Abstract
Recurrent AF after catheter ablation occurs in at least 20 to 40% of patients. Repeat ablation is primarily considered for those with symptomatic AF recurrences (often drug-refactory) occurring at least 3 months or more post-ablation. Pulmonary vein reconnection is almost universally encountered, and repeat isolation of electrically connected pulmonary veins should be the primary ablation strategy. Beyond repeat PVI and possible ablation of non-PV triggers, there is little to no evidence that additional substrate modification improves outcomes. In addition to repeat ablation, it is critical to address and treat comorbid conditions which increase arrhythmia risk post-ablation. Specifically, obesity, hypertension, and sleep-disordered breathing should be targeted and modified to increase the likelihood of success.
Keywords: Atrial Fibrillation Ablation, Repeat Catheter Ablation, Pulmonary Vein Reconnection, Atrial Fibrillation Lifestyle Modification
Introduction
Catheter ablation of atrial fibrillation (AF) has become an increasingly frequent procedure per-formed in electrophysiology laboratories worldwide. It is most often performed for maintenance of sinus rhythm in patients with symptomatic, drug-refractory paroxysmal or persistent AF or as an initial rhythm control strategy in lieu of anti-arrhythmic drug therapy in patients with paroxys-mal AF.[1] The increased efficacy of catheter ablation over anti-arrhythmic drug therapy to main-tain sinus rhythm has been demonstrated in a number of randomized, controlled trials and meta-analyses.[2-12] Unfortunately, recurrent atrial fibrillation or atrial tachycardia after an index AF ab-lation procedure results in repeat ablation in 20 to 40% of patients.[13] A number of dilemmas are presented by patients with recurrent AF after catheter ablation: Which patients should be con-sidered for a second procedure and when should repeat ablation be performed? What is the op-timal approach to ablation in a patient undergoing a repeat procedure? What additional interven-tions may reduce the likelihood of recurrence post-ablation? The purpose of this review is to summarize the available relevant data surrounding repeat ablation for atrial fibrillation and identi-fy areas needing further investigation.
Rationale For Repeat Catheter Ablation
The primary ablation strategy for AF is creation of electrical isolation of all pulmonary veins (PVs) with demonstration of bidirectional (entrance and exit) conduction block post-ablation.[1] The most commonly reported finding at repeat catheter ablation is resumption of con-duction to (and from) previously targeted pulmonary veins.[14-17] Durable PV isolation (PVI) may be so difficult to achieve after a single AF ablation that some have reported recovery of conduc-tion in 1 or more PVs in all patients undergoing repeat ablation.[18-19] Amazingly, pulmonary vein reconnection has been identified in up to 92% of patients undergoing a third or greater proce-dure.[20] Electrical isolation of the pulmonary veins is more likely to be permanent after a repeat ablation procedure. Consequently, one rationale for repeat ablation is to “finish” what was started during the first procedure and attempt to ensure permanent electrical isolation of all pulmonary veins. In addition, studies have shown incremental success with higher rates of long-term free-dom from AF with repeat ablation possibly resulting from a higher rate of permanent PV isola-tion.[12,19,21]
Timing Of Repeat Catheter Ablation
Among patients with recurrent arrhythmias post-ablation, there are a number of consid-erations impacting patient management. First, the patient’s symptoms should heavily influence subsequent management strategies. Patients with minimal to no symptoms who are adequately rate-controlled may be suitable for a rate-control and anticoagulation strategy rather than con-tinuing to pursue sinus rhythm. The timing of recurrence is also important when considering a repeat procedure. Recurrent arrhythmias within the first two to three months post-ablation may resolve spontaneously or not recur after cardioversion so a repeat procedure is often deferred in this timeframe.[1] The mechanism of recurrent arrhythmia (AF versus atrial tachycardia/flutter) may also play a role in decision-making. Patients typically considered for repeat ablation have recurrent, symptomatic AF more than 3 months after initial ablation. Early repeat ablation may be considered for recurrent arrhythmia (particularly atrial tachycardia or atrial flutter) that is diffi-cult to manage medically and recurs despite cardioversion. Recurrent atrial flutter or tachycar-dia post-ablation may be better managed with a repeat procedure as such arrhythmias can be difficult to rate control, frequently recur after cardioversion, and are often due to gaps in areas of prior ablation and have a relatively high success rate with repeat ablation. The focus of this re-view is recurrent atrial fibrillation after catheter ablation and not management of post-ablation atrial flutter or tachycardia.
An additional consideration is the likelihood of success with repeat catheter ablation. Factors shown to negatively impact recurrence rates include left atrial properties (volume, fibro-sis), associated systemic disease (hypertension, obstructive sleep apnea), concomitant heart disease (particularly mitral valve disease and hypertrophic cardiomyopathy), and duration of atrial fibrillation (e.g., longstanding persistent AF has a higher recurrence rate than paroxysmal AF, [table 1]).[1] Patients with multiple negative prognostic factors for recurrence perhaps are best managed medically (if possible) rather than exposed to the risks of ablation with low likelihood of success. It would not be appropriate to pursue repeat ablation in asymptomatic patients with the hope of obviating need for long-term oral anticoagulation when the CHA2DS2-VASc score indi-cates a moderate to high risk of stroke. Repeat catheter ablation is most commonly accepted for patients with well-documented arrhythmia recurrences who are symptomatic (despite a trial of anti-arrhythmic drug therapy) and are more than 3 months removed from the initial proce-dure.[1]
Table 1. Risk factors for atrial fibrillation recurrence after ablation.
| Age | Increased risk of recurrence with advancing age |
|---|---|
| AF duration and type | (Longstanding persistent > persistent > paroxysmal) |
| Cardiac structural changes | Left atrial dilatation; left ventricular function; hypertrophic cardiomyopathy; valvular heart disease |
| Clinical features | Hypertension; obesity; obstructive sleep apnea/sleep disordered breathing; metabolic syndrome; thyroid disease |
Strategies For Repeat Catheter Ablation
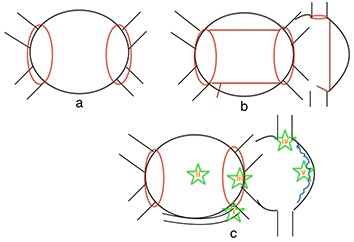
When AF recurs after PVI and PV reconnection is identified at repeat ablation it seems prudent to re-isolate any reconnected PVs. If the PVs have reconnected, however, how does one know that PV reconnection is the cause of recurrent arrhythmia? Going a step further, should additional ablation beyond repeat PVI be performed? If the PVs have not reconnected what ablation strategy should be employed? Considerations include using different energy deliv-ery sources to repeat PVI (e.g., using cryoablation if radiofrequency was used initially), creation of linear lesions in the left and/or right atrium, isolation of the superior vena cava or coronary si-nus, ablation at atrial sites with fractionated electrograms during AF, ablation at sites of vagal in-puts to the atria, and targeting non-PV triggers ([figure 1]). It is important to note there are no randomized controlled trials addressing these issues in patients with recurrent AF. The data re-porting outcomes with repeat AF ablation are derived from retrospective and observational co-hort and case-control studies. The most recent consensus statement on catheter ablation of AF suggests the first step when performing a repeat procedure is to check each PV for electrical reconduction followed by reisolation of PVs as necessary as there is data showing reasonably good outcomes with repeat PVI alone.[1,15] If there is little to no evidence of PV reconduction, non-PV foci should be sought and consideration should be given to modification of the ar-rhythmogenic substrate although no particular linear lesion set or alternative ablation approach is recommended in the guidelines.[1]
Figure 1. Potential ablation strategies during repeat AF procedures: a) repeat pulmonary vein isolation only with confirmation of entrance and exit block from each vein; b) pulmonary vein isolation with ad-ditional linear lesions (posterior wall isolation with linear lesions connecting the superior and infe-rior pulmonary veins; mitral isthmus ablation; +/- right atrial linear lesions); c) pulmonary vein iso-lation and ablation of non-pulmonary vein triggers (i = coronary sinus; ii = LA posterior wall (and left atrial appendage, not pictured); iii = fossa ovalis/interatrial septum; iv = crista terminalis/right atrium; v = superior vena cava).
Techniques To Enhance Durability Of Pulmonary Vein Isolation
As pulmonary vein reconnection is near universal among patients undergoing repeat ab-lation, it is prudent when re-isolating PVs to employ techniques shown to increase the likelihood of durable PVI. This is more likely to occur with the delivery of contiguous, transmural lesions regardless of the energy deliver system. It is postulated that improved acute lesion delivery will translate to enhanced long-term outcomes. A number of procedural techniques have been ad-vocated to improve the likelihood of transmural lesion formation thereby increasing the likelihood of durable PVI and (hopefully) freedom from arrhythmia. General anesthesia compared to con-scious sedation lowers reconnection rates among patients with recurrences who underwent re-peat ablation (19 vs 42%).[22] Efforts to minimize respiratory motion, particularly using high-frequency jet ventilation, have also been shown to improve freedom from AF at 1 year post-ablation.[23] Catheter stability may be further enhanced by manipulation through a steerable sheath, and use of such technology has been shown to improve short-term AF freedom rates post-ablation.[24] Ablation using multi-pore irrigated tip catheter technologies results in lower peri-procedural PV reconnection rates compared to standard irrigated tip catheters.[25] Contact force sensing technologies provide continuous feedback regarding catheter contact force and stability, and ablating with a contact force > 10 grams is associated with a lower likelihood of acute pul-monary vein reconnection and improved outcomes at 1 year.[26,27] Pulmonary vein reconnection rates were no different between standard radiofrequency ablation (using an open-irrigation RF catheter) and the first generation cryoballoon system among patients presenting for repeat abla-tion in a small study of 50 patients with paroxysmal AF.[28]
Rigorous testing to confirm bidirectional (entrance and exit) conduction block post-ablation improves long-term success rates.[29] A reasonable post-ablation wait period to assess for acute PV electrical reconnection seems to improve outcomes, and a study of 181 patients sug-gests waiting at least 35 minutes after acute isolation is the optimal observation time.[30]
Assessing for non-capture along the circumferential lesion set is one method for testing the integ-rity of the ablation line, and re-ablating sites of pace capture resulted in greater AF freedom (83 vs 52%) at 1-year follow-up in a prospective study.[31] Administration of adenosine to assess for dormant conduction can be useful for identifying gaps in the ablation line and pulmonary veins with higher risk of reconnection.[32] Additional ablation of acutely reconnected pulmonary veins after adenosine administration may or may not improve long-term outcomes as data is mixed.[33,34]
It is important to note that none of these approaches has been systematically studied to determine their true impact on promoting durable pulmonary vein isolation. It is also worth noting that absence of AF recurrence does not necessarily indicate permanent pulmonary vein isola-tion, and PV reconnection noted at repeat procedure may be incidental and not causative with regard to arrhythmia recurrence. That being said our initial approach during a repeat AF ablation procedure is to first and foremost ensure pulmonary vein isolation by ablating any reconnected pulmonary veins and confirming bidirectional conduction block ([figures 2] and [3]). Our standard approach is to use a contact force sensing catheter within a steerable sheath guided by an elec-troanatomic mapping system and intracardiac echocardiography. A circular mapping catheter is used to confirm bidirectional conduction block, and adenosine is routinely administered with re-ablation of any sites exhibiting dormant conduction. A comprehensive EP study is then per-formed to assess for other inducible arrhythmias or non-PV triggers with additional ablation as needed.
Figure 2. Rational approach to a repeat AF ablation procedure.
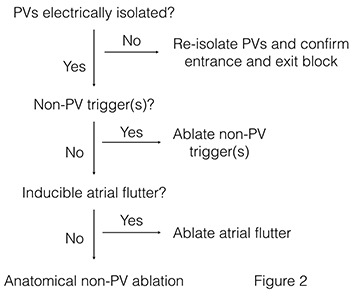
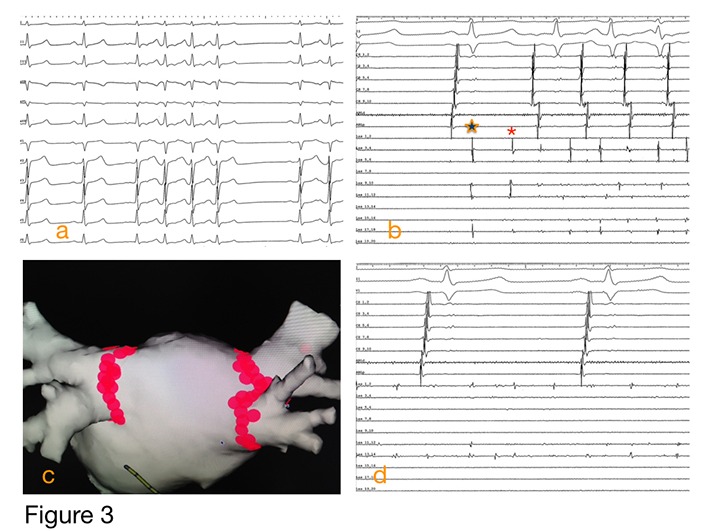
Figure 3. Illustrative case of a 47 year-old man undergoing repeat catheter ablation for atrial fibrillation. Paroxysmal AF had been diagnosed 2 years prior, and the patient underwent catheter ablation approximately 12 months earlier at another institution. He was AF free for nearly 9 months but then began having recurrent symptoms with paroxysmal AF documented. a) baseline rhythm at the start of the procedure under general anesthesia; frequent short bursts of AF noted; b) dis-played are 3 surface ECG leads and intracardiac recordings from a decapolar catheter in the coronary sinus (labeled cs 9,10 through cs 1,2) and a circular mapping catheter (labeled Las 19,20 through Las 1,2) placed in the right superior pulmonary vein; note the delayed pulmonary vein potential (star) and initiation of AF triggered by spontaneous firing from the RSPV (asterisk); the other 3 PVs remained electrically isolated from the prior procedure; c) electroanatomic map with a posterior view of the left atrium; the RSPV was re-isolated using RF ablation and addition-al tags were placed at sites around the remaining pulmonary veins were there was bipolar volt-age < 0.2 mV and no pace capture; 4) the circular mapping catheter in the right superior pulmo-nary vein demonstrates AF in the RSPV with exit block while the atria remain in sinus rhythm.
Options Beyond Pulmonary Vein Isolation: Ablation Of Non-Pulmonary Vein Triggers And Substrate Modification
As pulmonary vein electrical reconnection is a common finding at repeat ablation, it seems prudent to re-isolate any reconnected PVs as an initial repeat ablation strategy as men-tioned above. The decision to pursue additional ablation beyond PVI is difficult, and there is little data to guide whether additional ablation, if any, should be performed during a repeat procedure. Several studies have reported improved outcomes with a strategy of PVI and additional ablation of spontaneous or inducible non-pulmonary vein AF triggers.[15,16,20,35,36] One of these studies reported outcomes among 169 patients with recurrent AF despite 2 or more prior ablation proce-dures.[20] Astonishingly, only 8% of patients had all PVs isolated at baseline despite more than 1 prior ablation. Non-pulmonary vein triggers were rigorously sought with incremental doses of isoproterenol (3, 6, 12, and 20 µg/min and/or burst atrial pacing to provoke AF followed by cardi-oversion with or without low-dose isoproterenol). The majority of AF triggers localized to the pulmonary veins, although other triggers were identified (Eustachian ridge and crista terminalis; coronary sinus; SVC; LA posterior wall; left atrial appendage; interatrial septum). With a strategy of repeat PVI and targeting non-PV triggers, 81% of patients had arrhythmia control at up to 1-year follow-up.
Beyond PVI and ablation of non-PV triggers, there is very little data to guide whether ad-ditional substrate modification should be performed during a repeat ablation procedure. On one hand, it could be argued that recurrent AF is a failure of the initial strategy so a different strategy (i.e., substrate modification) should be attempted. Alternatively, one could postulate that the pri-mary goal of repeat ablation is to ensure durable PVI, and non-PV based ablation strategies should be reserved for patients without PV reconnection. Extensive ablation may come with the costs of altering atrial contractile properties, increasing the risk for procedural complications, and placing the patient at risk for iatrogenic atrial flutter(s) if bidirectional block is not achieved across linear lesions.[37] Ultimately, the critical question is how important the PVs are in driving a given patient’s arrhythmia. Comparing the cycle length of PV triggers to the cycle length in the coro-nary sinus during AF may provide some indication as to the role of the PVs in supporting a pa-tient’s arrhythmia.[38] Pulmonary vein electrogram frequency tends to be much higher than the coronary sinus early in the disease process (suggesting PV isolation will result in a high likelihood of arrhythmia control), whereas the PV electrogram frequency is often lower than the coronary sinus as the disease process becomes more advanced (suggesting non-PV sources may be of increased importance and PV isolation alone may not result in optimal outcome).
There are no randomized controlled trials evaluating the efficacy of substrate modifica-tion techniques in patients with recurrent AF. The available data for non-PV based ablation come from patients undergoing de novo ablation procedures. Substrate modification techniques such as left atrial linear ablation, focal impulse or rotor modulation, ablation of complex fraction-ated atrial electrograms (CFAEs), and ganglionated plexi modification have been evaluated pri-marily in patients undergoing initial ablation for persistent and longstanding persistent AF. Ex-trapolation of these results to patients undergoing repeat ablation should be done with caution. Electrical isolation of the LA posterior wall has been evaluated primarily in patients with persistent AF with mixed results.[39,40] Ablation of areas with complex fractionated activity (CFAEs) have been investigated in patients with paroxysmal and persistent AF. Nademanee et al. targeted CFAEs defined as sites with low-amplitude potentials and continuous electrical activity or cycle length < 120 ms and reported a success rate of 91% at 1-year follow-up.[41] A more recent study evaluated adjunctive ablation of CFAE sites (identified with an automated mapping system) ver-sus ablation of sites with continuous electrical activity.[42] At 1-year follow-up, freedom from ar-rhythmia, although modest, was higher with CFAE ablation compared with ablation of sites with continuous electrical activity (50 vs 28%). Adjunctive CFAE ablation has not been uniformly demonstrated to improve outcomes as one study randomly assigned 156 patients to PVI plus ablation of inducible non-PV triggers versus one of two additional strategies: PVI + empiric abla-tion of common non-PV AF trigger sites or PVI + ablation of left atrial CFAE sites.[43] Ablation of CFAE sites did not result in improved arrhythmia control at 1-year follow-up. In addition, a more recent randomized trial (STAR AF II) further assessed the role of adjunctive CFAE and linear ablation among patients with persistent atrial fibrillation.[44] At 18 months follow-up, 59% of PVI only patients were free from recurrent arrhythmia as opposed to 49% of patients who underwent PVI + CFAE ablation and 46% of patients who underwent PVI + empiric linear ablation.
The autonomic nervous system may play a role in initiating and maintaining AF through several mechanisms: facilitating spontaneous premature atrial depolarizations; shortening of atrial and PV effective refractory periods; and increasing heterogeneity of refractoriness. Con-sequently, a number of authors have investigated the role of adjunctive ganglionated plexus (GP) ablation. A randomized trial involving 67 patients with paroxysmal AF assigned to PVI ver-sus PVI plus GP ablation showed improved outcomes at 10 month follow-up (45.5 vs 73.5%).[45] This is an area of active investigation and additional data involving a larger number of patients is needed to determine if GP ablation truly improves outcomes.
Recent studies have reported the presence of stable reentrant circuits (“rotors”) within the atria of AF patients which may provide an additional target during AF ablation.[46-48] The CON-FIRM trial reported initial experience in 92 patients treated either with FIRM-guided ablation with PVI versus PVI alone.[49] FIRM ablation was associated with slowing or termination of AF in 86% of patients, and over follow-up 82% of FIRM patients remained free of AF compared with 45% in the PVI-only group. More recently, two additional studies evaluated the efficacy of FIRM abla-tion on early and long-term outcomes and reported less optimistic results. One study reported 6-month outcomes among 29 patients (20 persistent, 9 longstanding persistent) undergoing FIRM-identified rotor ablation alone.[50] Single-procedure freedom from atrial tachyarrhythmias without anti-arrhythmic drugs was 17%. The other study reported outcomes among 43 patients (56% paroxysmal) who underwent FIRM ablation and PVI.[51] At 18 month follow-up only 21% of pa-tients were free from arrhythmia off antiarrhythmic drugs.
Additional studies have evaluated the benefit of assessing for low-voltage areas at the time of ablation and performing additional substrate modification of these sites. A study involving 178 patients (65% persistent) found low voltage abnormalities in 35% and 10% of persistent and paroxysmal patients, respectively.[52] Low voltage areas were defined as sites with ≥ 3 adjacent points with bipolar voltage < 0.5 mV. Catheter ablation of low voltage areas in addition to PVI resulted in 12-month arrhythmia freedom of 70% compared with 27% among 26 patients with low voltage abnormalities who did not undergo further substrate modification. Another study as-sessed outcomes among 85 patients who underwent PVI and ablation of low voltage areas as-sociated with either fractionated or discrete rapid local activity within or along the border zones of low voltage areas compared with 42 “control” patients with persistent AF who underwent PVI alone.[53] Arrhythmia freedom at 13 months was 69% among patients who underwent ablation of low voltage areas compared with 47% in the PVI-alone control group.
Substrate modification techniques (i.e., linear ablation; targeting of CFAEs or low voltage areas) have yielded conflicting results among patients undergoing de novo ablation and have not been studied and are of unclear benefit in patients undergoing repeat AF ablation. Given that the majority of studies investigating non-PV based ablation have shown little to no improvement over PVI alone (e.g., STAR AF II), it is hard to advocate for extensive atrial ablation. The majority of evidence suggests repeat PVI (if the PVs have reconnected) and ablation of non-PV triggers seems to be the most effective strategy. If the PVs have not reconnected, it seems reasonable to perform a comprehensive EP study to assess for inducible atrial flutter(s) or atrial tachycar-dia(s) and reserve substrate modification for patients in whom the PVs have not reconnected and other arrhythmias are not inducible. If linear ablation is performed it is imperative that bidi-rectional block be confirmed to avoid creating the substrate for iatrogenic atrial arrhythmias.
Repeat AF Ablation: Cryoablation Versus Radiofrequency?
When patients have recurrent AF after ablation is a certain energy delivery system pre-ferred for repeat PVI? If cryoablation was used in the index procedure, should radiofrequency (RF) be employed in a subsequent procedure or vice versa? There is limited data addressing this subject but one interesting study is worth mention. Pokushalov et al. randomly assigned 80 patients with recurrent paroxysmal AF after a first PVI using radiofrequency ablation to repeat PVI with either cryoablation or RF.[54] Study participants had implantable loop recorders to moni-tor for recurrence. At 1-year follow-up more patients randomized to repeat ablation with RF (58%) were AF-free compared with those who underwent cryoablation (43%). This finding sug-gests repeat PVI with RF, as opposed to cryoablation, results in improved outcomes although this study is small and the results should be validated in a larger number of patients.
Pre-Procedural And Intra-Procedural Imaging To Guide Ablation
Ideally pre-procedural imaging could be used to identify sites of PV reconnection or pro-vide clues to the mechanism of recurrent arrhythmia to guide repeat ablation. Late gadolinium-enhanced (LGE) MRI has been used to identify gaps in lesion sets which may be targeted acute-ly or with repeat ablation.[55-57] One study involving 15 patients undergoing repeat ablation for AF found pre-ablation late gadolinium-enhanced MRI accurately identified gaps in areas of prior ab-lation resulted in shorter procedure times by allowing more targeted ablation.[58] In addition, as previously mentioned there is some evidence suggesting improved outcomes if areas of low voltage are targeted in addition to PVI. If this is validated in subsequent studies and found bene-ficial in patients undergoing repeat ablation, LGE-MRI may be useful for pre-procedure planning by helping identify abnormal substrate which could be targeted for ablation.
A critical step forward may be noninvasive imaging of electrical activation to identify the processes essential to maintaining an individual’s arrhythmia. Identification of focal drivers or rotational activities prior to entering the electrophysiology laboratory may facilitate a tailored abla-tion strategy more likely to be successful than empirically applying the same lesion sets to each patient regardless of arrhythmia mechanism. Medtronic, Inc. and CardioInsight’s ECVUETM is a noninvasive system which captures body surface electrical data to create and visualize epicardi-al 3D electroanatomic maps. The system has proven successful in mapping and ablation of persistent AF in a multicenter study.[59] In the study, 118 persistent AF patients underwent pre-ablation body surface mapping with data used to guide ablation of AF drivers. Acute success (AF termination) was achieved in 64% with driver-based ablation alone. At mean 6 months’ fol-low-up, 83% of patients were AF free including recurrent atrial tachycardia in 38%. Although ad-ditional work needs to be done to validate the accuracy of noninvasive electrical mapping, the concepts and available data are intriguing. Noninvasive electrical mapping may become a valu-able pre-ablation tool for both de novo and repeat AF ablation procedures by potentially identify-ing areas critical to a patient’s AF mechanism(s) prior to entry into the EP laboratory.
Ancillary Interventions To Minimize AF Recurrence
In addition to procedural interventions to treat AF, one should also screen for and modify any comorbid conditions which may increase the likelihood of AF recurrence ([table 1]). Specifi-cally, it is prudent to address obesity; sleep-disordered breathing/obstructive sleep apnea; hyper-tension; smoking and alcohol consumption. Obesity is a clearly defined risk factor for AF. It increases the risk of hypertension, metabolic syndrome/diabetes mellitus, and obstructive sleep apnea (OSA), all of which have also been associated with development of atrial fibrillation. Weight reduction has been shown to reduce AF symptom burden and severity. Obstructive sleep apnea independently increases the risk of incident atrial fibrillation and increases the risk of recurrent AF after ablation. OSA promotes atrial structural and electrical remodeling includ-ing atrial enlargement and low-voltage areas with conduction abnormalities. Treatment of OSA with continuous positive airway pressure (CPAP) improves arrhythmia-free survival post-catheter ablation. A recent study demonstrated aggressive risk factor modification including weight reduction (initial goal to reduce body weight by 10% followed by target BMI < 25 kg/m2); blood pressure management with target < 130/80 mmHg; aggressive lipid and glycemic control; treatment of obstructive sleep apnea if the apnea-hypopnea index (AHI) was > 30/hour; and ab-stinence from smoking and alcohol significantly improved arrhythmia-free survival. Additional research is needed to define optimal targets for management of AF risk factors, but it is clear that treatment of comorbid conditions optimizes AF control.
Conclusions
Recurrent AF after catheter ablation occurs in at least 20 to 40% of patients. Repeat ab-lation is primarily considered for those with symptomatic AF recurrences (often drug-refactory) occurring at least 3 months or more post-ablation. Pulmonary vein reconnection is almost uni-versally encountered, and repeat isolation of electrically connected pulmonary veins should be the primary ablation strategy. Beyond repeat PVI and possible ablation of non-PV triggers, there is little to no evidence that additional substrate modification improves outcomes. If substrate modification and linear lesions are created, however, it is imperative to confirm bidirectional con-duction block to avoid creating substrate for iatrogenic atrial arrhythmias. In addition to repeat ablation, it is critical to treat the “whole” patient by addressing comorbid conditions which in-crease arrhythmia risk post-ablation. Specifically, obesity, hypertension, and sleep-disordered breathing should be targeted and modified to increase the likelihood of success.
Disclosures
None.
References
- 1.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Pappone Carlo, Augello Giuseppe, Sala Simone, Gugliotta Filippo, Vicedomini Gabriele, Gulletta Simone, Paglino Gabriele, Mazzone Patrizio, Sora Nicoleta, Greiss Isabelle, Santagostino Andreina, LiVolsi Laura, Pappone Nicola, Radinovic Andrea, Manguso Francesco, Santinelli Vincenzo. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J. Am. Coll. Cardiol. 2006 Dec 5;48 (11):2340–7. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Wazni Oussama M, Marrouche Nassir F, Martin David O, Verma Atul, Bhargava Mandeep, Saliba Walid, Bash Dianna, Schweikert Robert, Brachmann Johannes, Gunther Jens, Gutleben Klaus, Pisano Ennio, Potenza Dominico, Fanelli Raffaele, Raviele Antonio, Themistoclakis Sakis, Rossillo Antonio, Bonso Aldo, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005 Jun 1;293 (21):2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 4.Jaïs Pierre, Cauchemez Bruno, Macle Laurent, Daoud Emile, Khairy Paul, Subbiah Rajesh, Hocini Mélèze, Extramiana Fabrice, Sacher Fréderic, Bordachar Pierre, Klein George, Weerasooriya Rukshen, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008 Dec 9;118 (24):2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 5.Oral Hakan, Pappone Carlo, Chugh Aman, Good Eric, Bogun Frank, Pelosi Frank, Bates Eric R, Lehmann Michael H, Vicedomini Gabriele, Augello Giuseppe, Agricola Eustachio, Sala Simone, Santinelli Vincenzo, Morady Fred. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 2006 Mar 2;354 (9):934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 6.Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 7.Krittayaphong Rungroj, Raungrattanaamporn Ongkarn, Bhuripanyo Kiertijai, Sriratanasathavorn Charn, Pooranawattanakul Sukanya, Punlee Kesaree, Kangkagate Charuwan. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai. 2003 May;86 Suppl 1 ():S8–16. [PubMed] [Google Scholar]
- 8.Noheria Amit, Kumar Abhishek, Wylie John V, Josephson Mark E. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch. Intern. Med. 2008 Mar 24;168 (6):581–6. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 9.Stabile Giuseppe, Bertaglia Emanuele, Senatore Gaetano, De Simone Antonio, Zoppo Franco, Donnici Giovanni, Turco Pietro, Pascotto Pietro, Fazzari Massimo, Vitale Dino Franco. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur. Heart J. 2006 Jan;27 (2):216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 10.Piccini Jonathan P, Lopes Renato D, Kong Melissa H, Hasselblad Vic, Jackson Kevin, Al-Khatib Sana M. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 11.Piccini Jonathan P, Lopes Renato D, Kong Melissa H, Hasselblad Vic, Jackson Kevin, Al-Khatib Sana M. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 12.Calkins Hugh, Reynolds Matthew R, Spector Peter, Sondhi Manu, Xu Yingxin, Martin Amber, Williams Catherine J, Sledge Isabella. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 13.Kobza Richard, Hindricks Gerhard, Tanner Hildegard, Schirdewahn Petra, Dorszewski Anja, Piorkowski Christopher, Gerds-Li Jin-Hong, Kottkamp Hans. Late recurrent arrhythmias after ablation of atrial fibrillation: incidence, mechanisms, and treatment. Heart Rhythm. 2004 Dec;1 (6):676–83. doi: 10.1016/j.hrthm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Verma Atul, Kilicaslan Fethi, Pisano Ennio, Marrouche Nassir F, Fanelli Raffaele, Brachmann Johannes, Geunther Jens, Potenza Domenico, Martin David O, Cummings Jennifer, Burkhardt J David, Saliba Walid, Schweikert Robert A, Natale Andrea. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005 Aug 2;112 (5):627–35. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 15.Callans David J, Gerstenfeld Edward P, Dixit Sanjay, Zado Erica, Vanderhoff Mark, Ren Jian-Fang, Marchlinski Francis E. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1050–5. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 16.Gerstenfeld Edward P, Callans David J, Dixit Sanjay, Zado Erica, Marchlinski Francis E. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J. Cardiovasc. Electrophysiol. 2003 Jul;14 (7):685–90. doi: 10.1046/j.1540-8167.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 17.Nanthakumar Kumaraswamy, Plumb Vance J, Epstein Andrew E, Veenhuyzen George D, Link Dale, Kay G Neal. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004 Mar 16;109 (10):1226–9. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 18.Hussein Ayman A, Saliba Walid I, Martin David O, Bhargava Mandeep, Sherman Minerva, Magnelli-Reyes Christina, Chamsi-Pasha Mohammed, John Seby, Williams-Adrews Michelle, Baranowski Bryan, Dresing Thomas, Callahan Thomas, Kanj Mohamed, Tchou Patrick, Lindsay Bruce D, Natale Andrea, Wazni Oussama. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):271–8. doi: 10.1161/CIRCEP.111.962100. [DOI] [PubMed] [Google Scholar]
- 19.Tzou Wendy S, Marchlinski Francis E, Zado Erica S, Lin David, Dixit Sanjay, Callans David J, Cooper Joshua M, Bala Rupa, Garcia Fermin, Hutchinson Mathew D, Riley Michael P, Verdino Ralph, Gerstenfeld Edward P. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):237–42. doi: 10.1161/CIRCEP.109.923771. [DOI] [PubMed] [Google Scholar]
- 20.Lin David, Santangeli Pasquale, Zado Erica S, Bala Rupa, Hutchinson Mathew D, Riley Michael P, Frankel David S, Garcia Fermin, Dixit Sanjay, Callans David J, Marchlinski Francis E. Electrophysiologic findings and long-term outcomes in patients undergoing third or more catheter ablation procedures for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2015 Apr;26 (4):371–7. doi: 10.1111/jce.12603. [DOI] [PubMed] [Google Scholar]
- 21.Bhargava Mandeep, Di Biase Luigi, Mohanty Prasant, Prasad Subramanyam, Martin David O, Williams-Andrews Michelle, Wazni Oussama M, Burkhardt J David, Cummings Jennifer E, Khaykin Yaariv, Verma Atul, Hao Steven, Beheiry Salwa, Hongo Richard, Rossillo Antonio, Raviele Antonio, Bonso Aldo, Themistoclakis Sakis, Stewart Kelly, Saliba Walid I, Schweikert Robert A, Natale Andrea. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009 Oct;6 (10):1403–12. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Di Biase Luigi, Conti Sergio, Mohanty Prasant, Bai Rong, Sanchez Javier, Walton David, John Annie, Santangeli Pasquale, Elayi Claude S, Beheiry Salwa, Gallinghouse G Joseph, Mohanty Sanghamitra, Horton Rodney, Bailey Shane, Burkhardt J David, Natale Andrea. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011 Mar;8 (3):368–72. doi: 10.1016/j.hrthm.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson Mathew D, Garcia Fermin C, Mandel Jeff E, Elkassabany Nabil, Zado Erica S, Riley Michael P, Cooper Joshua M, Bala Rupa, Frankel David S, Lin David, Supple Gregory E, Dixit Sanjay, Gerstenfeld Edward P, Callans David J, Marchlinski Francis E. Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm. 2013 Mar;10 (3):347–53. doi: 10.1016/j.hrthm.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 24.Piorkowski Christopher, Eitel Charlotte, Rolf Sascha, Bode Kerstin, Sommer Philipp, Gaspar Thomas, Kircher Simon, Wetzel Ulrike, Parwani Abdul Shokor, Boldt Leif-Hendrik, Mende Meinhard, Bollmann Andreas, Husser Daniela, Dagres Nikolaos, Esato Masahiro, Arya Arash, Haverkamp Wilhelm, Hindricks Gerhard. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol. 2011 Apr;4 (2):157–65. doi: 10.1161/CIRCEP.110.957761. [DOI] [PubMed] [Google Scholar]
- 25.Sciarra Luigi, Golia Paolo, Natalizia Andrea, De Ruvo Ermenegildo, Dottori Serena, Scarà Antonio, Borrelli Alessio, De Luca Lucia, Rebecchi Marco, Fagagnini Alessandro, Bandini Alberto, Guarracini Fabrizio, Galvani Marcello, Calò Leonardo. Which is the best catheter to perform atrial fibrillation ablation? A comparison between standard ThermoCool, SmartTouch, and Surround Flow catheters. J Interv Card Electrophysiol. 2014 Apr;39 (3):193–200. doi: 10.1007/s10840-014-9874-2. [DOI] [PubMed] [Google Scholar]
- 26.Park Chan-Il, Lehrmann Heiko, Keyl Cornelius, Weber Reinhold, Schiebeling Jochen, Allgeier Juergen, Schurr Patrick, Shah Ashok, Neumann Franz-Josef, Arentz Thomas, Jadidi Amir S. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J. Cardiovasc. Electrophysiol. 2014 Jul;25 (7):701–8. doi: 10.1111/jce.12396. [DOI] [PubMed] [Google Scholar]
- 27.Natale Andrea, Reddy Vivek Y, Monir George, Wilber David J, Lindsay Bruce D, McElderry H Thomas, Kantipudi Charan, Mansour Moussa C, Melby Daniel P, Packer Douglas L, Nakagawa Hiroshi, Zhang Baohui, Stagg Robert B, Boo Lee Ming, Marchlinski Francis E. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J. Am. Coll. Cardiol. 2014 Aug 19;64 (7):647–56. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 28.Kühne Michael, Suter Yves, Altmann David, Ammann Peter, Schaer Beat, Osswald Stefan, Sticherling Christian. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010 Dec;7 (12):1770–6. doi: 10.1016/j.hrthm.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Chen Songwen, Meng Weidong, Sheng He Ding, Chen Gang, Zhang Feng, Yan Yiwen, Zhu-Ge Ying, Liu Shaowen. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol. 2012 May;35 (5):524–31. doi: 10.1111/j.1540-8159.2012.03343.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Kohki, Naito Shigeto, Kaseno Kenichi, Tsukada Naofumi, Sasaki Takehito, Hayano Mamoru, Nishiuchi Suguru, Fuke Etsuko, Miki Yuko, Sakamoto Tamotsu, Nakamura Keijiro, Kumagai Koji, Kataoka Akihisa, Takaoka Hiroyuki, Kobayashi Yoshio, Funabashi Nobusada, Oshima Shigeru. Optimal observation time after completion of circumferential pulmonary vein isolation for atrial fibrillation to prevent chronic pulmonary vein reconnections. Int. J. Cardiol. 2013 Oct 15;168 (6):5300–10. doi: 10.1016/j.ijcard.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Steven Daniel, Sultan Arian, Reddy Vivek, Luker Jakob, Altenburg Manuel, Hoffmann Boris, Rostock Thomas, Servatius Helge, Stevenson William G, Willems Stephan, Michaud Gregory F. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J. Am. Coll. Cardiol. 2013 Jul 2;62 (1):44–50. doi: 10.1016/j.jacc.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Cheung Jim W, Lin Frank S, Ip James E, Bender Seth R, Siddiqi Faisal K, Liu Christopher F, Thomas George, Markowitz Steven M, Lerman Bruce B. Adenosine-induced pulmonary vein ectopy as a predictor of recurrent atrial fibrillation after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2013 Dec;6 (6):1066–73. doi: 10.1161/CIRCEP.113.000796. [DOI] [PubMed] [Google Scholar]
- 33.McLellan Alex J A, Kumar Saurabh, Smith Catherine, Morton Joseph B, Kalman Jonathan M, Kistler Peter M. The role of adenosine following pulmonary vein isolation in patients undergoing catheter ablation for atrial fibrillation: a systematic review. J. Cardiovasc. Electrophysiol. 2013 Jul;24 (7):742–51. doi: 10.1111/jce.12121. [DOI] [PubMed] [Google Scholar]
- 34.Macle Laurent, Khairy Paul, Weerasooriya Rukshen, Novak Paul, Verma Atul, Willems Stephan, Arentz Thomas, Deisenhofer Isabel, Veenhuyzen George, Scavée Christophe, Jaïs Pierre, Puererfellner Helmut, Levesque Sylvie, Andrade Jason G, Rivard Lena, Guerra Peter G, Dubuc Marc, Thibault Bernard, Talajic Mario, Roy Denis, Nattel Stanley. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015 Aug 15;386 (9994):672–9. doi: 10.1016/S0140-6736(15)60026-5. [DOI] [PubMed] [Google Scholar]
- 35.Mainigi Sumeet K, Sauer William H, Cooper Joshua M, Dixit Sanjay, Gerstenfeld Edward P, Callans David J, Russo Andrea M, Verdino Ralph J, Lin David, Zado Erica S, Marchlinski Francis E. Incidence and predictors of very late recurrence of atrial fibrillation after ablation. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):69–74. doi: 10.1111/j.1540-8167.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 36.Takigawa Masateru, Takahashi Atsushi, Kuwahara Taishi, Okubo Kenji, Takahashi Yoshihide, Nakashima Emiko, Watari Yuji, Yamao Kazuya, Nakajima Jun, Takagi Katsumasa, Kimura Shigeki, Hikita Hiroyuki, Hirao Kenzo, Isobe Mitsuaki. Impact of Non-Pulmonary Vein Foci on the Outcome of the Second Session of Catheter Ablation for Paroxysmal Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2015 Jul;26 (7):739–46. doi: 10.1111/jce.12681. [DOI] [PubMed] [Google Scholar]
- 37.Sawhney Navinder, Anousheh Ramtin, Chen Wei, Feld Gregory K. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):243–8. doi: 10.1161/CIRCEP.109.924878. [DOI] [PubMed] [Google Scholar]
- 38.Bunch T Jared, Cutler Michael J. Is pulmonary vein isolation still the cornerstone in atrial fibrillation ablation? J Thorac Dis. 2015 Feb;7 (2):132–41. doi: 10.3978/j.issn.2072-1439.2014.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oral Hakan, Chugh Aman, Good Eric, Igic Petar, Elmouchi Darryl, Tschopp David R, Reich S Scott, Bogun Frank, Pelosi Frank, Morady Fred. Randomized comparison of encircling and nonencircling left atrial ablation for chronic atrial fibrillation. Heart Rhythm. 2005 Nov;2 (11):1165–72. doi: 10.1016/j.hrthm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Bai Rong, Di Biase Luigi, Mohanty Prasant, Trivedi Chintan, Dello Russo Antonio, Themistoclakis Sakis, Casella Michela, Santarelli Pietro, Fassini Gaetano, Santangeli Pasquale, Mohanty Sanghamitra, Rossillo Antonio, Pelargonio Gemma, Horton Rodney, Sanchez Javier, Gallinghouse Joseph, Burkhardt J David, Ma Chang-Sheng, Tondo Claudio, Natale Andrea. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016 Jan;13 (1):132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 2;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 42.Verma Atul, Sanders Prashanthan, Champagne Jean, Macle Laurent, Nair Girish M, Calkins Hugh, Wilber David J. Selective complex fractionated atrial electrograms targeting for atrial fibrillation study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):55–62. doi: 10.1161/CIRCEP.113.000890. [DOI] [PubMed] [Google Scholar]
- 43.Dixit Sanjay, Marchlinski Francis E, Lin David, Callans David J, Bala Rupa, Riley Michael P, Garcia Fermin C, Hutchinson Mathew D, Ratcliffe Sarah J, Cooper Joshua M, Verdino Ralph J, Patel Vickas V, Zado Erica S, Cash Nancy R, Killian Tony, Tomson Todd T, Gerstenfeld Edward P. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol. 2012 Apr;5 (2):287–94. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 44.Verma Atul, Jiang Chen-yang, Betts Timothy R, Chen Jian, Deisenhofer Isabel, Mantovan Roberto, Macle Laurent, Morillo Carlos A, Haverkamp Wilhelm, Weerasooriya Rukshen, Albenque Jean-Paul, Nardi Stefano, Menardi Endrj, Novak Paul, Sanders Prashanthan. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015 May 7;372 (19):1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 45.Katritsis Demosthenes G, Giazitzoglou Eleftherios, Zografos Theodoros, Pokushalov Evgeny, Po Sunny S, Camm A John. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011 May;8 (5):672–8. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 46.Skanes A C, Mandapati R, Berenfeld O, Davidenko J M, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998 Sep 22;98 (12):1236–48. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 47.Ryu Kyungmoo, Shroff Sunil C, Sahadevan Jayakumar, Martovitz Nichole L, Khrestian Celeen M, Stambler Bruce S. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: evidence for a role of driver regions. J. Cardiovasc. Electrophysiol. 2005 Dec;16 (12):1348–58. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 48.Shivkumar Kalyanam, Ellenbogen Kenneth A, Hummel John D, Miller John M, Steinberg Jonathan S. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J. Cardiovasc. Electrophysiol. 2012 Dec;23 (12):1277–85. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianni Carola, Mohanty Sanghamitra, Di Biase Luigi, Metz Tamara, Trivedi Chintan, Gökoğlan Yalçın, Güneş Mahmut F, Bai Rong, Al-Ahmad Amin, Burkhardt J David, Gallinghouse G Joseph, Horton Rodney P, Hranitzky Patrick M, Sanchez Javier E, Halbfaß Phillipp, Müller Patrick, Schade Anja, Deneke Thomas, Tomassoni Gery F, Natale Andrea. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm. 2016 Apr;13 (4):830–5. doi: 10.1016/j.hrthm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 51.Buch Eric, Share Michael, Tung Roderick, Benharash Peyman, Sharma Parikshit, Koneru Jayanthi, Mandapati Ravi, Ellenbogen Kenneth A, Shivkumar Kalyanam. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm. 2016 Mar;13 (3):636–41. doi: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rolf Sascha, Kircher Simon, Arya Arash, Eitel Charlotte, Sommer Philipp, Richter Sergio, Gaspar Thomas, Bollmann Andreas, Altmann David, Piedra Carlos, Hindricks Gerhard, Piorkowski Christopher. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Oct;7 (5):825–33. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 53.Jadidi Amir S, Lehrmann Heiko, Keyl Cornelius, Sorrel Jérémie, Markstein Viktor, Minners Jan, Park Chan-Il, Denis Arnaud, Jaïs Pierre, Hocini Mélèze, Potocnik Clemens, Allgeier Juergen, Hochholzer Willibald, Herrera-Sidloky Claudia, Kim Steve, Omri Youssef El, Neumann Franz-Josef, Weber Reinhold, Haïssaguerre Michel, Arentz Thomas. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circ Arrhythm Electrophysiol. 2016 Mar;9 (3) doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 54.Pokushalov Evgeny, Romanov Alexander, Artyomenko Sergey, Baranova Vera, Losik Denis, Bairamova Sevda, Karaskov Alexander, Mittal Suneet, Steinberg Jonathan S. Cryoballoon versus radiofrequency for pulmonary vein re-isolation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013 Mar;24 (3):274–9. doi: 10.1111/jce.12038. [DOI] [PubMed] [Google Scholar]
- 55.Ranjan Ravi, Kato Ritsushi, Zviman Menekhem M, Dickfeld Timm M, Roguin Ariel, Berger Ronald D, Tomaselli Gordon F, Halperin Henry R. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):279–86. doi: 10.1161/CIRCEP.110.960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ranjan Ravi, Kholmovski Eugene G, Blauer Joshua, Vijayakumar Sathya, Volland Nelly A, Salama Mohamed E, Parker Dennis L, MacLeod Rob, Marrouche Nassir F. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ Arrhythm Electrophysiol. 2012 Dec;5 (6):1130–5. doi: 10.1161/CIRCEP.112.973164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vergara Gaston R, Marrouche Nassir F. Tailored management of atrial fibrillation using a LGE-MRI based model: from the clinic to the electrophysiology laboratory. J. Cardiovasc. Electrophysiol. 2011 Apr;22 (4):481–7. doi: 10.1111/j.1540-8167.2010.01941.x. [DOI] [PubMed] [Google Scholar]
- 58.Bisbal Felipe, Guiu Esther, Cabanas-Grandío Pilar, Berruezo Antonio, Prat-Gonzalez Susana, Vidal Bárbara, Garrido Cesar, Andreu David, Fernandez-Armenta Juan, Tolosana Jose María, Arbelo Elena, de Caralt Teresa M, Perea Rosario J, Brugada Josep, Mont Lluís. CMR-guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc Imaging. 2014 Jul;7 (7):653–63. doi: 10.1016/j.jcmg.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Knecht S, Sohal M, Arentz T, Jadidi A, Rostock T, Deisendorfer IV, Cauchemez B, Albenque JP, Neumann T, Ernst S, Packer D, Tavernier R, Duytschaever M. Noninvasive mapping prior to ablation for persistent atrial fibrillation: The AFACART multicenter study (PO 06-52). Heart Rhythm. 2015;0:0–0. [Google Scholar]


