Abstract
Background:
The nosological nature of “idiopathic” arrhythmias and the effect of etiotropic and pathogenetic treatment are often unknown.
Methods And Results:
19 patients (42.6±11.3 years, 9 women) with atrial fibrillation (n = 16), supraventricular (n = 10) and ventricular (n = 4) premature beats, supraventricular (n = 2) and ventricular tachycardia (n = 1), left bundle branch block (n= 2), AV block (n = 2) without structural heart changes. Viruses were identified (polymerase chain reaction, PCR) along with measurement of anti-heart antibodies (AHA) and endomyocardial biopsy (EMB).
EMB allowed to establish diagnosis in all patients:
infectious-immune myocarditis (n = 11, parvovirus-positive in 1),
parvovirus-positive endomyocarditis (n = 1),
systemic (n = 2) and myocardial (n = 1) vasculitis,
Fabry’s disease (n = 1),
arrhythmogenic right ventricular dysplasia (n = 1),
unspecified genetic cardiomyopathy (n = 2, herpes virus 6 one positive).
Level of AHA had the greatest significance for myocarditis diagnostics. All patients with myocarditis/vasculitis had background therapy: acyclovir (n = 10), IV immunoglobulin (n = 2), meloxicam (n = 12), hydroxychloroquine (n = 15), steroids (n = 14, 31.1±12.5 mg/day), azathioprine 150 mg/day (n = 2). Median follow-up was 4 years. Treatment significantly reduced the rate of arrhythmias (8 [5;8] to 3 [1.25;7.75] points); disappearance of bundle branch block was noted.
Conclusion:
EMB allowed to diagnose immune-mediated inflammatory diseases in 78.9% patients with ‘idiopathic’ arrhythmias and genetic diseases in 21.1%. Background therapy of myocarditis improved the antiarrhythmic efficiency, and allowed the best premed for interventional treatment.
Keywords: Idiopathic Arrhythmias, Lone Atrial Fibrillation, Endomyocardial Biopsy, Myocarditis, Myocardial Vasculitis, Immunosuppressive Therapy
Background
Methods of diagnosis and treatment of arrhythmias are actively developing, therefore etiology often goes on the back burner. As Ivan Pavlov had noted, “The etiology is the weakest part of medicine.” The concept of “idiopathic” arrhythmia has being mentioned in literature for at least 50 years (including “lone atrial fibrillation”[1]); it means arrhythmias in patients (usually younger than 60 years) without structural heart changes (“with a healthy heart”.) A more rigorous definition considers that causes scope has come to the end, but it also includes unexplained arrhythmias, or primary electrical heart disease. Incidence of idiopathic arrhythmias ranges from 3-17% to 20-45% for atrial fibrillation (AF),[2,3] and from 5% to 10-30% for ventricular arrhythmias.[4] The absence of obvious cause does not mean a favorable course and prognosis.[5,6]
Attempts to establish the etiology of «idiopathic» arrhythmias using myocardial biopsy are few, and their results are contradictory: in isolated works myocarditis in AF was revealed in 66% of atrial biopsies and 22-25% of ventricular biopsies.[7,8] In ventricular arrhythmias signs of myocarditis, cardiomyopathy and normal findings were 0-80%.[9,10] Attempts to speculate about the inflammatory etiology of «idiopathic» arrhythmias only due increased blood C-reactive protein (CRP) and cytokines are inconsistent. Genetic channelopathies can be the other causes of «idiopathic» arrhythmias. Options of causal and pathogenetic treatment of idiopathic arrhythmia remain unclear.
Study Objective
To establish the nosological nature of “idiopathic” arrhythmias with endomyocardial biopsy of the right ventricle and to evaluate the effect of etiotropic and pathogenetic treatment.
Materials And Methods
Stugy included 19 patients of 24-60 years (mean age 42.6 ±11.3 years, 9 women) with different arrhythmias and conduction abnormalities ([Table 1]), and without: left ventricular hypertrophy (>14 mm), dilatation with reduced contractility, myocardial infarction, endocarditis, history of open heart surgery, valvular heart disease, hypertrophic and various types of restrictive cardiomyopathies, diffuse connective tissue diseases and vasculitis, thyrotoxic and hypertensive heart. Hypertension (53.6%) and grade 1 obesity (26.3%) were not considered as a leading cause of arrhythmia. All patients underwent standard screening (including thyroid function), and following additional tests:
Table 1. Patients distribution by arrhythmia type and clinic date.
AF – atrial fibrillation, SVT – paroxysmal supraventricular tachycardia, PAB – premature atrial beats, RBBB – right bundle branch block, LBBB – left bundle branch block, AV – atrioventricular, PVB – premature ventricular beats, VT – ventricular tachycardia, CRP – C-reactive protein (mg/dl), L - leukocytes in the blood (x109/ml), EDV – end-diastolic volume of left ventricle (ml), IVS - interventricular septum (mm), LA – left atria (ml), EF – ejection fraction of left ventricle (%)
| N | Type of arrhythmia | age | sex | CRP | L | EDV | IVS | LA | EF |
|---|---|---|---|---|---|---|---|---|---|
| 1 | paroxysmal AF | 24 | M | 0 | 5.0 | 4.4 | 7 | 32 | 71 |
| 2 | paroxysmal AF | 38 | F | 0 | 5.3 | 4.7 | 7 | 65 | 59 |
| 3 | persistent AF | 50 | M | 0 | 6.1 | 5.2 | 10 | 69 | 56 |
| 3 | persistent AF | 50 | M | 0 | 6.1 | 5.2 | 10 | 69 | 56 |
| 4 | persistent AF | 45 | M | 0 | 5.8 | 4.9 | 8 | 63 | 65 |
| 5 | paroxysmal AF + SVT + PAB | 54 | F | 0.8 | 6.8 | 4.4 | 8 | 38 | 70 |
| 6 | paroxysmal AF + SVT + PAB | 33 | F | 0 | 7.3 | 4.1 | 6 | 42 | 58 |
| 7 | paroxysmal AF + SVT + PAB | 29 | M | 0 | 7.5 | 4.8 | 9 | 51 | 66 |
| 8 | paroxysmal AF + PAB +RBBB | 64 | F | 0 | 4.6 | 4.7 | 10 | 63 | 58 |
| 9 | paroxysmal AF + PAB | 50 | M | 0 | 6.2 | 5.3 | 8 | 67 | 58 |
| 10 | paroxysmal AF + PAB | 60 | F | 0 | 6.9 | 4.9 | 9 | 35 | 64 |
| 11 | paroxysmal AF + PAB | 32 | M | 0 | 5.4 | 4.8 | 9 | 60 | 62 |
| 12 | persistent AF + AV block +RBBB | 32 | M | 0 | 5.7 | 4.8 | 14 | 203 | 68 |
| 13 | persistent AF + AV block | 45 | F | 0 | 7.7 | 5.2 | 9 | 42 | 73 |
| 14 | paroxysmal AF + PAB + PVB + 1st degree AV block | 46 | M | 0 | 6.8 | 5.3 | 10 | 59 | 73 |
| 15 | paroxysmal AF + PAB + PVB + unsustained VT | 54 | F | 0 | 5.2 | 4.5 | 8 | 46 | 72 |
| 16 | paroxysmal AF + PAB + PVB + unsustained VT | 49 | M | 0.1 | 7.0 | 5.0 | 9 | 80 | 69 |
| 17 | PVB + VT | 31 | F | 0 | 6.1 | 4.6 | 10 | 40 | 58 |
| 18 | transient LBBB + SVT | 46 | F | 0 | 4.1 | 4.9 | 10 | 52 | 63 |
| 19 | transient LBBB | 34 | M | 0 | 4.4 | 5.4 | 7 | 62 | 56 |
IgG to herpes viruses, Coxsakie B viruses and genome of herpes viruses types 1,2,6, Epstein-Barr virus, herpes zoster, cytomegalovirus in the blood (PCR);
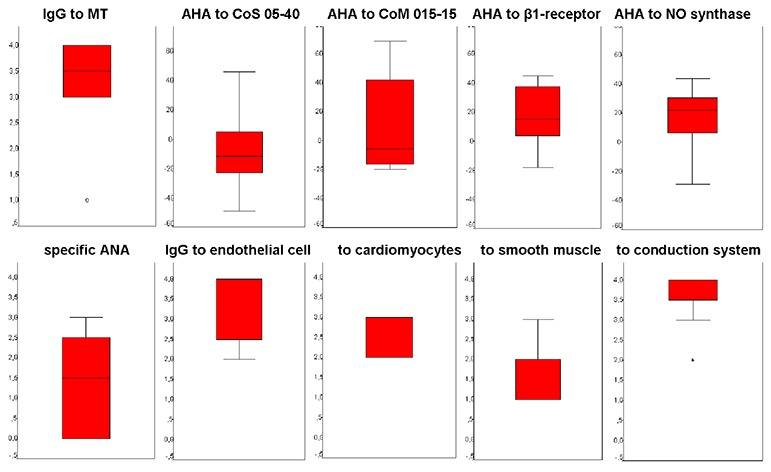
anti-heart antibodies (AHA) by direct and indirect ELISA in 3 laboratories: IgM and IgG to myocardial antigen (normal 1:100); IgG to cardiomyocytes cytoplasmic protein CoS05-40 and membrane protein CoM015-15; IgG to NO-synthase; IgG to β1-adrenergic receptor (n = 8, normal -30 to +20); anti-endothelial cell IgG, anti-cardiomyocyte IgG, anti-smooth muscle IgG, conduction heart system (normal 1:40), and antigen-specific anti-nuclear antibodies (ANA) with bovine heart (normally absent). We used pure FITC (fluorescein isothiocyanate)-labelled anti-human IgG and luminescent anti-human serum. Accounting was performed with fluorescent microscope Leica (Laborlux and DM4000V) at x 400 and 600;
endomyocardial biopsy (EMB) of the 5 sites in the right ventricle. Samples underwent PCR (including parvovirus B19 identifying), morphological study with hematoxylin-eosin, Van Gieson, periodic acid-Schiff (PAS) reaction staining, in some cases, electron microscopy.
Moreover, geneticist consultation and DNA (deoxyribonucleic acid) diagnosis (n = 4), treadmill test (n = 5), transesophageal (n = 5) and intracardiac electrophysiological study (n = 3), myocardial scintigraphy with 99mTc-MIBI (n = 10), magnetic resonance imaging (MRI, n = 3), cardiac multi-slice computer tomography (CT, n = 3), coronary angiography (n = 6), and skin biopsy (n = 1) were performed when appropriate. Paroxismal tachycardias (incl. AF and sustained ventricular tachycardia, VT), atrioventricular (AV) block and bundle brunch blocks were identified in ECG and/or Holter monitoring. The premature ventricular beats (PVB) and premature atrial beats (PAB) assessed by means of Holter monitoring.
SPSS 11.5 for Microsoft Windows was used for statistical analysis. Quantitative signs are presented as M+6 (average + one standard deviation) or as median with indication of the first and the third quartiles. Kolmogorov-Smirnoff test was applied for normalcy of distribution check. Criteria of Student, Mann-Whitney and Wilcoxon were used for estimation of differences significance. Differences have been considered significant in case of p<0.05. Local ethics committee approved this study and all patients signed written informed consent.
Results
Arrhythmia was the first disease sign in 73.7% of patients, acute onset was observed in 52.6%, relationship with infection - in 36.8%, disease duration less than a year - in 14.8%. Average duration of arrhythmia was 6 years (72 months, 30 to 144), average age of arrhythmia’s onset was 34.8 ± 10.4 years, age of AF onset – 36.2 ±10.8 years. Patients received an average of 5 antiarrhythmic drugs (AAD, 1 to 8), including 14 patients who received amiodarone. At enrollment, one patient had permanent pacemaker due to AV block, 3 patients had a history of radio frequency ablation (RFA, 2 - due to paroxysmal AV tachycardia, one - due to stable VT; three patients (15.8%) had syncope.
Minimal levels of acute-phase reactants were found in 21.1% patients; T-wave changes on the ECG - in 73.7%, isolated left atrial enlargement - in 52.6% ([Table 1]), diffuse uneven or focal perfusion impairment on scintigraphy - in 50%. Only one patient (with AF and premature ventricular beats, PVB) revealed 70% stenosis of the right coronary artery in the absence of angina pectoris and stress-induced ischemia. One patient had late contrast enhacenment (CT) and fat inclusions in the myocardium (MRI).
Cardiotropic virus genome (Epstein-Barr virus and herpes virus type 6, HHV6) was detected in the blood in two patients. Anti-herpes IgG levels were 2-3 times higher in 70-80% patients, anti-Coxsackie IgG levels - 2 times higher in 50% patients. Titers of anti-myocardial IgG were increased 3-4 times higher ([Fig. 1]). Extended analysis revealed that anti-endothelial cell IgG and anti-conduction system IgG had the highest titers, anti-cardiomyocyte IgG increased in a lesser extent. Levels of anti-smooth muscle IgG were normal that suggested selectivity of autoimmune reactions. Two thirds of patients had positive specific ANA (in the absence of standard ANA). Level of natural autoantibodies tended to slight increase. Increased AHA levels seen as sign of possible myocarditis, and one of the indication for EMB.
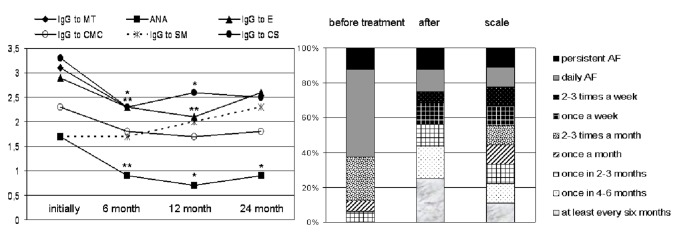
Figure 1. Median of increase of different types of anti-heart antibodies. The abscissa is the level of titer increase (x fold). AHA, anti-heart antibodies, MT, myocardial tissue, ANA, anti-nuclear antibodies.
Biopsy results are shown in [Table 2]: no patient had normal histology. Different combinations of morphological features allowed nosological diagnosis in all patients ([Table 3]).
Table 2. Results of endomyocardial biopsy.
| PCR results / morphological changes | n | % |
|---|---|---|
| parvovirus B19 / herpes simplex virus type 6 (PCR) | 2/1 (of 18) | 16.7% |
| endocardium sclerosis / thickening | 8 | 42.1% |
| lymphohistiocytic infiltration in endocardium | 2 | 10.5% |
| subendocardial / interstitial lipomatosis | 12 | 63.2% |
| interstitial lymphohistiocytic infiltration (>14 and 7-10) | 11/3 | 73.7% |
| necrosis / myolysis / apoptosis of cardiomyocytes | 6 | 31.6% |
| immune cytolysis (emperipolesis, peripolesis) | 2 | 10.5% |
| mitosis in cardiomyocytes | 1 | 5.3% |
| disarray of cardiomyocytes | 2 | 10.5% |
| dystrophy / hypertrophy of cardiomyocytes | 14/7 | 73.7/36.8% |
| productive vasculitis / arteriosclerosis / angiopathy | 7/7/1 | 36.8/36.8/5.3% |
| interstitial edema / swelling | 12 | 63.2% |
| interstitial sclerosis | 14 | 73.7% |
| fat (up to 50%) + fibrosis (up to 25%) + cell reorganization | 1 | 5.3% |
| PAS-positive substance in cardiomyocytes | 1 | 5.3% |
| no changes | 0 | 0 |
| Total | 19 | 100% |
Table 3. Distribution of patients according to nosological diagnosis based on EMB.
| initial diagnosis | diagnosis according to EMB | n | % patients | |
|---|---|---|---|---|
| myocarditis? | chronic / subacute infectious-immune myocarditis, including virus-positive (parvo B19 in myocardium/ EBV in the blood) active / borderline lupus-myocarditis |
11 2 8/3 2 |
57.89% | 78.9% |
| chronic viral autoimmune endomyocarditis (parvo B19) | 1 | 5.26% | ||
| systemic vasculitis, including | 2 | 10.53% | ||
| myocarditis with predominant vasculitis? | hypersensitive | 1 | ||
| myocardial vasculitis (with minimal signs of myocarditis) | 1 | 5.26% | ||
| myocarditis? genetic cardiomyopathy? | viral (HHV6) + genetic cardiomyopathy | 1 | 5.26% | 21.1% |
| genetic cardiomyopathy | 1 | 5.26% | ||
| Fabry disease | 1 | 5.26% | ||
| myocarditis? ARVD? | arrhythmogenic right ventricular dysplasia | 1 | 5.26% | |
| Total | 19 | 100% | ||
Chronic infectious-immune myocarditis based on lymphohistiocytic infiltration, including perivascular infiltration, was diagnosed in 11 patients, and in 8 patients it was active ([Fig. 2]). These patients included one with hemodynamically significant coronary atherosclerosis ([Fig. 2a]). Parvovirus B19 was identified in 2 patients. Twelve patients (almost exclusively with signs of myocarditis) had adipocytes concentrates under endocardium or in myocardial wall ([Fig. 2i]) with rare nuclei debris ([Fig. 2j]). Lipomatosis was considered as a marker and irreversible substrate of arrhythmias. Endocardium sclerosis and thickening, small focal interstitial sclerosis reflected duration of inflammation. In 2 patients morphological diagnosis was “lupus-myocarditis” (similarly with autoimmune “lupus-hepatitis”): it was characterized by signs of immune cytolysis (peripolesis and emperipolesis, [Fig. 2b]), vasculitis with perivascular bulbous sclerosis ([Fig. 3d]), and in one case - mitosis in cardiomyocytes ([Fig. 2c]). Immune cytolysis (with nuclear antigens exposure that may interact with intracellular lymphocytes) was morphological confirmation of antinuclear antibodies development: levels of specific ANA, other AHA in these patients were the highest (1:160-1:320), including anti-cardiomyocyte IgG. They had a history of autoimmune thyroiditis and polyvalent allergy, higher titers of anti-DNA IgG, anti-anticardiolipin IgM, and fibrinogen.
Chronic viral-immune (parvovirus B19) endomyocarditis with predominant endocardial infiltration but without eosinophils and neutrophils ([Fig. 2f]). Unusually low titer of anti-endothelial cell IgG (1:40) probably reflects massive AHA release in the immune complexes into the endothelium from the blood. Arrhythmias significantly varied (AF, atrial tachycardias, premature atrial beats PAB, PVB, and unsustained VT) and were resistant to all AAD. The second parvovirus-positive patient with myocarditis had minimal signs of endocarditis ([Fig. 3f]). Seven patients showed signs of active productive vasculitis ([Fig. 3,a-c]), other 7 patients had vessels sclerosis with significant lumen narrowing ([Fig. 3,d-e]). No patients had necrotising vasculitis. In 3 patients vasculitis was the leading morphological sign that was reflected in diagnosis.
Systemic vasculitis with cardiac injury: myocarditis with predominant productive vasculitis without necrosis and secondary (ischemic?) myocardial degeneration with foci of myolysis in one patient ([Fig. 3h]). In patient with more active vasculitis, systemic disease was confirmed by biopsy of the clear skin (leukoclastic vasculitis, [Fig. 3j]) without general clinical signs, or anti-neutrophil cytoplasmic antibodies (ANCA). Mild transient eosinophilia (6%), as well as eosinophils in the infiltrates ([Fig. 3i]), signs of endocarditis ([Fig. 3g]), and single blood clots in the myocardial microvasculature allowed to consider hypersensitive vasculitis and Loeffler’s endomyocarditis. Daily paroxysmal AF was resistant to all AAD and combined with polytopic extrasystoles. The patient with less active vasculitis and myocarditis had a history of atopic asthma, hemorrhagic rash episode, hay fever, eosinophilia up to 20%, and typical angina with unchanged coronary arteries and paroxysmal AF. Periods of exacerbation alternated with long and often spontaneous remission. At the time of biopsy, eosinophils and ANCA were normal that did not allow to diagnose “gross” vasculitis (eg, Churg-Strauss syndrome).
Myocardial vasculitis (with minimal signs of myocarditis) in one patient was isolated ([Fig. 3b]) with concomitant thickening and sclerosis of the endocardium. Signs of the disease included transient (stress-induced) complete LBBB and stress-induced ischemia on myocardial perfusion scintigraphy without coronary atherosclerosis. Low titer of anti-endothelial cell IgG (1:80; antibodies’ fixing in the vascular and endocardial endothelium?) was combined with specific ANA (1:80).
Unspecified genetic cardiomyopathy was diagnosed in two patients. Diagnose was based on the disarray of cardiomyocytes ([Fig. 4,a-c]) without clinical signs of hypertrophic cardiomyopathy. One of these patients (HHV6-positive in the blood and myocardium) also had small (viral?) inclusions in cardiomyocytes ([Fig. 4d]) and tender focal cardiosclerosis ([Fig. 4e]) thay may suggest history of myocarditis. Clinically, possible genetic nature of the disease was confirmed by AF development at the age of 16 years, and moderate mental retardation. At the same time, AF paroxysms recurred during sore throats and frequent acute respiratory viral infections. Titer of anti-endothelial cell IgG was increase up to 1:320, and titer of anti-myocardial IgG - up to 1:300. The second patient (with transient left bundle brunch block (LBBB) and history of paroxysmal supraventricular tachycardia) had fatty tissue in the myocardium, but no fibro-fatty replacement, and ventricular arrhythmias.
Fabry disease. EMB in patient of 32 years with persistent AF, AV block and minimal left ventricular hypertrophy revealed central myolysis foci in cardiomyocytes, PAS-positive substance in the membrane of cardiomyocytes ([Fig. 4g]), suggesting that storage disease. Also differential diagnosis included myocarditis, “athlete’s heart” and mesenchymal dysplasia syndrome. Geneticist suggested Fabry disease taking into account biopsy data, which was confirmed by biochemical (level of A-galactosidase 5.1 nM/mg/h, normally - 48.6-150.3) and genetic (Glu283Lys (Q283E) mutation in the gene X-Gal) studies. Increased titer of anti-smooth muscle IgG up to 1:160 may reflect involvement of small vessels with accumulation of the globotriaosilceramid. Titers of other AHA were normal.
Morphological features of arrhythmogenic right ventricular dysplasia (ARVD) were observed in patients with frequent PVC and history of RFA of sustained right ventricular tachycardia, and included: subendocardial adipocytes up to 50% separating myocardium into segments; fibrotic septa with architectonics abnormalities of the myocardium and fibrosis area up to 25%; sclerosis and thickening of the endocardium, as well as no signs of myocarditis ([Fig. 4f]). Fat inclusions in the right ventricle on MRI supported ARVD. At the same time, patient had a history of ovarian dysfunction, subfebrile episode regarded as myocarditis, positive effects of NSAIDs; ECG revealed small negative T-waves in V1-V4, which lability allowed suggesting myocarditis. Levels of AHA remained normal.
Figure 2. Key morphological changes in patients with myocarditis. Stained with hematoxylin and eosin (a-j, l) and Van Gieson (k). Large (a-d, f-h, j, l) and low (e, i, k) magnification. interstitial lymphohistiocytic infiltration > 14 cells (a, b, e), focal lysis (b, l) and necrosis (h) of cardiomyocytes, immune cytolysis with emperipolesis (intracellular lymphocytes locations are indicated by arrows) and loss of cardiomyocytes (c), mitosis in cardiomyocytes (d) significant endothelial thickening, swelling with focal proliferation (including villous proliferation, g) and infiltration (f), subendocardial and intramyocardial lipomatosis (i, k) with nuclei debris (j), focal fibrosis (k).
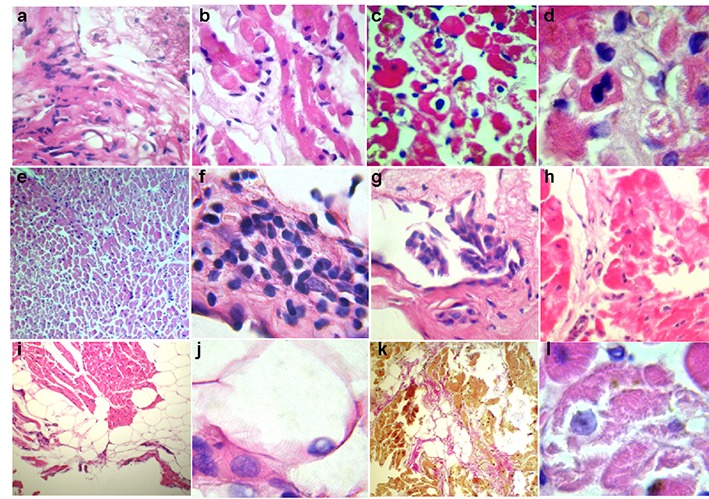
Figure 3. Key morphological changes in patients with vasculitis. EMB: light microscopy (a-i), and electron microscopy (k, l). Skin biopsy (j). Stained with hematoxylin and eosin (a-c, e-j) and Van Gieson (d). Large magnification. Cardiomyocytes with cytoplasmic homogenization, foci of myolysis with formation of cytoplasmic clefts (h), lymphohistiocytic myocardial (i) and endocardial (g) infiltration with deposits of eosinophils, vessels with endothelial proliferation, luminal stenosis, perivascular infiltrates (a-c) and sclerosis (d, e), microvessel ectasia with swollen endothelium and thrombotic lumen occlusion with loose clots (f). Skin: leukoclastic vasculitis (j). Explanation of electron-diffraction photographs - see text.
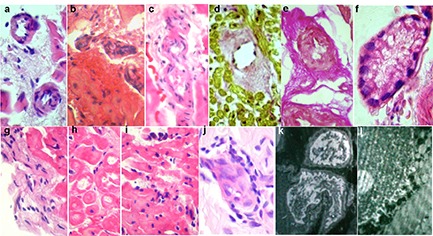
Figure 4. Key morphological changes in patients with genetic cardiomyopathies. Stained with hematoxylin and eosin (a-d, f), Van Gieson (e), and PAS-reaction (g). Large (a, b, d, e) and low (c, f, g) magnification. Disarray of cardiomyocytes with glomerular structures formation, cells arborization (disarray, a-c), cardiomyocytes with disappearance of the cross-striation and small intranuclear inclusions (d), mild interstitial sclerosis (e), accumulation of PAS-positive membrane-bound substance in cardiomyocytes (g).

Thus, EMB allowed to diagnose different immune-mediated inflammatory heart diseases in 78.9% patients and genetic – in 21.1% patients ([Table 3]), and their ratio was about 4:1.
At the end of the study, we obtained results of electron microscopy in three patients with myocarditis ([Fig. 3,k]-l): lysosomal myolysis, microbodies in cardiomyocytes, mitochondrial wrinkling, cristae adhesion, absence of matrix; vascular endothelium swelling, thickening of the microvessels basic membrane, precipitate on the endothelium surface, increased pinocytosis. The most marked changes were revealed in the mitochondria that are, apparently, both play a key role in the pathogenesis of metabolic disorders, and also are potential target for drug exposure.
Comparison of the results of non-invasive and morphological studies showed that AHA had the highest diagnostic value ([Table 4]). We found not only correlation between the diagnosis and specific ANA (r = 0.65; p <0.01), but also a clear correlation between immune and morphological activity of myocarditis. In patients without viral genome, specificity of ANA was 100%. Other signs had a high specificity but low sensitivity. Following factors were the most important for diagnosis of the genetic nature of arrhythmias: age younger than 40 years (sensitivity 75%, negative predictive value of 88.9%), isolated nature of arrhythmia (75% and 90.9%, respectively), family history and possible genetic markers (specificity and positive predictive value of 100%), and early repolarization syndrome (specificity 78.6%, negative predictive value of 84.6%).
Table 4. The sensitivity and specificity of various clinical, laboratory and instrumental signs in the diagnosis of myocarditis.
«+», positive predictive value, «-», negative predictive value, DND, diffuse non-uniform distribution, RP, radiopharmaceutical
| diagnostic signs | sensitivity | specificity | + | - |
|---|---|---|---|---|
| anamnestic triad | 16.7% | 100% | 100% | 25% |
| duration of history < 1 year | 21.4% | 100% | 100% | 26.6% |
| acute onset | 64.3% | 75% | 90% | 37.5% |
| onset correlation with infection | 50% | 75% | 87.5% | 30% |
| history of sore throat / tonsillitis | 50% | 75% | 87.5% | 30% |
| systemic immune signs | 35.7% | 100% | 100% | 30.8% |
| microvascular angina / ischemia | 35.7% | 100% | 100% | 30.8% |
| increased level of anti-O-streptolysin | 21.4% | 100% | 100% | 26.4% |
| increased CRP | 14.3% | 100% | 100% | 25% |
| non-specific inflammatory signs | 35.7% | 75% | 83.3% | 25% |
| viral genome in blood | 7.1% | 75% | 50% | 18.8% |
| specific ANA | 78.6% | 66.7% | 91.7% | 40% |
| anti-endothelial cell IgG ≥ 1:160 | 78.6% | 66.7% | 91.7% | 40% |
| anti-cardiomyocyte IgG ≥ 1:160 | 64.3% | 100% | 100% | 37.5% |
| anti-conduction system IgG ≥1:160 | 92.9% | 66.7% | 92.9% | 66.7% |
| isolated atriomegaly | 64.3% | 75% | 90% | 37.5% |
| pericardial effusion | 21.4% | 75% | 75% | 21.4% |
| DND of RP (scintigraphy) | 22.2% | 0 | 66.7% | 0 |
| local perfusion disorders (scintigraphy) | 33.3% | 100% | 100% | 14.3% |
Drug treatment included selection of the most effective AAD and basic myocarditis therapy. IC class AADs were the most effective: aethacizine, allapinine (including combination with sotalol), propafenone; 4 patients received amiodarone, 3 patients (with treatment-resistant AF) - β-blocker. Warfarin was prescribed in 9 patients.
Antiviral therapy (acyclovir, 750 mg/day IV and/or 1.6-2.0 g/day per os for 10-21 days) was administered to 10 patients with viral genome in the blood/myocardium or high titers of antiherpetic antibodies. Epstein-Barr virus was eliminated from the blood, but not the herpes virus type 6. Parvovirus-positive patients underwent IV infusion of immunoglobulin, 10 and 12.5 g, respectively.
All patients with verified myocarditis received hydroxychloroquine 200 mg/day for 15.0 [7.0; 24.0] months. Steroids were administered to 14 patients: mean dose was 31.1 ± 12.5 mg/day, maintenance dose 6.2 ± 2.2 mg/day, median duration of therapy - 18.0 [4.0, 25.5] months. Patients with systemic vasculitis had pulse therapy with metylprednisone 1000 mg No. 3 (n=1) or azathioprine 150 mg/day (n=2).
Median follow-up of patients was 4 years (48.0 [31.0, 62.0] months). The level of all AHA, except anti-cardiomyocyte IgG and anti-smooth muscle IgG, decreased significantly in six months ([Fig. 5]); in 1 and 2 years effect of treatment remained, but weakened (with decreasing doses and drug withdrawal). In 4 women side effects caused early steroid dose decline: peripheral myopathy in two patients and severe sweats and hot flashes in 2 cases. More frequent intercurrent infections were observed only with azathioprine. Causes of exacerbations were “common” infection in 10 patients, amiodarone-induced thyrotoxicosis - in 3 patients, dose reduction or drug withdrawal -in 9 patients. Median number of exacerbations per patient during follow-up was 3 [14 3], and they required to increase the dose of steroids by 1/3-1/2 from baseline.
Figure 5. Dynamics of anti-heart antibodies and the frequency of atrial fibrillation during therapy. Left - titers IgG to anti-myocardial tissue (MT), anti-cardiomyocytes (CMC), anti-smooth muscle (SM), anti-endothelial cells (E), anti-conducting system (CS) antibodies and specific anti-nuclear antibodies (ANA), measured as multiplies of normal; * significance of differences compared with baseline value with p <0.05, ** - p <0.01. Right - frequency of AF paroxysms during complex treatment (estimates after 6 months from the start of basic therapy).
In 16 patients AF at baseline was resistant to AAD (daily paroxysmal AF or sustained AF more than in 60% patients, [Fig. 5]). Basic therapy in 14 patients with myocarditis resulted in significant decrease of median AF episodes from 8 [5; 8] to 3 [1.25; 7.75] (see scale in [Fig. 5]): more than 40% patients had AF paroxysms once a month or less. After persistent suppression of inflammatory activity, 4 patients underwent RFA of pulmonary vein with complete AAD withdrawal. In 2 patients AF remained constant. In other patients satisfactory antiarrhythmic effect was achieved with drug therapy. In one patient with vasculitis, tachycardia-dependent LBBB disappeared but recurred after steroids withdrawal. A patient with ARVD had ICD implantation, a patient with Fabry disease – pacemaker implantation.
Discussion
Despite the predominance of supraventricular arrhythmias (AF in 16 patients, only in 3 - in combination with PVC), right ventricular EMB was informative in 100% of patients, including 2 patients with isolated LBBB. In literature, there are only two consistent articles discussing biopsies in resistant to treatment “idiopathic” AF performed in 1991 and 1997.[7,8] Biopsy of interatrial septum revealed signs of myocarditis in 66% of cases, while in ventricles myocarditis was found only in 22% of patients in the first article and in 25% - in the second article. The number of patients in both studies by A.Frustaci was 26. There are no other published biopsy data in “idiopathic” AF.
This study showed a greater frequency of myocarditis in ventricular biopsy specimens (78.9%) compared with those of atrial biopsies by A.Frustaci, and in 60% active myocarditis was revealed. We suggest that the cause was in patients’ selection: EMB was performed only in those with high levels of anti-heart antibodies. Our patients had significantly longer history of arrhythmias and consistently more frequent fibrosis (73.7% compared to 57.2% by A.Frustaci). Biopsy also allowed to differ genetic disease including ARVD from myocarditis in 4 patients: frequency of errors in the diagnosis of ARVD in such patients is known to reach 50%.[11] Disarray was revealed in 2 patients, and in the absence of hypertrophic cardiomyopathy clinical signs clearly indicated the genetic nature of the disease. One can expect signs of cardiomyopathy progression of in these patients over time.
There are no published data on the high frequency of subendocardial lipomatosis (63.2%) and productive vasculitis (36.8%) in “idiopathic” arrhythmias. Both signs correlated with inflammatory infiltration (r = 0.88, p <0.001 and r = 0.58, p <0.05, respectively). There are only a few mentions of isolated myocardial vasculitis as the cause of life-threatening arrhythmias[12 and “idiopathic» PVBs.[13 Perhaps, adipose tissue is formed instead of ceased cardiomyocytes, from stem cells that present in the myocardium.[14,15] Therefore, data on the lipogenesis mechanism in ARVD are interesting: nuclear translocation of plakoglobin suppresses the signaling pathway involved in the development of the right ventricle outflow tract, and leads to induction of stem cells transformation into adipocytes.[16] The activation of stem cells may be confirmed by mitosis identification in lupus-myocarditis: myocardium is a tissue with very low level of regeneration (about 0.05% of the cells), but in diseases the intensity of this process increases 10-70-fold.
In one patient with “idiopathic” arrhythmia we observed such active endocarditis that had never been previously described, although the possibility of viral valves infection was confirmed experimentally and clinically.[17] Immune endocarditis (Libman-Sacks, as part of systemic vasculitis) is also known. Some of our patients, of course, are associated with systemic diseases but do not reach all criteria; this may be due to genetically determined response features.
We proved high significance of AHA in the diagnosis of myocarditis that is fundamentally important. Data on the increased level of AHA in “idiopathic” arrhythmias and conduction abnormalities have been obtained previously,[18] but the case of AHA development was unclear. Some study proved correlation between titer of anti-endothelial cell IgG and severity of humoral rejection of the transplanted heart .[19] Significantly higher frequency of AHA compared with control (15.7%) in patients with ventricular (64.2%) and supraventricular arrhythmias (44.0%), as well as high frequency of myocarditis (81%) in these patients were proved only in 2012.[20] Anti-β1-receptor antibodies had 90% positive predictive value regarding histological changes.
In Russian articles significantly higher AHA titers in patients compared to healthy donors along with no between patients with coronary artery disease, myocarditis and cardiomyopathy were described.[21] The role of abzymes - catalytic anti-DNA antibodies that can cause double-stranded DNA hydrolysis and detorsion, and thus have a direct cytotoxic effect – was studied. Two-fold increase in their level in patients with immune myocarditis was confirmed ,[22] as well as correlation with severity of the disease: high level in malignant myocarditis, normal level in benign myocarditis (with increase of catalytic activity in 1/3 of patients) and post-myocarditis cardiosclerosis (without increase of activity). Thus, normal level of these antibodies does not exclude myocarditis that we observed in our study but suggests less activity.
The sensitivity of acute-phase proteins, including CRP, was very low: in majority of patients with active myocarditis they remained within normal limits. Primary chronic disease was observed in 57.1% (including patients with lupus-myocarditis and active endocarditis).
According to the diagnosed myocarditis, background therapy was administered to all patients. Clinical effect was seen in patients with baseline ineffectiveness of antiarrhythmic therapy. Formally, these patients had indications for RFA, but unrecognized myocarditis significantly reduces its effectiveness. In addition, 4 patients with all formal indications for RFA achieved satisfactory antiarrhythmic effect without surgery.
Doses of steroids in the treatment of myocarditis, especially its arrhythmic type, have not been studied. In some patients with “idiopathic” arrhythmias and verified myocarditis aggressive therapy was used.[7,8,10,23]In low doses, prednisone shows predominantly anti-inflammatory but not immunosuppressive properties. However, metipred 16 mg/day in 104 patients with “idiopathic” AF and elevated CRP in combination with constant propafenone therapy resulted in significantly less AF recurrences compared to the control group (9.6% and 50%, respectively), and AF transformation to permanent form (2% and 29%, respectively) with mean follow-up about 2 years.[24] Nature of AF also remained unclear.
Our study showed that clinical effect in myocarditis can be achieved with different regimens of background therapy (from hydroxychloroquine monotherapy to combination of metipred pulse therapy with azathioprine), and intensity of this effect depended not only on the disease activity, but also on the duration of the disease and irreversible structural changes in the myocardium (fibrosis, lipomatosis).
Conclusions
Thus, EMB confirmed nosological diagnosis in all patients with “idiopathic” arrhythmias (predominantly AF, as well as PVBs and LBBB). Prevalence of immuno-mediated inflammatory diseases (78.9%) over genetic diseases (21.1%) was found; in one case their combination can not be excluded. Level of various anti-heart antibodies, including specific ANA, had the greatest diagnostic value among noninvasive markers. Lack of correlation of arrhythmia onset with infection, acute onset, and coronary atherosclerosis and hypertension could not rule out myocarditis; more than half of the patients had primary chronic myocarditis. Antiviral and immunosuppressive therapy tailored according to myocarditis severity, could improve the effectiveness of antiarrhythmic therapy in patients resistant to AAD or prepare them for the RFA.
Disclosures
None.
References
- 1.EVANS W, SWANN P. Lone auricular fibrillation. Br Heart J. 1954 Apr;16 (2):189–94. doi: 10.1136/hrt.16.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weijs Bob, Pisters Ron, Nieuwlaat Robby, Breithardt Günter, Le Heuzey Jean-Yves, Vardas Panos E, Limantoro Ione, Schotten Ulrich, Lip Gregory Y H, Crijns Harry J G M. Idiopathic atrial fibrillation revisited in a large longitudinal clinical cohort. Europace. 2012 Feb;14 (2):184–90. doi: 10.1093/europace/eur379. [DOI] [PubMed] [Google Scholar]
- 3.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffery L, Antman Elliott M, Halperin Jonathan L, Hunt Sharon Ann, Nishimura Rick, Ornato Joseph P, Page Richard L, Riegel Barbara, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm A John, Dean Veronica, Deckers Jaap W, Despres Catherine, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais Joao, Osterspey Ady, Tamargo Juan Luis, Zamorano José Luis. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114 (7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 4.Chugh S S, Kelly K L, Titus J L. Sudden cardiac death with apparently normal heart. Circulation. 2000 Aug 8;102 (6):649–54. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 5.Scardi S, Mazzone C, Pandullo C, Goldstein D, Poletti A, Humar F. Lone atrial fibrillation: prognostic differences between paroxysmal and chronic forms after 10 years of follow-up. Am. Heart J. 1999 Apr;137 (4 Pt 1):686–91. doi: 10.1016/s0002-8703(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 6.Bikkina M, Larson M G, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann. Intern. Med. 1992 Dec 15;117 (12):990–6. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 7.Frustaci A, Caldarulo M, Buffon A, Bellocci F, Fenici R, Melina D. Cardiac biopsy in patients with "primary" atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest. 1991 Aug;100 (2):303–6. doi: 10.1378/chest.100.2.303. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo M A, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997 Aug 19;96 (4):1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 9.Drago F, Mazza A, Gagliardi M G, Bevilacqua M, Di Renzi P, Calzolari A, Francalanci P, Boldrini R, Bosman C, Di Liso G, Ragonese P. Tachycardias in children originating in the right ventricular outflow tract: lack of clinical features predicting the presence and severity of the histopathological substrate. Cardiol Young. 1999 May;9 (3):273–9. doi: 10.1017/s1047951100004935. [DOI] [PubMed] [Google Scholar]
- 10.Thongtang V, Chiathiraphan S, Ratanarapee S, Panchavinnin P, Srivanasont N, Jootar P, Sahasakul Y, Charoenchob N, Tresukosol D. Prevalence of myocarditis in idiopathic dysrhythmias: role of endomyocardial biopsy and efficacy of steroid therapy. J Med Assoc Thai. 1993 Jul;76 (7):368–73. [PubMed] [Google Scholar]
- 11.Pieroni Maurizio, Dello Russo Antonio, Marzo Francesca, Pelargonio Gemma, Casella Michela, Bellocci Fulvio, Crea Filippo. High prevalence of myocarditis mimicking arrhythmogenic right ventricular cardiomyopathy differential diagnosis by electroanatomic mapping-guided endomyocardial biopsy. J. Am. Coll. Cardiol. 2009 Feb 24;53 (8):681–9. doi: 10.1016/j.jacc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Hosenpud J D, McAnulty J H, Niles N R. Unexpected myocardial disease in patients with life threatening arrhythmias. Br Heart J. 1986 Jul;56 (1):55–61. doi: 10.1136/hrt.56.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Biase M, Chiddo A, Caruso G, Tritto M, Marchese A, Rizzon P. Ventricular premature beats in young subjects without evidence of cardiac disease: histological findings. Eur. Heart J. 1992 Jun;13 (6):732–7. doi: 10.1093/oxfordjournals.eurheartj.a060248. [DOI] [PubMed] [Google Scholar]
- 14.Frustaci Andrea, Chimenti Cristina, Pieroni Maurizio, Salvatori Luisa, Morgante Emanuela, Sale Patrizio, Ferretti Elisabetta, Petrangeli Elisa, Gulino Alberto, Russo Matteo A. Cell death, proliferation and repair in human myocarditis responding to immunosuppressive therapy. Mod. Pathol. 2006 Jun;19 (6):755–65. doi: 10.1038/modpathol.3800594. [DOI] [PubMed] [Google Scholar]
- 15.Leri Annarosa, Hosoda Toru, Kajstura Jan, Anversa Piero, Rota Marcello. Identification of a coronary stem cell in the human heart. J. Mol. Med. 2011 Oct;89 (10):947–59. doi: 10.1007/s00109-011-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardi Raffaella, Marian A J. Molecular genetics and pathogenesis of arrhythmogenic right ventricular cardiomyopathy: a disease of cardiac stem cells. Pediatr Cardiol. 2011 Mar;32 (3):360–5. doi: 10.1007/s00246-011-9890-2. [DOI] [PubMed] [Google Scholar]
- 17.Burch G E, Sun S C, Colcolough H L, Sohal R S, DePasquale N P. Coxsackie B viral myocarditis and valvulitis identified in routine autopsy specimens by immunofluorescent techniques. Am. Heart J. 1967 Jul;74 (1):13–23. doi: 10.1016/0002-8703(67)90035-x. [DOI] [PubMed] [Google Scholar]
- 18.Ristic A D, Maisch B. Cardiac rhythm and conduction disturbances: what is the role of autoimmune mechanisms? Herz. 2000 May;25 (3):181–8. doi: 10.1007/s000590050005. [DOI] [PubMed] [Google Scholar]
- 19.Fredrich R, Toyoda M, Czer L S, Galfayan K, Galera O, Trento A, Freimark D, Young S, Jordan S C. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation. 1999 Feb 15;67 (3):385–91. doi: 10.1097/00007890-199902150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Brisinda Donatella, Sorbo Anna Rita, Venuti Angela, Ruggieri Maria Pia, Manna Raffaele, Fenici Peter, Wallukat Gerd, Hoebeke Johan, Frustaci Andrea, Fenici Riccardo. Anti-β-adrenoceptors autoimmunity causing 'idiopathic' arrhythmias and cardiomyopathy. Circ. J. 2012;76 (6):1345–53. doi: 10.1253/circj.cj-11-1374. [DOI] [PubMed] [Google Scholar]
- 21.Topkara Veli K, Dang Nicholas C, Barili Fabio, Cheema Faisal H, Martens Timothy P, George Isaac, Bardakci Hasmet, Oz Mehmet C, Naka Yoshifumi. Predictors and outcomes of continuous veno-venous hemodialysis use after implantation of a left ventricular assist device. J. Heart Lung Transplant. 2006 Apr;25 (4):404–8. doi: 10.1016/j.healun.2005.11.457. [DOI] [PubMed] [Google Scholar]
- 22.Mal'tsev K A, Khitrov A N, Vvedenskaia O Iu, Ponomarenko N A, Isaeva M A, Klimova M V, Tret'iak E B, Shogenov Z S, Alekberova Z S, Gabibov S V, Suchkov S V. [Catalytic autoantibodies--a new molecular instrument in cardiology and ophthalmology]. Ter. Arkh. 2006;78 (11):70–6. [PubMed] [Google Scholar]
- 23.Balaji S, Wiles H B, Sens M A, Gillette P C. Immunosuppressive treatment for myocarditis and borderline myocarditis in children with ventricular ectopic rhythm. Br Heart J. 1994 Oct;72 (4):354–9. doi: 10.1136/hrt.72.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dernellis John, Panaretou Maria. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur. Heart J. 2004 Jul;25 (13):1100–7. doi: 10.1016/j.ehj.2004.04.025. [DOI] [PubMed] [Google Scholar]