Abstract
Objective:
To study whether physical activity during adulthood or early life is associated with multiple sclerosis (MS) incidence in 2 prospective cohorts of women.
Methods:
Women in the Nurses' Health Study (NHS) (n = 81,723; 1986–2004) and NHS II (n = 111,804; 1989–2009) reported recent physical activity at baseline and in selected follow-up questionnaires. Using this information, we calculated total metabolic equivalent hours of physical activity per week, a measure of energy expenditure. There were 341 confirmed MS cases with first symptoms after baseline. Participants also reported early-life activity. To estimate relative rates (RRs) and 95% confidence intervals (CIs), we used Cox proportional hazards models, adjusting for age, latitude of residence at age 15, ethnicity, smoking, supplemental vitamin D, and body mass index at age 18.
Results:
Compared with women in the lowest baseline physical activity quartile, women in the highest quartile had a 27% reduced rate of MS (RRpooled = 0.73, 95% CI 0.55–0.98; p-trend 0.08); this trend was not present in 6-year lagged analyses. Change in physical activity analyses suggested that women reduced activity before onset of MS symptoms. In NHS and NHS II, higher strenuous activity at ages 18–22 years was weakly associated with a decreased MS rate. However, in NHS II, total early-life activity at ages 12–22 was not associated with MS.
Conclusions:
Though higher physical activity at baseline was weakly associated with lower MS risk, this may have been due to women reducing physical activity in response to subclinical MS.
Clinical research has found that among patients with multiple sclerosis (MS) with mild or moderate disability, exercise improves muscular strength and aerobic capacity and may improve mobility, fatigue, and health-related quality of life.1 Previous work has suggested exercise or physical activity might even slow progression of MS, though evidence is inconsistent.2 Exercise could potentially modify MS disease activity via multiple mechanisms, including modulating immune factors and stress hormones and mediating expression of neuronal growth factors.3,4
Little research has assessed the association of physical activity with the risk of incident MS.5–7 Two case-control studies found that, before diagnosis, individuals with MS tended to be more physically active than controls.5,6 Another found no difference in pre-onset physical activity levels between cases and controls.7 These studies, however, collected physical activity exposure after MS diagnosis and did not use detailed or validated questionnaires to assess physical activity. Given this background, we prospectively examined the association between physical activity during early life and during adulthood with MS incidence in 2 large cohorts of US women: the Nurses' Health Study (NHS) and the NHS II (NHS II).
METHODS
Participants.
NHS began in 1976; investigators recruited 121,701 female nurses, 30–55 years old. NHS II began in 1989; investigators recruited 116,430 female nurses, 25–42 years old. Every 2 years, women complete a questionnaire to update lifestyle exposures and medical conditions. The first assessment of current levels of physical activity was in 1986 in NHS and 1989 in NHS II; the dates of return of these questionnaires were the start of follow-up (baseline) for each cohort, respectively. We excluded participants who were missing or had incomplete baseline physical activity or extreme baseline physical activity values (>4 h/d8 or ≥125 metabolic equivalent [MET]–h/wk). After exclusions, there were 81,723 women in NHS and 111,804 women in NHS II.
Standard protocol approvals, registrations, and patient consents.
The institutional review board of Brigham and Women's Hospital approved this study.
Ascertainment of MS.
Participants with newly diagnosed MS self-report their diagnoses on biennial questionnaires. We asked women for permission to contact their neurologists for medical records, clinical history (including diagnosis date and date of first symptoms), and the neurologist's certainty of diagnosis (definite, probable, possible, not MS). Since 2003, the study neurologist (T.C.) has reviewed all medical records. If a neurologist did not respond, we sent a questionnaire to the participant's internist. For 90% of women with MS, the treating physician was a neurologist. In most cases, diagnosis was supported by MRI findings (>70% NHS; >90% NHS II). For these analyses, we defined confirmed cases as those with definite or probable MS according to neurologist or internist and with onset of first neurologic symptoms after baseline, in an effort to avoid the potential effect of reverse causation.9 There were 50 cases of MS (28 definite, 22 probable) in NHS and 291 cases (200 definite, 91 probable) in NHS II with first MS symptoms after baseline that had complete information on baseline physical activity, yielding 341 MS cases.
Assessment of physical activity.
At baseline, participants reported average time per week (7 categories, 0–≥11 hours) spent during the previous year participating in the following activities: walking or hiking outdoors; jogging (>10 minutes per mile); running (≤10 minutes per mile); bicycling; lap swimming; tennis, squash, or racquetball; calisthenics, aerobics, aerobic dance, or rowing machine; and (NHS II only) other aerobic recreation. They also reported usual outdoor walking pace (easy [<2 miles per hour (mph)], average [2–2.9 mph], brisk [3–3.9 mph], very brisk [≥4 mph]) and average flights of stairs walked daily (categories from ≤2 to >15). Similar questions were asked in NHS in 1988, 1992, 1994, 1996, 1998, and 2000 and in NHS II in 1991, 1997, 2001, and 2005. Based on reported hours of participation and assigned MET score10 for each activity, total MET-h/wk, a measure of energy expenditure, was derived.11
In a validation study of a random sample from NHS II, participants completed past-week activity recalls and 1-week activity diaries 4 times throughout 1 year.12 Among 149 participants, correlations between questionnaire-reported activity and that reported by past-week activity recalls and activity diaries were 0.79 and 0.62, respectively.12 The correlation between questionnaires administered 2 years apart was 0.59.
In 1988 (NHS) and 1989 (NHS II), women provided information about early-life physical activity: the number of months per year (0, 1–3, 4–6, 7–9, 10–12) they engaged in strenuous aerobic physical activity at least twice per week during high school (ages 14–17; NHS II) and between the ages of 18 and 22 (NHS/NHS II). In NHS II, in 1997, women reported more detailed early-life activity, specifically the average time per week (categories, 0–≥11 hours) spent walking to/from school/work, doing moderate recreational activity, and doing strenuous recreational activity in grades 7–8 (ages 12–13), grades 9–12 (ages 14–17), and at ages 18–22. For each age period, we created a total MET-h/week activity score weighted by intensity.13 In analyses, we used all early-life activity assessments (NHS [1988] and NHS II [1989, 1997]).
Covariates.
Women reported information on state of residence at age 15 (1992, NHS; 1993, NHS II) and ancestry (1992, NHS; 1989, NHS II; South European, Scandinavian, other Caucasian, African American, Hispanic, Asian, Native American [NHS only], other) and were categorized as previously described.9 Women updated smoking status every 2 years; pack-years of smoking were derived from this information. Women completed comprehensive validated semiquantitative food frequency questionnaires14 every 4 years and supplemental vitamin D intake (IU/d) was derived using reported intake. In 1980 (NHS) and 1989 (NHS II), women reported their current height and weight at age 18; body mass index (BMI) at age 18 was calculated by dividing age 18 weight (in kilograms) by reported height (in meters) squared.
Statistical analyses.
For adult physical activity, person-time accrued from the date of return of the first physical activity questionnaire (baseline) to the date of first symptoms of MS, death from any cause, or loss to or end of follow-up (May 31, 2004, for NHS and May 31, 2009, for NHS II), whichever occurred first. For early-life activity, however, we followed women until date of MS diagnosis, rather than first MS symptoms, as most women would not have MS symptoms in early life, thereby minimizing concerns about reverse causation. In sensitivity analyses, we used date of first MS symptoms. We used Cox proportional hazards models to estimate relative rates (RR). To account for potential confounding by age and time, models were stratified by 5-year age categories and 2-year time intervals. We additionally adjusted for other potential MS risk factors defined above.15–17 We used missing indicators for any covariates with missing information. In secondary analyses, we further adjusted for ultraviolet B (UVB) flux in state of residence in 1986 (NHS) or 1989 (NHS II).
Trend tests were carried out by modeling median values of physical activity quartiles as a continuous variable. We included product terms of exposure and age groups to test for violations of the proportional hazards assumption of the Cox models. We carried out analyses separately in each cohort and pooled RRs using a fixed effects model with the inverse of the variance of the rate estimates as the weight; we used a Q statistic to assess estimate heterogeneity.18 We conducted all analyses using SAS, version 9.3. All p values are 2-sided.
Adult physical activity.
The main physical activity exposure was quartiles of baseline MET-h/wk. We also examined the association of incident MS with quartiles of cumulative average MET-h/wk, derived from all available activity questionnaires up to the start of each 2-year follow-up interval.19 As women may have changed physical activity in response to subclinical MS, we performed lagged analyses of baseline and cumulative average physical activity by excluding the first 4 and 6 years of follow-up. We chose these lag periods to assess potential influence of subclinical MS on physical activity levels, while maintaining sufficient cases to have reasonable power. We also separately assessed associations between baseline vigorous (≥6 METs) activity (jogging; running; bicycling; lap swimming; tennis, squash, or racquetball; calisthenics, aerobics, aerobic dance, or rowing machine; and stair climbing)20 and moderate (2.5–4.5 METs) activity (walking or hiking outdoors) with incident MS.
Early-life activity.
Strenuous early-life activity (months per year of strenuous activity reported in NHS [1988] and NHS II [1989]) was categorized as 0, 1–3, 4–6, or 7–12 months per year. There were 130 cases of MS (84 definite, 46 probable; ages 18–22) in NHS and 445 cases (320 definite and 125 probable; high school and ages 18–22) in NHS II with diagnosis of MS after 1986 or 1989, respectively, yielding 575 MS cases for strenuous activity analyses at ages 18–22. For analyses of total early-life physical activity reported in 1997 in NHS II, we categorized total activity during each age period into previously used categories (<21, 21–36, 36–48, 48–72, ≥72 MET-h/wk)13 and started follow-up in 1997; there were 195 cases (124 definite and 71 probable in NHS II) with date of MS diagnosis after the 1997 questionnaire.
Activity change analysis.
We assessed whether participants with MS changed activity levels after MS onset. We calculated the difference between date of each questionnaire return and date of first symptoms (and separately for date of diagnosis). For each questionnaire cycle, we standardized total physical activity (MET-h/wk) as the percentage of the mean total activity of individuals who did not develop MS during follow-up. For years in which physical activity was not asked (NHS: 1990, 2002, 2006; NHS II: 1993, 1999, 2005, 2009), we averaged activity from the closest questionnaire cycles.21 We fit linear regression models separately in each cohort, regressing percent physical activity on age, age2, pack-years, latitude of residence at age 15, ethnicity, BMI at age 18, and supplemental vitamin D intake. For each patient, the residual from this model is the difference between her standardized activity and the average standardized activity of participants without MS of the same age and with the same other characteristics at the same questionnaire cycle. We calculated mean residuals for cases for each questionnaire period in both NHS and NHS II cohorts combined and plotted mean residuals according to time from first symptoms (or diagnosis). We used generalized estimating equations to test the linearity of physical activity changes over time. We conducted all analyses using SAS, version 9.3 (SAS Institute, Cary, NC).
RESULTS
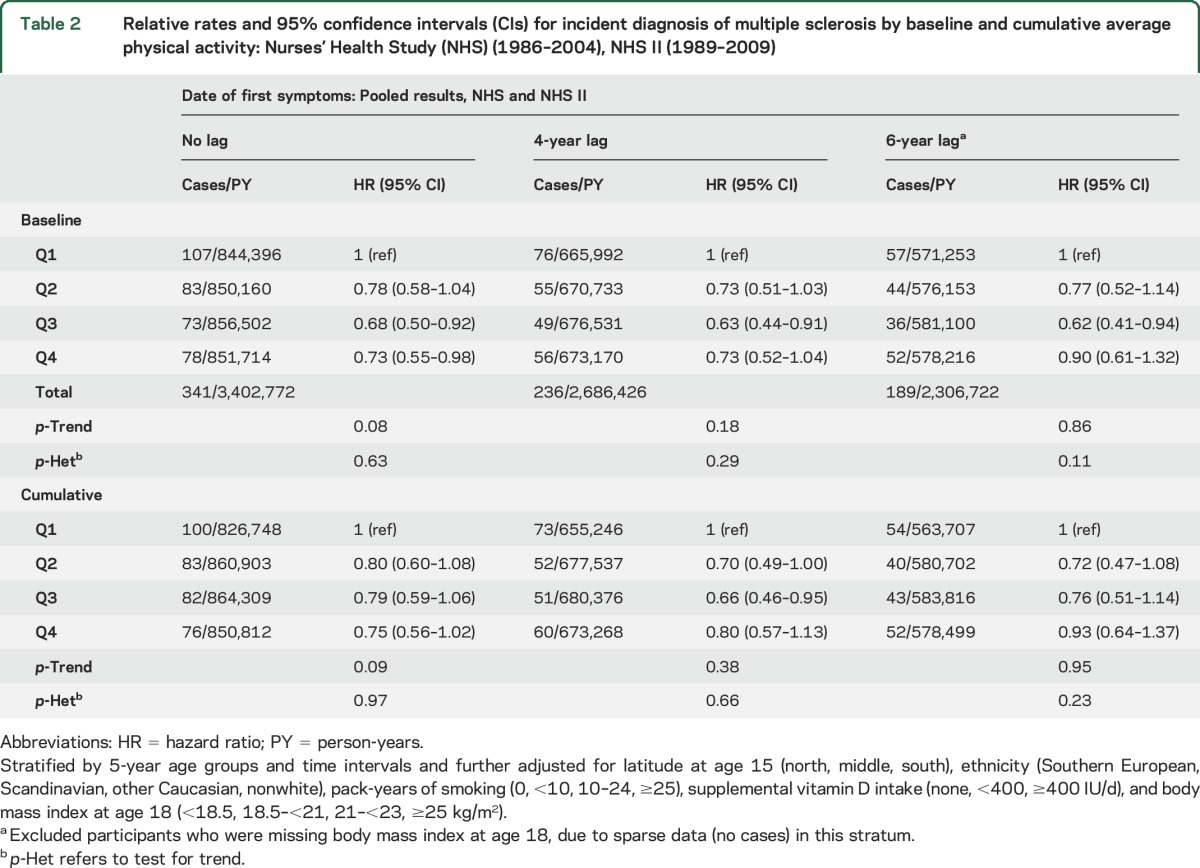
Compared with women who were less active at baseline, the most active participants had higher intake of supplemental vitamin D (table 1). We followed NHS participants for up to 18 years (average 16.7 years) and NHS II participants for up to 20 years (average 18.2 years). Total baseline physical activity and cumulative average physical activity were associated with a reduced MS rate (table 2). Compared with women in the lowest quartile of baseline activity, those in the highest quartile had a 27% reduced rate of MS (table 2). In pooled analyses, higher baseline vigorous activity was associated with only a modest and nonsignificant reduction in MS incidence (RR, comparing women in the highest quartile of vigorous activity with those in the lowest, 0.85; 95% confidence interval [CI] 0.62–1.17; p-trend 0.21). There was no association between moderate activity and incident MS (RR 0.90; 95% CI 0.66–1.22; p-trend 0.34). When we carried out 6-year lagged analyses, there was no longer a trend of association between baseline activity and MS (table 2) or between vigorous activity and MS (p-trend 0.67). However, the pooled relative rates were still less than 1.
Table 1.
Selected baseline characteristics of 81,723 women in Nurses' Health Study (NHS) and 111,804 women in NHS II by total physical activity quartiles (1986 for NHS, 1989 for NHS II)
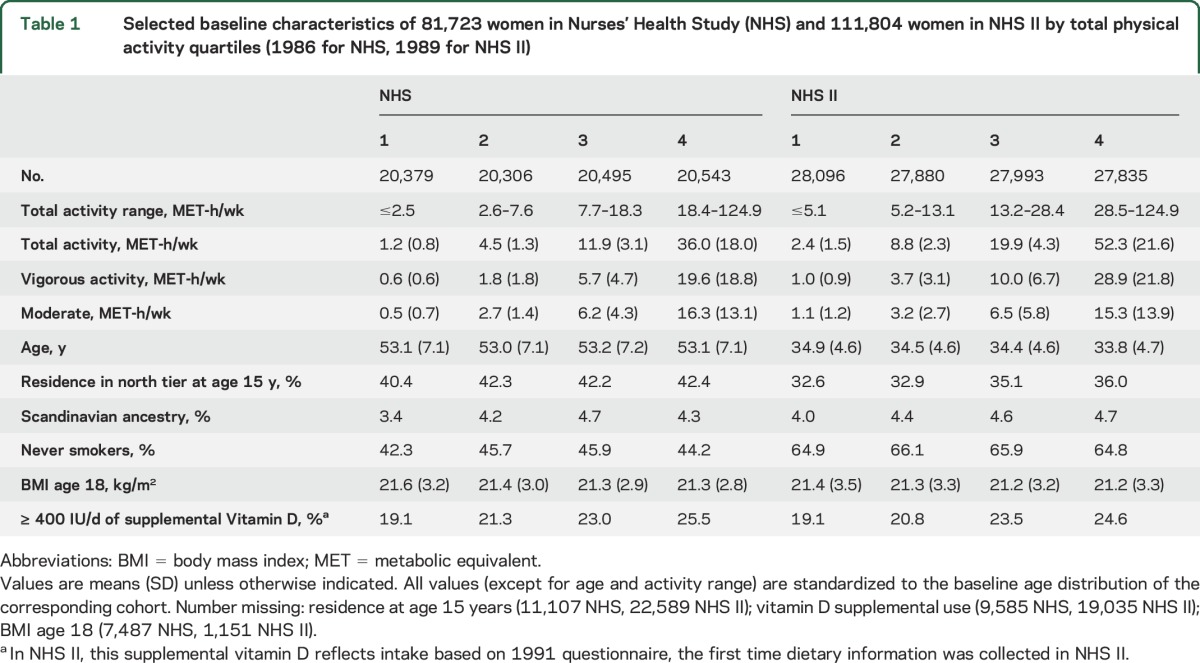
Table 2.
Relative rates and 95% confidence intervals (CIs) for incident diagnosis of multiple sclerosis by baseline and cumulative average physical activity: Nurses' Health Study (NHS) (1986–2004), NHS II (1989–2009)
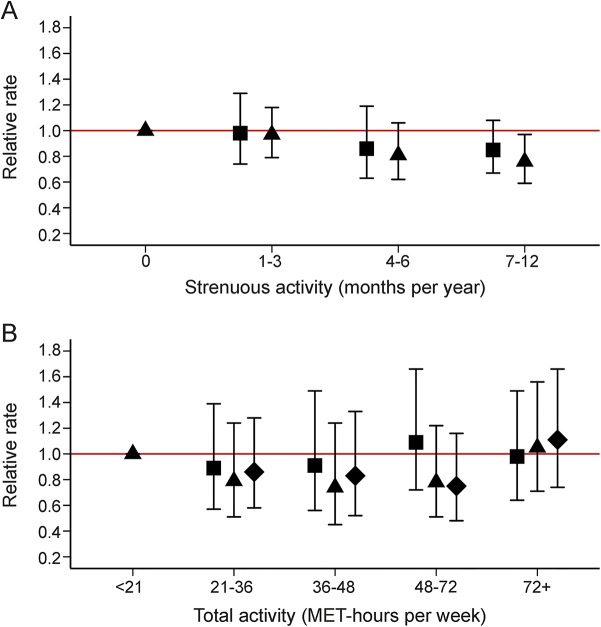
In pooled analyses, MS risk decreased with increasing number of months of strenuous aerobic activity at ages 18–22 (figure 1A). However, in NHS II, higher levels of activity (MET-h/wk) reported during ages 12–13, 14–17, and 18–22 were not associated with MS (figure 1B); results were similar when we started follow-up in 1989 or when using date of first symptoms.
Figure 1. Relative rates and 95% confidence intervals for diagnosis of multiple sclerosis (MS) by early-life activity.
(A) Early-life strenuous activity and rate of MS. Months per year engaged in strenuous aerobic physical activity at least twice per week. Follow-up 1986–2004 in Nurses' Health Study (NHS); 1989–2009 in NHS II. Ages 14–17 (squares): 445 NHS II cases; p-trend 0.14. Ages 18–22 (triangles): 575 cases (130 NHS, 445 NHS II); p-trend 0.01, p-het 0.44. (B) NHS II early-life activity and rate of MS. Follow-up 1997–2009; 195 cases. Categories of metabolic equivalent (MET)–h/wk of total activity at the following ages: 12–13 (squares; p-trend 0.81), 14–17 (triangles; p-trend 0.45), 18–22 (diamonds; p-trend 0.73). Analyses stratified by 5-year age groups and time intervals and further adjusted for latitude at age 15 (north, middle, south), ethnicity (Southern European, Scandinavian, other Caucasian, nonwhite), pack-years of smoking (0, <10, 10–24, ≥25), supplemental vitamin D intake (none, <400, ≥400 IU/d), and body mass index at age 18 (<18.5, 18.5–<21, 21–<23, ≥25 kg/m2).
The association between baseline activity and MS was not materially changed when we adjusted for UVB flux. When we restricted analyses to definite cases in NHS II, results were similar for baseline and cumulative average exposures (sample size in NHS too small for a comparable analysis). Analyses of definite cases for early-life physical activity in NHS and NHS II also yielded similar results.
Change in physical activity analyses suggested that physical activity decreased among women with MS over time, relative to women without MS (figure 2). There was a decrease in activity up to 6 years before the first symptoms of MS.
Figure 2. Relative physical activity by time of multiple sclerosis (MS) symptoms or diagnosis.
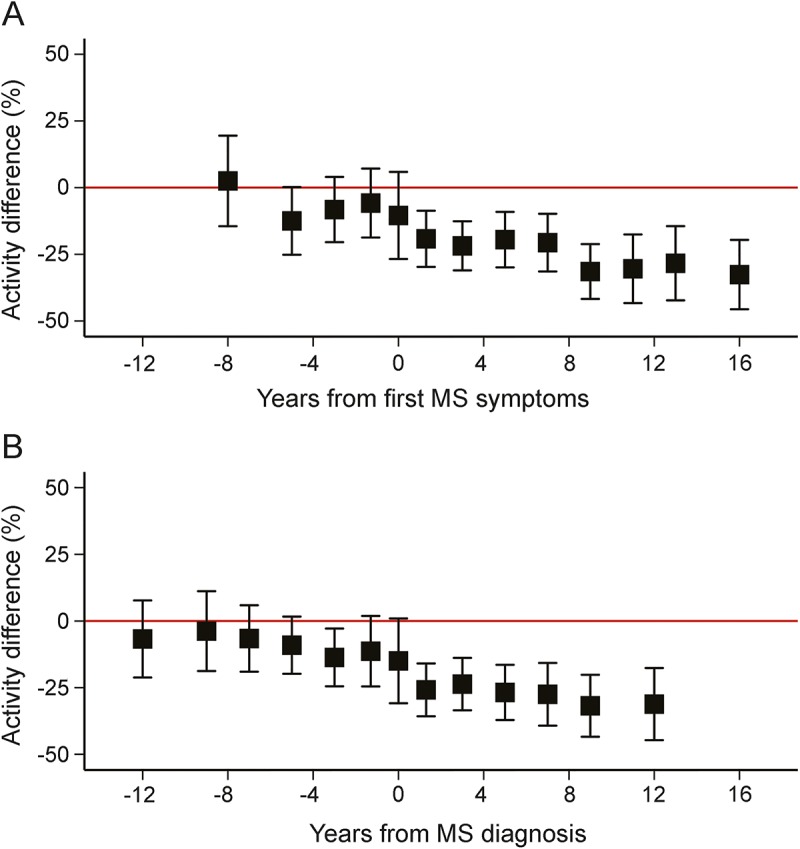
(A) Relative physical activity by time of MS symptoms. p-Trend overall: 0.0002. p-Trend up to and including the year of first symptoms: 0.64. p-Trend after first symptoms: 0.06. (B) Relative physical activity by time of MS diagnosis. p-Trend overall: 0.0003. p-Trend up to and including the year became a case: 0.35. p-Trend after becoming a case: 0.56. Adjusted for age (age, age2), latitude at age 15 (north, middle, south), ethnicity (Southern European, Scandinavian, other Caucasian, nonwhite), pack-years of smoking (0, <10, 10–24, ≥25), cumulative average supplemental vitamin D intake (none, <400, ≥400 IU/d), and body mass index at age 18 (<18.5, 18.5–<21, 21–<23, ≥25 kg/m2).
DISCUSSION
In this large, longitudinal study, women who reported higher average physical activity levels in adulthood had a lower risk of MS than less active women. However, this trend was not present in 6-year lagged analyses, suggesting lower physical activity could have been an early sign, rather than cause, of MS. There was no consistent association of early-life activity with MS. Furthermore, we observed decreased physical activity at or before MS onset.
In an Israeli study, compared to controls from the 1961 census, 241 individuals with prevalent MS residing in Israel in 1966 did not report different physical activity levels before MS onset.7 In a study of 100 MS cases in Canada, cases were more likely to report spending leisure time doing physical activities before the onset of MS symptoms than were controls (patients with rheumatoid arthritis).5 In another more recent Canadian study, newly diagnosed patients with MS reported being more physically active (relative to the general population of the same age and sex) during the year before MS diagnosis than did controls.6 These studies might have been biased due to recall bias, selection bias, and exposure measurement error. In addition, none of the studies used detailed or validated questionnaires to assess physical activity.
In contrast, our study consisted of 2 large cohorts with long follow-up. Though information on leisure-time physical activity was self-reported, it was collected via structured questionnaires. Physical activity as assessed by these questionnaires has been associated with a decreased risk of coronary heart disease,22 stroke,23 diabetes,24 and cancer.13,25–27 Adult physical activity exposures were prospectively reported and have been validated.12,28 We were also able to assess physical activity changes before and after reported onset of MS symptoms and MS diagnosis.
The biological mechanisms through which physical activity could relate to MS risk are unknown. Exercise could potentially modify MS disease activity by mediating expression of neuroactive proteins, such as insulin-like growth factor-I, which appears to be neuroprotective, and other neurotrophic factors that are likely involved in neuronal survival and activity-dependent plasticity,3 as well as via effects on inflammation, stress hormones, and immune function.4 These same mechanisms could also potentially influence the development of MS.
Evidence suggests that relative to nondiseased populations, patients with MS are less physically active.29,30 A recent study among 118 patients with MS found that, after diagnosis, 38% of participants stopped physical activity and many reduced frequency of activity.31 In our study, participants could have possibly reduced physical activity in response to prediagnosis symptoms.31 In an effort to address this, we carried out several analyses. Though total physical activity was weakly associated with a lower rate of MS in non-lagged analyses, this trend was no longer present when we carried out 6-year lagged analyses. We found no consistent pattern of association between early-life physical activity and incident MS, which does not support biological plausibility. Additionally, change in physical activity analyses suggested a decrease in activity at or before MS symptom onset. Overall, these results are supportive of women reducing physical activity in response to MS symptoms.
Limitations to our study include that early-life physical activity was not validated. Adult activity was not validated against accelerometry data or fitness in these cohorts. However, a study among middle-aged women reported good correlations of the NHS II questionnaire-estimated physical activity with average accelerometer counts (0.42) and estimated and measured maximal oxygen uptake (0.49, 0.54).28 In a validation study of men, there was a stronger correlation of physical activity estimated by questionnaire with that estimated by diary for vigorous activity than for moderate activity.20 As a result, we expect less measurement error for vigorous activity than for moderate or total activity analyses. Given the prospective nature of this study, we would expect any measurement error to be nondifferential.
Some MS cases could possibly have been misdiagnosed. However, analyses of baseline and cumulative average physical activity yielded similar results when we restricted to definite cases. All participants in our cohorts are registered nurses, likely with similar access to health care; of MS cases diagnosed after 1990, more than 90% have MRI findings supporting MS diagnosis. In addition, in these cohorts, the HLA-DRB1*1501 allele has been associated with the expected increased risk of MS.32 We cannot rule out possible bias due to confounding. In addition to vitamin D intake and smoking, other behaviors are likely related to physical activity. However, with the exception of Epstein-Barr virus antibody titers, 25-hydroxyvitamin D (25[OH]D) levels, and HLA-DRB1 genotype, we adjusted for all major known MS risk factors. In 25(OH)D prediction models developed in NHS and NHS II, physical activity quintiles were associated with higher predicted 25(OH)D levels (p < 0.0001).33 Though we did not adjust for 25(OH)D levels, we adjusted for supplemental vitamin D intake, which is associated with higher predicted 25(OH)D levels33 and MS risk.16 It remains unclear how much of the association between physical activity and MS might have been due to more active women having more sunlight exposure, a major vitamin D source,34 than less active women. When we adjusted for UVB flux, results for total baseline physical activity did not change materially. This study only includes women and over 95% were Caucasian, so our results may not be generalizable to men or other race groups.
In this large prospective study with validated measures of physical activity, we found weak and nonconvincing evidence that higher physical activity reduces the risk of incident MS. Although prior work has shown that physical activity has numerous health benefits,35 it seems unlikely that it protects against risk of developing MS. Our study does not directly address whether physical activity has any benefits in patients with MS, but physical activity should be recommended for individuals with MS because of its beneficial effects on cardiovascular outcomes, which are a major cause of morbidity and mortality in individuals with MS.
ACKNOWLEDGMENT
The authors thank the participants in the Nurses' Health Study and Nurses' Health Study II for their continuing cooperation and Leslie Unger for technical assistance.
GLOSSARY
- 25[OH]D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- MS
multiple sclerosis
- MET
metabolic equivalent
- mph
miles per hour
- NHS
Nurses' Health Study
- RR
relative rate
- UVB
ultraviolet B
AUTHOR CONTRIBUTIONS
K.L.M., A.A., K.S.D., and J.M. conceived of and designed the study. K.L.M., A.A., and T.C. acquired the data. K.S.D. and K.L.M. analyzed the data. K.S.D., K.L.M., and A.A. drafted the manuscript and figures. J.M. and T.C. provided critical revision of the manuscript.
STUDY FUNDING
Supported by the US National Institutes of Health (grants T32 HL007575, UM1 CA186107, UM1 CA176726, R01NS046635).
DISCLOSURE
K. Dorans reports support from research grants from the National Institutes of Health during the conduct of the study. J. Massa reports no disclosures relevant to the manuscript. T. Chitnis has received personal compensation for advisory board/consulting for Biogen-Idec, Genzyme-Sanofi, and Roche, and has received research support from Merck-Serono and Novartis Pharmaceuticals. A. Ascherio reports research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Department of Defense and served on a medical advisory board for Bayer HealthCare. K. Munger has received a research grant from the National Multiple Sclerosis Society. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil 2013;94:1800–1828. [DOI] [PubMed] [Google Scholar]
- 2.Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord 2012;5:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White LJ, Castellano V. Exercise and brain health: implications for multiple sclerosis: part 1: neuronal growth factors. Sports Med 2008;38:91–100. [DOI] [PubMed] [Google Scholar]
- 4.White LJ, Castellano V. Exercise and brain health: implications for multiple sclerosis: part II: immune factors and stress hormones. Sports Med 2008;38:179–186. [DOI] [PubMed] [Google Scholar]
- 5.Warren SA, Warren KG, Greenhill S, Paterson M. How multiple sclerosis is related to animal illness, stress and diabetes. Can Med Assoc J 1982;126:377. [PMC free article] [PubMed] [Google Scholar]
- 6.Ghadirian P, Dadgostar B, Azani R, Maisonneuve P. A case-control study of the association between socio-demographic, lifestyle and medical history factors and multiple sclerosis. Can J Public Health 2001;92:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alter MAA, Leibowitz U. Epidemiology of multiple sclerosis in Israel. In: Alter MK, ed. The Epidemiology of Multiple Sclerosis. Springfield, IL: CC Thomas; 1968:83–109. [Google Scholar]
- 8.Rockhill B, Willett WC, Hunter DJ, et al. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst 1998;90:1155–1160. [DOI] [PubMed] [Google Scholar]
- 9.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–1718. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 11.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA 2002;288:2300–2306. [DOI] [PubMed] [Google Scholar]
- 12.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999. [DOI] [PubMed] [Google Scholar]
- 13.Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst 2008;100:728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 15.Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol 2001;154:69–74. [DOI] [PubMed] [Google Scholar]
- 16.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65. [DOI] [PubMed] [Google Scholar]
- 17.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp S. sbe23: meta-analysis regression. Stata Tech Bull 1998;March:16–22. [Google Scholar]
- 19.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–1499. [DOI] [PubMed] [Google Scholar]
- 20.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–86. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–658. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA 2000;283:2961–2967. [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282:1433–1439. [DOI] [PubMed] [Google Scholar]
- 25.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' health study research group. J Natl Cancer Inst 1997;89:948–955. [DOI] [PubMed] [Google Scholar]
- 26.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 27.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med 2010;170:1758–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettee Gabriel K, McClain JJ, Lee CD, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc 2009;41:1403–1412. [DOI] [PubMed] [Google Scholar]
- 29.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler 2005;11:459–463. [DOI] [PubMed] [Google Scholar]
- 30.Nortvedt MW, Riise T, Maeland JG. Multiple sclerosis and lifestyle factors: the Hordaland health study. Neurol Sci 2005;26:334–339. [DOI] [PubMed] [Google Scholar]
- 31.Frau J, Coghe G, Lorefice L, et al. Attitude towards physical activity in patients with multiple sclerosis: a cohort study. Neurol Sci 2015;36:889–893. [DOI] [PubMed] [Google Scholar]
- 32.De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A. Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology 2008;70:1113–1118. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand KA, Giovannucci E, Liu Y, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr 2012;108:1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80:1678s–1688s. [DOI] [PubMed] [Google Scholar]
- 35.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]