relative risk of 1.554 (95% CI: 0.801 to 3.016, p=0.192).
Figure 5. Relative Risk of Developing Atrial Fibrillation after Cardiac Surgery: Sotalol versus Amiodarone The studies and data elements are organized as in Figure 2. The studies were homogeneous (I2 = 0%), thus the fixed effect model was employed for meta-analysis. The combined (weighted mean) relative risk is 1.214 (95% CI: 0.815 to 1.808) indicating no significant difference between sotalol and amiodarone in preventing atrial fibrillation following cardiac surgery (p<0.339) .
Adverse Events and Drug Toxicity Following Acute and Chronic Administration of Amiodarone and Sotalol
The number of studies is limited in this meta-analysis and most of the studies had short duration. Therefore they do not provide a full picture about the incidence of adverse events that may be anticipated with these drugs. Therefore, we performed a thorough review of the literature to provide estimates of adverse events and toxicities with acute and long term administration of sotalol and amiodarone that should be considered when administering these drugs to patients for conversion of AF and to maintain NSR. The results are summarized in Table 2. Most of the adverse events are related to the pharmacologic effects of these drugs. Both sotalol and amiodarone are Vaughan-Williams Class III (action potential prolonging) agents with Class II (beta receptor blocking) properties. With acute, predominantly IV administration, hypotension, bradycardia, AV block and new onset CHF can be anticipated with both drugs. Both drugs prolongs the QTc interval, and Torsades de Pointes (TdP) ventricular tachycardia may occur with both, but to a much lesser extent for IV sotalol (<1%) than observed with chronic oral sotalol administration for the suppression of AF (0.3-3.2%) or ventricular tachycardia (2-4%).[15,16] Adult Respiratory Distress Syndrome (ARDS) is a potentially lethal complication which is due to amiodarone toxicity. It is especially frequent with IV administration following pulmonary surgery (11%),[17] but the lower end of the estimate is still considerable with a 2% incidence of ARDS.[14] Other serious amiodarone toxicities like optic neuropathy, optic neuritis and thyrotoxicosis are realitve rare with acute IV administration, hepatic injury may occur in 2.8-4.2% of the patients.[14] With chronic oral administration for maintenance of NSR after AF conversion, Tdp is the most serious adverse event that occurs between 0.3 to 3.2% of the patients with sotalol, and extremely rare with amiodarone.[16,18] Non allergic bronchospasm occurs only with sotalol administration.[16] Less serious adverse events show a similar incidence between sotalol and amiodarone therapy including gastrointestinal complains (sotalol ≈20%, amiodarone≈ 30%), dizziness, headache, insomnia malaise may occur as high as ≈ 30%, as well as fatigue ≈10% of the patients by both drugs. On the other hand, pulmonary toxicity, hypo- and hyperthyroidism, hepatitis and cirrhosis, may occur frequently with amiodarone therapy and not at all with sotalol. Aplastic anemia is relatively rare with amiodarone but can be fatal and does not occur with sotalol.[14,19] Other frequent, but potentially non fatal amiodarone toxicities manifested as photophobia, corneal microdeposits, photosensitivity and skin discoloration (See [Table 2]). Optic neuropathy or optic neuritis (estimated incidence <1% to 2%) caused by amiodarone may lead to blindness.[20] In summary, both drugs may cause serious adverse events, but amiodarone therapy may result in a number of potentially fatal non cardiovascular organ toxicities, while sotalol therapy has not been associated with potentially fatal organ toxicity.
Table 2. Adverse Events and Drug Toxicity Following Acute and Chronic Administration of Amiodarone and Sotalol.
* indicates major adverse event/toxicity. Superscript numbers indicate the reference numbers of the data source. 0% indicates that no report of that adverse event has been found. “infrequent” and “extremely rare” indicate that events were occasionally reported as part of either the post marketing experience or foreign experience and the actual incidence have not been or cannot be estimated. ARDS; Adult Respiratory Distress Syndrome, CHF; congestive heart failure, IV; intravenous. TdP; Torsades de Pointes ventricular tachycardia
| Administration | Amiodarone | Sotalol | |
|---|---|---|---|
| Acute (Predominantly IV) | |||
| *Hypotension | 12-20%[14] | 6.3%[15] | |
| *TdP | <2%[14] | <1% (0.1%)[15] | |
| Bradycardia/AV block | 4.9%[14] | 12-13%[16] | |
| *Cardiac Arrest | 3%[14] | 0.1%[15] | |
| Heart Failure | 2%[14] | 1.2%[16] | |
| *ARDS | 214-11%[17] | 0% | |
| *Optic neuropathy/neuritis | infrequent[14] | 0% | |
| Peripheral neuropathy | infrequent[14] | extremely rare[16] | |
| *Thyrotoxicosis | infrequent[14] | 0% | |
| Hepatic injury | 2.8-4.2%[14] | extremely rare[16] | |
| Chronic (Oral) | |||
| *Proarrhythmia (TdP) | <1%[18] | 0.3-3.2%[16] | |
| CHF (new onset) | 2-2.2%[14] | 1.2-3.3%[16] | 0% |
| *Pulmonary toxicity | 1-17%[19] | 0% | |
| Non-Allergic Bronchospasm | 0% | 1.8-2.4%[16] | |
| *Optic neuropathy/neuritis | <1-2%[20] | 0% | |
| Photophobia, corneal microdeposits | >90%[14,20] | 0% | |
| Gastrointestinal Complaints | 30%[18] | 20.5-20.7%[16] | |
| Elevated Liver Enzyme Levels | 15-30%[14] | extremely rare[16] | |
| *Hepatitis and Cirrhosis | <3%[14] | 0% | |
| *Hypothyroidism | 4-22%[14] | 0% | |
| *Hyperthyroidism | 2-12%[14] | 0% | |
| Neurologic Events (i.e. Dizziness, Headache, Insomnia, Malaise, etc.) | 3-30%[18] | 22-29%[16] | |
| Fatigue | 4-9%[21] | 10-11%[16] | |
| Tremor, ataxia | 3-35%[20] | 0% | |
| Peripheral neuropathy | 0.3%[20] | extremely rare[16] | |
| Photosensitivity | 25-75%[14,20] | extremely rare[16] | |
| Skin discoloration | 4-9%[20] | 0% | |
| *Aplastic anemia | rare[1r] | 0% |
Discussion
With the availability of IV, as well as oral sotalol and amiodarone, both agents can be employed in a number of different situations with flexibility in the route of delivery. We thus undertook a systematic literature review and meta-analysis to evaluate the efficacy of sotalol for the pharmacologic conversion of AF, and reviewed meta-analysis of the maintenance of sinus rhythm after cardiac surgery.[6] The efficacy of sotalol IV and oral was similar to amiodarone in AF conversion and sotalol and amiodarone were equally effective in the maintenance of normal sinus rhythm after cardiac surgery.
While the focus of this study was AF conversion and prevention of AF following cardiac surgery, we also hade limited data on the efficacy of both drugs in the long term maintenance of NSR with oral administration following cardioversion of AF. We found a better long term suppression of AF with oral amiodarone compared to oral sotalol treatment. Our findings are in agreement with a recent meta-analysis, which evaluated the long term efficacy of both drugs in the maintenance of NSR following cardioversion.[22]
An important consideration is that amiodarone has numerous none cardiovascular adverse events, some of them can be fatal such as amiodarone-induced pulmonary toxicity. Our review of the adverse events and toxicity of the two drugs indicates that sotalol has much less serious non cardiovascular adverse effects. This is confirmed in a study on the reassessment of clinical outcomes by initial antiarrhythmic drug therapy in the AFFIRM Trial, which concluded that death, intensive care unit hospitalization and non-cardiovascular death were more frequent with amiodarone.[23]
The estimates of the incidence of adverse events and toxicities can have a wide range (see [Table 2]). This can be explained by the underlining disease status of a patient group, as well as the wide range of doses employed of the two agents. For example, the incidence of TdP was less when it used for maintenance of NSR after AF conversion (0.3-3.2%) than in the treatment of ventricular tachycardia (2-4%).[15,16] Furthermore, sotalol has a linear pahramacokinetic profile and the QT prolongation caused by sotalol is dose related. Consequently, with high sotalol doses the risk of developing TdP is higher than with lower daily doses of sotalol. Similarly, pulmonary toxicity of amiodarone has been reported more frequently when high maintenance doses are employed and declined when the daily dose was reduced to 400 mg or below. Still, pulmonary toxicity may happen at any dose at any time with amiodarone therapy. Given the long elimination half life of amiodarone, toxicities may occur after discontinuation of amiodarone for up to one year.
Side effects contribute an important dimension in the decision to employ sotalol, or amiodarone. The cardiac side effects of Sotalol are bradycardia, hypotension and QT prolongation with a 1-3% incidence of TdP tachycardia, more frequently seen in low EF patients. Amiodarone also causes bradycardia, as well as hypotension, but proarrhythmia and TdP is much more infrequent. The non-cardiac side effects are significantly different between sotalol and amiodarone. Amiodarone is well known to cause a long list of side effects from photosensitivity and skin discoloration to blood dyscrasias (neutropenia and agranulocytosis), to hypo or hyperthyroidism , hepatic toxicity and pulmonary toxicity (ARDS like picture or pulmonary fibrosis). Neuropathies, including optic and peripheral are also reported. Often the non-cardiac side effects of amiodarone are such as to influence the choice of which agent to employ.
Clinical Implications
Given the similar efficacy of sotalol and amiodarone and the short and long term toxicity of amiodarone, consideration should be given to employing IV and oral sotalol in the treatment of AF in patients with adequate left ventricular function.
Perhaps the greatest utility of IV sotalol is in the treatment of AF is in preventing AF post coronary artery bypass surgery and valve surgery where AF remains a problem.[6] Currently, IV amiodarone is often employed, though even brief periods of amiodarone use can lead to optic and peripheral neuropathies,[24,25] as well as rarely an acute respiratory distress syndrome like picture.[26,27] The efficacy of oral sotalol has been demonstrated in patients post CABGS28 and it is possible that with IV loading a pharmacoeconomic advantage may be found with IV sotalol over oral sotalol.
Limitations
One of the limitations of this systematic review is the relatively small number of studies in our meta-analyses. However, the studies were homogenous for all outcomes and publication bias was not found, which support the creditability of our findings. Another limitation is that most of the studies in our meta-analysis had short follow up time. Given the limited number of studies and the short follow up times, we had limited data on the adverse event profile of amiodarone and sotalol in AF conversion and during long term administration.
Disclosures
Dr. Somberg lead the team developing IV Sotalol and has since sold his proprietary interest. He also has investments in generic drug companies manufacturing amiodarone.
References
- 1.Mozaffarian Dariush, Benjamin Emelia J, Go Alan S, Arnett Donna K, Blaha Michael J, Cushman Mary, de Ferranti Sarah, Després Jean-Pierre, Fullerton Heather J, Howard Virginia J, Huffman Mark D, Judd Suzanne E, Kissela Brett M, Lackland Daniel T, Lichtman Judith H, Lisabeth Lynda D, Liu Simin, Mackey Rachel H, Matchar David B, McGuire Darren K, Mohler Emile R, Moy Claudia S, Muntner Paul, Mussolino Michael E, Nasir Khurram, Neumar Robert W, Nichol Graham, Palaniappan Latha, Pandey Dilip K, Reeves Mathew J, Rodriguez Carlos J, Sorlie Paul D, Stein Joel, Towfighi Amytis, Turan Tanya N, Virani Salim S, Willey Joshua Z, Woo Daniel, Yeh Robert W, Turner Melanie B. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015 Jan 27;131 (4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 2;130 (23):e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Allen Lapointe N, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, Lopes RD, Povsic TJ, Raju SS, Shah BR, Kosinski A, McBroom AJ, Chobot MM, Gray R, Sanders GD. Treatment of Atrial Fibrillation. Comparative Effectiveness Review 119. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I.) AHRQ Publication No.13-EHC095- EF. Rockville, MD: Agency for Healthcare Research and Quality; Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/358/1559/ atrial-fibrillation-report-130628.pdf (Accessed: Aug 2015) 2013;0:0–0. [Google Scholar]
- 4.Hagens Vincent E, Ranchor Adelita V, Van Sonderen Eric, Bosker Hans A, Kamp Otto, Tijssen Jan G P, Kingma J Herre, Crijns Harry J G M, Van Gelder Isabelle C. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J. Am. Coll. Cardiol. 2004 Jan 21;43 (2):241–7. doi: 10.1016/j.jacc.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan Sean D, Orme Michelle E, Morais Edith, Mitchell Stephen A. Interventions for the treatment of atrial fibrillation: a systematic literature review and meta-analysis. Int. J. Cardiol. 2013 May 10;165 (2):229–36. doi: 10.1016/j.ijcard.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 6.Kerin Nicholas Z, Jacob Sony. The efficacy of sotalol in preventing postoperative atrial fibrillation: a meta-analysis. Am. J. Med. 2011 Sep;124 (9):875.e1–9. doi: 10.1016/j.amjmed.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Joseph A P, Ward M R. A prospective, randomized controlled trial comparing the efficacy and safety of sotalol, amiodarone, and digoxin for the reversion of new-onset atrial fibrillation. Ann Emerg Med. 2000 Jul;36 (1):1–9. doi: 10.1067/mem.2000.107655. [DOI] [PubMed] [Google Scholar]
- 8.Singh Bramah N, Singh Steven N, Reda Domenic J, Tang X Charlene, Lopez Becky, Harris Crystal L, Fletcher Ross D, Sharma Satish C, Atwood J Edwin, Jacobson Alan K, Lewis H Daniel, Raisch Dennis W, Ezekowitz Michael D. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 2005 May 5;352 (18):1861–72. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 9.Thomas Stuart P, Guy Duncan, Wallace Elisabeth, Crampton Roselyn, Kijvanit Pat, Eipper Vicki, Ross David L, Cooper Mark J. Rapid loading of sotalol or amiodarone for management of recent onset symptomatic atrial fibrillation: a randomized, digoxin-controlled trial. Am. Heart J. 2004 Jan;147 (1) doi: 10.1016/s0002-8703(03)00526-x. [DOI] [PubMed] [Google Scholar]
- 10.Vijayalakshmi Kunadian, Whittaker Victoria J, Sutton Andrew, Campbell Philip, Wright Robert A, Hall James A, Harcombe Alun A, Linker Nicholas J, Stewart Michael J, Davies Adrian, de Belder Mark A. A randomized trial of prophylactic antiarrhythmic agents (amiodarone and sotalol) in patients with atrial fibrillation for whom direct current cardioversion is planned. Am. Heart J. 2006 Apr;151 (4):863.e1–6. doi: 10.1016/j.ahj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Wong C-K, White H D, Wilcox R G, Criger D A, Califf R M, Topol E J, Ohman E M. Management and outcome of patients with atrial fibrillation during acute myocardial infarction: the GUSTO-III experience. Global use of strategies to open occluded coronary arteries. Heart. 2002 Oct;88 (4):357–62. doi: 10.1136/heart.88.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auer Johann, Weber Thomas, Berent Robert, Puschmann Rudolf, Hartl Peter, Ng Choi-Keung, Schwarz Christian, Lehner Ernst, Strasser Ulrike, Lassnig Elisabeth, Lamm Gudrun, Eber Bernd. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: the pilot study of prevention of postoperative atrial fibrillation (SPPAF), a randomized, placebo-controlled trial. Am. Heart J. 2004 Apr;147 (4):636–43. doi: 10.1016/j.ahj.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Mooss Aryan N, Wurdeman Richard L, Sugimoto Jeffrey T, Packard Kathleen A, Hilleman Daniel E, Lenz Thomas L, Rovang Karen S, Arcidi Joseph M, Mohiuddin Syed M. Amiodarone versus sotalol for the treatment of atrial fibrillation after open heart surgery: the Reduction in Postoperative Cardiovascular Arrhythmic Events (REDUCE) trial. Am. Heart J. 2004 Oct;148 (4):641–8. doi: 10.1016/j.ahj.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 14.NEXTERONE (Intravenous Amiodarone) Product Label. http://www. accessdata.fda.gov/drugsatfda_docs/label/2015/022325s009lbl.pdf. 2015;0:0–0. [Google Scholar]
- 15.Marill K A, Runge T. Meta-analysis of the Risk of Torsades de Pointes in patients treated with intravenous racemic sotalol. Acad Emerg Med. 2001 Feb;8 (2):117–24. doi: 10.1111/j.1553-2712.2001.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 16.Betapace AF. Product Label. http://www.accessdata.fda.gov/drugsatfda_docs/ label/2011/021151s010lbl.pdf (accessed Dec 2015) 0;0:0–0. [Google Scholar]
- 17.Van Mieghem W, Coolen L, Malysse I, Lacquet L M, Deneffe G J, Demedts M G. Amiodarone and the development of ARDS after lung surgery. Chest. 1994 Jun;105 (6):1642–5. doi: 10.1378/chest.105.6.1642. [DOI] [PubMed] [Google Scholar]
- 18.Goldschlager Nora, Epstein Andrew E, Naccarelli Gerald V, Olshansky Brian, Singh Bramah, Collard Harold R, Murphy Elizabeth. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007 Sep;4 (9):1250–9. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Van Cott Theodore E, Yehle Karen S, DeCrane Susan K, Thorlton Janet R. Amiodarone-induced pulmonary toxicity: case study with syndrome analysis. Heart Lung. 2013 Jul 10;42 (4):262–6. doi: 10.1016/j.hrtlng.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Vassallo Patricia, Trohman Richard G. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007 Sep 19;298 (11):1312–22. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- 21.Amiodarone HCL Tablets Product Label. http://www.accessdata.fda.gov/ drugsatfda_docs/label/2015/018972s047lbl.pdf. 2015;0:0–0. [Google Scholar]
- 22.Lafuente-Lafuente Carmelo, Valembois Lucie, Bergmann Jean-François, Belmin Joël. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015; (3) doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Saksena Sanjeev, Slee April, Waldo Albert L, Freemantle Nick, Reynolds Mathew, Rosenberg Yves, Rathod Snehal, Grant Shannon, Thomas Elizabeth, Wyse D George. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J. Am. Coll. Cardiol. 2011 Nov 1;58 (19):1975–85. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chassang B, Bonnin N, Moisset X, Citron B, Clavelou P, Chiambaretta F. Two cases of bilateral amiodarone-associated optic neuropathy. J Fr Ophtalmol. 2014 Mar;37 (3):231–6. doi: 10.1016/j.jfo.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Charness M E, Morady F, Scheinman M M. Frequent neurologic toxicity associated with amiodarone therapy. Neurology. 1984 May;34 (5):669–71. doi: 10.1212/wnl.34.5.669. [DOI] [PubMed] [Google Scholar]
- 26.Papiris Spyros A, Triantafillidou Christina, Kolilekas Likurgos, Markoulaki Despoina, Manali Effrosyni D. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010 Jul 1;33 (7):539–58. doi: 10.2165/11532320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Trappe Hans-Joachim, Brandts Bodo, Weismueller Peter. Arrhythmias in the intensive care patient. Curr Opin Crit Care. 2003 Oct;9 (5):345–55. doi: 10.1097/00075198-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Gomes J A, Ip J, Santoni-Rugiu F, Mehta D, Ergin A, Lansman S, Pe E, Newhouse T T, Chao S. Oral d,l sotalol reduces the incidence of postoperative atrial fibrillation in coronary artery bypass surgery patients: a randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 1999 Aug;34 (2):334–9. doi: 10.1016/s0735-1097(99)00213-2. [DOI] [PubMed] [Google Scholar]