Abstract
We previously evaluated Wilms’ tumor gene 1 (WT1) peptide vaccination in a large number of patients with leukemia or solid tumors and have reported that HLA‐A*24:02 restricted, 9‐mer WT1‐235 peptide (CYTWNQMNL) vaccine induces cellular immune responses and elicits WT1‐235‐specific cytotoxic T lymphocytes (CTLs). However, whether this vaccine induces humoral immune responses to produce WT1 antibody remains unknown. Thus, we measured IgG antibody levels against the WT1‐235 peptide (WT1‐235 IgG antibody) in patients with glioblastoma multiforme (GBM) receiving the WT1 peptide vaccine. The WT1‐235 IgG antibody, which was undetectable before vaccination, became detectable in 30 (50.8%) of a total of 59 patients during 3 months of WT1 peptide vaccination. The dominant WT1‐235 IgG antibody subclass was Th1‐type, IgG1 and IgG3. WT1‐235 IgG antibody production was significantly and positively correlated with both progression‐free survival (PFS) and overall survival (OS). Importantly, the combination of WT1‐235 IgG antibody production and positive delayed type‐hypersensitivity (DTH) to the WT1‐235 peptide was a better prognostic marker for long‐term OS than either parameter alone. These results suggested that WT1‐235 peptide vaccination induces not only WT1‐235‐specific CTLs as previously described but also WT1‐235‐specific humoral immune responses associated with antitumor cellular immune response. Our results indicate that the WT1 IgG antibody against the WT1 peptide may be a useful predictive marker, with better predictive performance in combination with DTH to WT1 peptide, and provide a new insight into the antitumor immune response induction in WT1 peptide vaccine‐treated patients.
Keywords: WT1 IgG antibody, predictive marker, WT1 peptide‐based immunotherapy, glioblastoma
Short abstract
What's new?
The Wilms' tumor gene 1 (WT1) antigen is a promising target for immunotherapeutic strategies against glioblastoma multiforme (GBM), a brain tumor with poor survival rates. The present study shows that vaccination with WT1‐235 peptide can induce WT1‐235‐specific humoral immune responses in GBM patients. WT1‐235 IgG antibody production was significantly associated with prolonged progression‐free survival and overall survival. Survival times were significantly longer in GBM patients with positive delayed‐type hypersensitivity (DTH) responses to WT1 peptide. Thus, in WT1 vaccine‐treated GBM patients, especially those exhibiting positive DTH responses, WT1‐235 IgG antibody production can predict long‐term survival.
Abbreviations
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DTH
delayed type‐hypersensitivity
- ELISA
enzyme‐linked immunosorbent assay
- GBM
glioblastoma multiforme
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- OS
overall survival
- PB
peripheral blood
- PFS
progression‐free survival
- TAA
tumor associated antigen
- WHO
World Health Organization
- WT1
Wilms' tumor gene 1
Immunotherapies that enhance pre‐existing antitumor immune responses represent an attractive therapeutic approach for cancer treatment. One of the most promising targets of tumor‐associated antigen (TAA)‐targeting cancer immunotherapy is the Wilms’ tumor gene 1 (WT1), which was ranked as the top antigen among a total of 75 in the National Cancer Institute pilot project to prioritize cancer antigens.1 Although WT1 was originally isolated as a tumor suppressor gene responsible for Wilms' tumor, a pediatric neoplasm,2 wild‐type WT1 is overexpressed in human leukemia,3 particularly in leukemia stem cells4 as well as a variety of solid tumors such as lung,5 colon,6 breast,7, 8 and pancreatic cancer9 and astrocytic tumors.10 Numerous studies lend support for an oncogenic rather than a tumor suppressor role for WT1 based on its functional roles such as the promotion of growth,11, 12 induction of resistance to cell death,13 promotion of DNA damage repair,14 inhibition of differentiation,15, 16 induction of cytoskeletal changes,17 and promotion of tumor angiogenesis.18, 19 Moreover, the WT1 protein elicits humoral20, 21, 22 and cellular immune responses in vitro 23, 24, 25 and in vivo.26 Based on these studies, WT1‐targeting cancer Immunotherapies, such as peptide‐based vaccines,27, 28, 29 WT1 mRNA‐electroporated dendritic cell (DC) therapy,30 and WT1 peptide‐pulsed DC therapy31 have been developed with clinical efficacy in various malignant tumor types.
Glioblastomas, a World Health Organization (WHO)‐defined grade IV astrocytoma, are the most common primary malignant brain tumor in adults. Despite current treatment modalities that typically include surgical resection, radiation, and systemic chemotherapy, almost all patients experience tumor progression or recurrence. The median survival of patients diagnosed with glioblastoma multiforme (GBM) remains at 12–15 months, with 2‐ and 5‐ year survival rates of 26–33% and <5%, respectively.32, 33, 34 We have previously reported the results of a phase II clinical trial of a WT1 peptide vaccine for recurrent GBM.35 WT1 peptide vaccine was well tolerated and induced clinical responses with 57.1% disease control rate (partial response [PR] in two patients and stable disease [SD] in 10 patients among 21 cases). The median progression‐free survival (PFS) time was 20.0 weeks, and the 6‐month PFS rate was 33.3%. Based on these promising results, 38 additional patients were recruited to the clinical trial.
The primary aim of immune monitoring during clinical trials for TAA‐targeted immunotherapy is to assess the correlation between the induction of TAA‐specific cellular immune responses and clinical antitumor efficacy of immunotherapy.36 In previous WT1 peptide vaccine clinical trials with immune monitoring to assess the induction of WT1 epitope‐specific cellular immune responses, the association of both delayed type hypersensitivity (DTH) to the WT1 peptide and WT1 peptide/MHC multimer (WT1‐tetramer) with clinical outcomes have been reported.37, 38 However, whether WT1 peptide vaccine induces humoral immune responses to produce IgG antibodies against the WT1 peptide and whether WT1 IgG production is associated with clinical outcomes remain undetermined.
In this study, humoral immune responses to the WT1 peptide (aa 235–243) were investigated in patients with GBM who were treated with the modified 9‐mer WT1‐235 peptide that was identified as an antigenic peptide for HLA‐A*24:02. The serum levels of the WT1‐235 IgG antibody were measured by enzyme‐linked immunosorbent assay (ELISA), and its association with clinical outcomes was analyzed.
Materials and Methods
WT1 peptide vaccine immunotherapy
WT1 peptide vaccine immunotherapy was performed under the approval by the Ethical Review Board of Osaka University Faculty of Medicine as described previously.35 This trial was registered at the UMIN Clinical Trials Registry as Umin000002001. In brief, the Good Manufacturing Practice (GMP)‐grade, 9‐mer modified WT1 peptide (aa 235–243, CYTWNQMNL; Peptide Institute, Ibaraki, Osaka, Japan and Multiple Peptide Systems, San Diego, CA, USA), in which Y was substituted for M at the second amino acid position (the anchor position) of the natural WT1 peptide to improve binding to HLA class I, HLA*A24:02, was used for immunization. Patients who met the criteria for the clinical study were intradermally injected with 3 mg of 9mer‐WT1 peptide emulsified with Montanide ISA51 adjuvant (Sepic, Paris, France) once a week for 12 consecutive weeks. The antitumor effect of the vaccine was assessed by determining the response of target lesions on magnetic resonance imaging (MRI) scans according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Fig. 1 a).39 When slowing or inhibition of tumor progression was observed during the first 3 months, WT1 peptide vaccination was further continued at 1‐ to 4‐week intervals. All patients were given the best supportive care after dropout from the WT1 peptide vaccine clinical trial. The PFS time was calculated from the day of the first WT1 vaccination to the day of the last MRI prior to the detection of disease progression. The OS period after WT1 vaccination and OS period after tumor recurrence in WT1‐vaccinated patients were also calculated. The characteristics of patients are summarized in Table 1.
Figure 1.
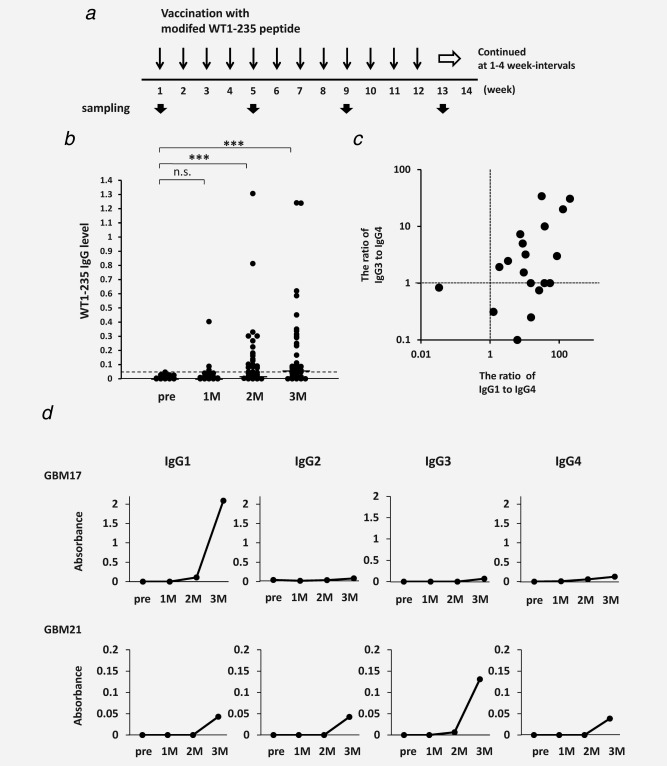
Induction of WT1‐specific humoral immune responses by WT1 peptide vaccination. (a) Vaccination and sampling schedules during WT1 peptide immunotherapy. (b) Increased serum WT1‐235 IgG antibody levels during vaccination with WT1 peptide. Serum WT1‐235 IgG antibody levels were determined by ELISA at the indicated time points and presented as values of absorbance at 440 nm. ***p < 0.001. (c) Two‐dimensional plot of the ratio of Th1‐ to Th2‐ type WT1‐235 IgG subclasses. Serum levels of WT1‐235 IgG1, IgG3, and IgG4 antibodies were measured by ELISA using subclass‐specific secondary antibodies, and the ratio of absorbance of WT1‐235 IgG1 or IgG3 antibody to that of WT1‐235 IgG4 antibody for each patient sample was plotted. (d) The levels of WT1 peptide IgG antibody subclasses. Data from two representative cases (patients 17 and 21) are shown.
Table 1.
Patient characteristics: A. Characteristics at baseline
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| Median | 51 |
| Range | 20–79 |
| Sex | |
| Male | 37 (62.7) |
| Female | 22 (37.3) |
| KPS | |
| ∼70 | 17 (28.8) |
| 80 | 7 (11.9) |
| 90 | 20 (33.9) |
| 100 | 9 (15.3) |
| Unknown | 6 (10.2) |
| Time to entry (days) | |
| Median | 459.5 |
| Range | 89–5,116 |
| Number of chemotherapy | |
| None | 10 (16.9) |
| 1 | 36 (61.0) |
| ≧2 | 13 (22.0) |
| Number of surgery | |
| 1 | 38 (64.4) |
| ≧2 | 21 (35.6) |
| Number of radiotherapy | |
| 1 | 44 (74.6) |
| ≧2 | 15 (25.4) |
| KPS; Karnofsky performance status score, time to entry; days from diagnosis to entry to the trial. | |
| B. WT1‐specific immune responses and clinical responses | |
|---|---|
| Responses | N (%) |
| Production of WT1‐235 IgG antibody a | |
| Positive | 28 (56.0) |
| Negative | 22 (44.0) |
| Delayed type hypersensitivity (DTH) to WT1 peptide skin test a | |
| Positive | 30 (60.0) |
| Negative | 18 (36.0) |
| NE | 2 (4.0) |
| Response during the first 3 months of vaccination | |
| PR | 2 (3.4) |
| SD | 20 (33.9) |
| PD | 36 (61.0) |
| NE | 1 (1.7) |
| Best response | |
| CR | 2 (3.4) |
| PR | 2 (3.4) |
| SD | 18 (30.5) |
| PD | 36 (61.0) |
| NE | 1 (1.7) |
| PFS (days) | |
| Median | 83 |
| Range | 24–2,583+ |
| OS (days) | |
| Median | 252 |
| Range | 35–2,583+ |
| Frequencies of WT1‐specific CD8+ CTLs before vaccination (%) | |
| Median: | 0.49 |
| Range: | 0.052–1.95 |
| Frequencies of WT1‐specific CD8+ CTLs at 1 month (%) | |
| Median | 0.27 |
| Range | 0.085–1.01 |
| Frequencies of WT1‐specific CD8+ CTLs at 3 months (%) | |
| Median | 0.31 |
| Range | 0.085–1.07 |
Positivity at 3 months of vaccination in patients who underwent 12 or more vaccinations, NE; not evaluated. Frequencies of WT1‐specific CD8+ CTLs were obtained from 15 patients including 10 responders and five nonresponders.35
Sera samples
Sera were obtained from 59 patients with recurrent or progressive GBM who provided informed consent and were treated with WT1 peptide vaccine immunotherapy at Osaka University Hospital at the indicated time points. Samples were stored at −80°C until use.
WT1 DTH skin test
WT1 DTH skin test was performed as previously reported.37 Briefly, 10 μg of WT1 peptide in saline and saline alone were intradermally injected in the forearm of the skin. DTH‐positivity was defined as erythema ≧2 mm in diameter measured after 48 h.
WT1 peptide/HLA‐a*24:02 tetramer assay for WT1‐specific cytotoxic T lymphocytes
The frequency of WT1‐specific CD8+ cytotoxic T lymphocytes (CTLs) was determined by the WT1 peptide/HLA‐A*24:02 tetramer assay, as previously described.35
ELISA for WT1‐235 IgG antibody levels
The WT1 aa 235–252 (CMTWNQMNLGATLKGVAA) peptide was synthesized as the capture antigen for WT1‐235 IgG antibody by PH Japan (Hiroshima, Japan) at a purity of >75%. For ELISA, briefly, 0.2 μg peptide was covalently linked to each well of 96‐well plates using the peptide coating kit from Takara (Shiga, Japan) according to the manufacturer's instructions. Plates were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) diluted with distilled water at a 1:5 ratio at room temperature for 2 h and washed with tris‐buffered saline with 0.05% Tween® 20 (TBST). Patient sera were diluted at 1:100 with the blocking buffer included in the peptide coating kit, and 100 μl of diluted sera were added to each well for overnight incubation at 4°C. All samples were measured in duplicate wells. Plates were then washed with TBST and incubated with horse radish peroxidase (HRP)‐conjugated rabbit antihuman IgG antibody (Santa Cruz Biotechnology, TX, USA) diluted at 1:1,000 in blocking buffer included in the Peptide Coating kit at room temperature for 2 h. After washing with TBST, the plates were incubated with HRP‐conjugated goat antirabbit IgG antibody (Santa Cruz Biotechnology) diluted at 1:1,000 in TBST at room temperature for 2 h. Bound WT1‐235 IgG antibody was colorimetrically detected using 50 μl TMB substrate (KPL, Baltimore, MD, USA) per well, and the reaction was stopped with the addition of 100 μl of 1N HCl. The absorbance at 450 nm was measured using a MTP‐310Lab microplate reader (Corona Electric, Ibaraki, Japan). Rabbit anti‐WT1‐235 peptide antibody40 was used as a positive control.
For measurement of IgG subclass‐specific WT1‐235 IgG antibody levels, IgG subclass‐specific mouse anti‐human IgG1, IgG2, IgG3, and IgG4 monoclonal antibodies (IgG1: #9052–05, IgG2: #9070–05, IgG3: #9210–05, and IgG4: #9190–05; Southern Biotech, Cambridge, UK) were used as secondary antibodies, and HRP‐conjugated goat anti mouse IgG antibody (#W4028, Promega, Madison, WI, USA) was used as the third antibody.
Cell lines
U373 MG and U‐87 MG glioblastoma cells were cultured in Dulbecco's modified essential medium supplemented with 10% fetal bovine serum (FBS). Parent T2 cells and T2 cells overexpressing HLA‐A24:02 (T2‐2402)41 were cultured in RPMI1640 medium supplemented with 10% FBS. The cell lines were authenticated by STR analysis.
Flow cytometry
U373 MG and U‐87 MG cells (1 × 105 cells) were incubated first with anti‐WT1 monoclonal antibody (6F‐H2; Dako Cytomation, Golstrup, Denmark) for 1 h, followed by incubation with fluorescein isothiocyanate (FITC)‐conjugated antimouse IgG antibody for 1 h. T2 and T2‐2402 cells (1 × 105 cells) were incubated in RPMI1640 medium containing 1 µg/ml of WT1‐235 peptide (aa 235–243, CYTWNQMNL) for 2 h. The cells were then incubated in RPMI1640 medium containing 1/100 volume of patient sera for 1 h, followed by incubation with FITC‐conjugated antihuman IgG antibody for 1 h. Cells were resuspended in PBS containing 2% FBS and analyzed for binding of antibody to cell surface using FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Statistical analysis
WT1‐235 IgG antibody production was analyzed by unpaired t test. Association of intermediate markers with survival time was analyzed by log‐rank test. Association of serum WT1‐235 IgG level with the frequencies of WT1‐specific CD8+ CTLs and DTH response to WT1 were analyzed by the Fisher's exact probability test and the χ2 for independence test, respectively. The difference in antibody binding to cells was analyzed by unpaired t test.
Results
ELISA for measurement of WT1‐235 IgG antibody
We first validated the ELISA developed to measure WT1‐235 IgG antibody in patient sera and determined that it was reproducible with a coefficient of variation of 10.2% from five independent assays using one postvaccination serum sample (Patient 10, collected at 3 months after the initiation of WT1 peptide vaccination). In this patient, serum WT1‐235 IgG antibody level was increased compared to the prevaccination sample. To determine the cutoff level for positivity for WT1‐235 IgG antibody, the prevaccination sample that showed similar or lower absorbance than the sample that had only dilutant was used as the negative control. The cutoff level for increase in WT1‐235 IgG antibody was determined as an increase of 0.05 in absorbance from the mean absorbance (0.041) + 2 standard deviations (SDs) in the negative control sample, as determined from five independent assays.
WT1‐235 IgG antibody production in response to WT1 peptide vaccination
In this study, WT1 peptide vaccine was administered weekly in 59 patients with GBM (Fig. 1 a). Of those, 50 patients received a minimum of 12 WT1 vaccinations, whereas the remaining nine patients dropped out due to disease progression. Measurement of sera collected prior to WT1 vaccination and at 1, 2, and 3 months after the initiation of WT1 vaccination indicated that serum WT1‐235 IgG antibody levels were below the cutoff level before WT1 vaccination in all cases but reached over the cutoff level in 30 (50.8%) out of 59 cases during the first 3 months of WT1 peptide vaccination (Fig. 1 b). These results indicated that WT1‐specific humoral immune responses were elicited that led to WT1‐235 IgG antibody production in response to WT1 peptide vaccination.
Th1‐type and WT1‐specific humoral immune responses in vaccinated GBM patients
To examine whether the WT1‐specific immune response against WT1‐235 peptide was Th1‐type, dominant subclasses of WT1‐235 IgG antibody were analyzed in randomly selected 19 patient sera among 30 cases with increased levels of WT1‐235 IgG antibody. All subclasses of IgG were detected in 14 of these 19 samples, whereas three subclasses except for IgG2 were detected in the remaining five samples. IgG1 and IgG3 are known as Th1‐type IgG subclasses, whereas IgG4 is considered as a Th2‐type IgG subclass. Thus, using the ratio of IgG1/IgG4 or IgG3/IgG4 of ≥1 as indicative of Th1‐type WT1‐specific humoral immune response, we determined that 18 (94.7%) out of 19 cases exhibited Th1‐type WT1‐specific responses, whereas the remaining one case was non‐Th1‐type (Fig. 1 c). These results indicated that Th1‐type WT1‐specific humoral immune responses were induced in the majority of the tested subjects. Figure 1d shows the levels of WT1‐235 IgG subclasses in two representative cases, patients 17 and 21; IgG1 and IgG3 were the predominant subclasses in sera obtained at 3 months after vaccination initiation in these cases.
WT1‐235 IgG antibody production was associated with good PFS and OS
We next examined the association of intermediate markers such as WT1‐235 IgG antibody, DTH to WT1 peptide, and tumor regression with survival time in GBM patients treated with WT1 peptide vaccine. WT1‐specific immune responses and clinical responses during WT1 peptide vaccination were summarized in Table 1. First, of those patients who received 12 or more WT1 vaccinations, the PFS and OS times were compared between the patients with WT1‐235 IgG antibody production (n = 28) and those without IgG antibody response (n = 22) during the initial 3 months of WT1 vaccination. Both the PFS and OS times in patients with WT1‐235 IgG antibody production were significantly longer than those who did not exhibit IgG production (p = 0.028 and 0.001, respectively) (Fig. 2 a).
Figure 2.
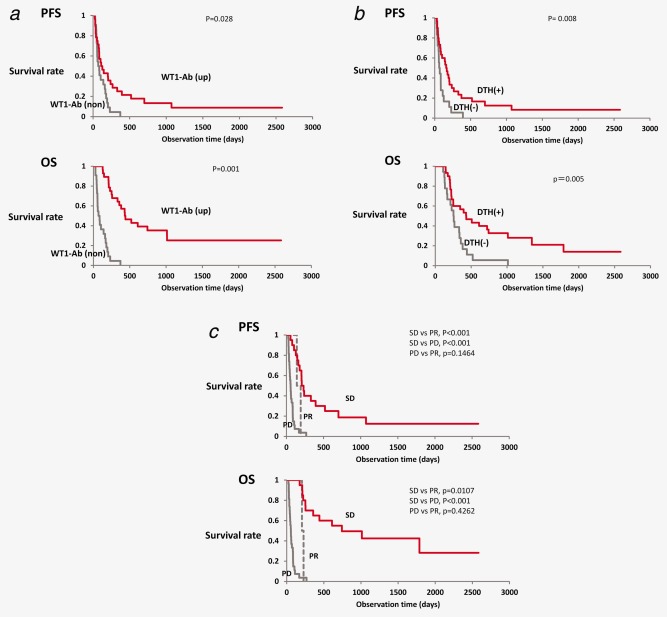
Association of WT1 IgG antibody production with prolonged survival. Association of intermediate markers, such as serum WT1‐235 IgG levels (a), delayed‐type hypersensitivity (DTH) response to WT1 peptide (b), and clinical responses according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (c), with progression‐free survival (PFS) and overall survival (OS) was analyzed by the log‐rank test. (a) Red and gray lines represent elevated (WT1 ab up) and nonelevated (WT1 ab non) serum WT1‐235 IgG antibody levels, respectively. (b) Red and gray lines represent positive and negative WT1‐DTH, respectively. (c) Red, gray dotted, and gray solid lines represent stable disease (SD), partial response (PR), and progressive disease (PD), respectively. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Next, we analyzed the difference in survival time between patients with (n = 30) and without (n = 18) positive DTH to WT1 peptide during the first 3 months after the initiation of WT1 peptide vaccination. Our analysis revealed that both the PFS and OS times were significantly longer in patients with positive DTH to WT1 peptide compared to those without positive DTH‐type response (p = 0.008 and p = 0.030, respectively) (Fig. 2 b).
Among 50 patients who underwent 12 or more WT1 vaccinations, 49 had MRI data in the initial 3 months of vaccination for tumor regression assessment according to the RECIST criteria. Two (4.0%), 20 (40.8%), and 27 (55.1%) patients had PR, SD, and progressive disease (PD), respectively, at 3 months after WT1 vaccination initiation. The complete response (CR) + PR rate was 4.0% with a disease control rate (CR + PR + SD) of 44.9%. When the difference in survival time was analyzed among patients who achieved PR, SD, or PD during the initial 3 months of WT1 vaccination, both the PFS and OS times were the longest in patients who achieved SD among the three groups (p < 0.001) (Fig. 2 c). Unexpectedly, both the PFS and OS times in patients who achieved PR were shorter than those in patients with SD and were not significantly different from those in patients with PD (Fig. 2 c). One patient with PR at 3 months after vaccination experienced PD and dropped out at 5 months. The other patient with PR at 3 months of vaccination developed a new tumor surrounding the cervical spinal cord and dropped out at 6 months. These results indicated that both WT1‐235 IgG antibody production and positive DTH to WT1 peptide were associated with prolonged survival times in GBM patients who were vaccinated with WT1 peptide and that they could be used as predictive markers for survival rates during WT1 peptide immunotherapy.
Association between WT1‐235 IgG antibody production and WT1‐specific CTL induction
In our previous report, we observed that the higher frequencies of WT1‐specific CTLs in the peripheral blood (PB) of patients before WT1 vaccination were associated with favorable clinical outcomes.35 We herein examined the correlation between the frequencies of WT1‐specific CTLs and WT1‐235 IgG antibody production during the initial 3 months of WT1 vaccination in 15 cases that had available data on WT1‐specific CTLs. As seen in Table 2, the frequencies of PB WT1‐specific CTLs before WT1 vaccination were significantly associated with increased serum WT1‐235 IgG antibody levels.
Table 2.
Serum WT1‐235 IgG levels associates with other markers
| Serum WT1‐235 IgG level | Up | Non | Sum |
|---|---|---|---|
| (A) Frequencies of WT1‐CTLs | |||
| High | 9 | 3 | 12 |
| Low | 0 | 3 | 3 |
| Sum | 9 | 6 | 15 |
| (B) DTH to WT1 peptide | |||
| Positive | 17 | 13 | 30 |
| Negative | 11 | 7 | 18 |
| Sum | 28 | 20 | 48 |
(A) p = 0.0045, (B) p = 0.0837.
Association of elevation of serum WT1‐235 IgG levels in the initial 3 months of WT1 peptide vaccine treatment and either frequencies of WT1‐specific CTLs before vaccination (A) or positive DTH to WT1‐235 peptide in the initial 3 months of WT1 peptide vaccine treatment (B) was analyzed by Spearman's correlation coefficient by rank test. Cutoff value for frequencies of WT1‐CTLs was determined as 0.16% which was mean + 2SD in 15 healthy control individuals.
Next, we determined whether DTH to WT1 peptide was associated with WT1‐235 IgG antibody production in 48 patients who received a minimum of 12 WT1 vaccinations and had available data on DTH responses. Albeit a correlation between positive DTH to WT1‐235 peptide and WT1‐235 IgG antibody production was observed, it did not reach statistical significance (p = 0.0837) (Table 2).
Combination of serum WT1‐235 IgG antibody production and positive DTH was a better predictor of survival in WT1‐vaccinated GBM patients
As positive DTH to WT1‐235 peptide was not significantly correlated with WT1‐235 IgG antibody production, survival time was analyzed in the following four groups that were stratified according to WT1‐235 IgG antibody production and DTH response to WT1‐235 peptide: (1) elevated WT1‐235 IgG/positive DTH [IgG‐up/DTH(+), n = 17], (2) elevated WT1‐235 IgG/negative DTH [IgG‐up/DTH(‐), n = 11], (3) nonelevated WT1‐235 IgG/positive DTH [IgG‐non/DTH(+), n = 13], and (4) nonelevated WT1‐235 IgG/negative DTH [IgG‐non/DTH(−), n = 7]. The PFS time was significantly longer in the IgG‐up/DTH(+) group compared to the other three groups (Fig. 3 a). In addition, 1‐year PFS rate was 35.3% in the IgG‐up/DTH(+) group, whereas it was 0% in the other three groups. Furthermore, the OS time was the longest in the IgG‐up/DTH(+) group, and this was statistically significant. Finally, there were no significant differences in the OS times between the IgG‐non/DTH(+) and IgG‐up/DTH(−) groups, whereas the OS time was the shortest in the IgG‐non/DTH(−) group (Fig. 3). These results indicated that production of WT1‐235 IgG antibody and positive DTH to WT1 peptide as a combination correlated with longer OS times. Indeed, the 2‐year OS rate was 52.3% in the IgG‐up/DTH(+) group, whereas it was 0% in the other three groups. These results clearly showed that, when in combination, serum WT1‐235 IgG antibody production and positive DTH to WT1‐235 peptide demonstrated better predictive performance for clinical outcomes in GBM patients receiving WT1 peptide immunotherapy.
Figure 3.
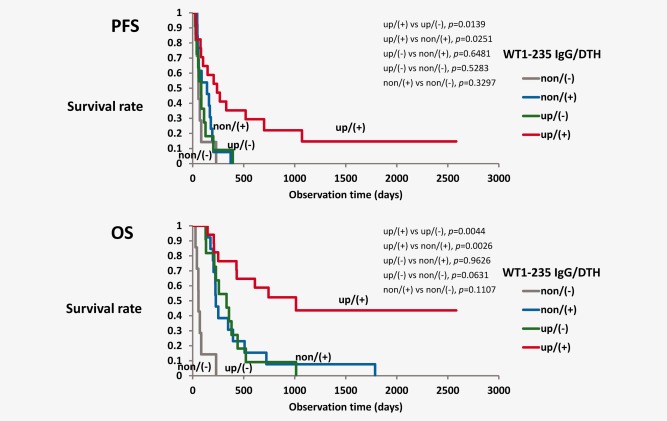
Combination of WT1‐235 IgG production and DTH to WT1 peptide is a better predictor of favorable prognosis. Progression‐free survival (PFS) and OS were analyzed in the following four groups: elevated WT1‐235 IgG antibody/positive DTH [up/(+), red lines], elevated WT1‐235 IgG antibody/negative DTH [up/(−), green lines], nonelevated WT1‐235 IgG antibody/positive DTH [non/(+), blue lines], and nonelevated WT1‐235 IgG antibody/negative DTH [non/(−), gray lines]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Undetectable binding of WT1‐235 IgG antibody to WT1 protein on cell surface
The results demonstrating that the production of WT1‐235 IgG antibody was significantly associated with favorable clinical outcomes raised the possibility that WT1‐235 IgG antibody might be involved in inhibition of tumor growth. Given that the binding of WT1‐235 IgG antibody to tumor cell surface is essential for its direct action, we assessed whether this occurred in two glioblastoma cell lines. Flow cytometric analysis using an anti‐WT1 monoclonal antibody (clone 6F‐H2) did not detect WT1 protein on the cellular surface in U87MG and U373MG glioblastoma cell lines (data not shown). Because T cell receptor‐like antibody, ESK 1 has been reported,42 we examined whether WT1‐235 IgG antibody could recognize WT1 peptide‐HLA class I complex that were presented on cell surface. After incubation with the WT1‐235 peptide, T2 or T2‐2402 cells were incubated with pre‐ or postvaccination sera from 10 patients in whom WT1‐235 IgG antibody increase was detected in response to vaccination. However, flow cytometric analysis showed that WT1‐235 IgG antibody did not bind to WT1 peptide‐HLA class I complex that were presented on the cell surface at detectable levels (data not shown).
Persistent production of serum WT1‐235 antibody in long‐term survivors
The vaccination interval in this trial was 1 week in the first 3 months, which was then extended to 2, 3, and 4 weeks. To examine whether serum WT1‐235 antibody induction was being maintained during WT1 vaccination despite extended vaccination intervals, serum WT1‐235 antibody levels were analyzed at the indicated time points in sera from two representative patients in whom tumor growth was being suppressed by WT1 vaccination for more than 1 year (Fig. 4). Serum WT1‐235 antibody production was maintained even after the incremental extension of vaccination interval from 1 to 4 weeks.
Figure 4.
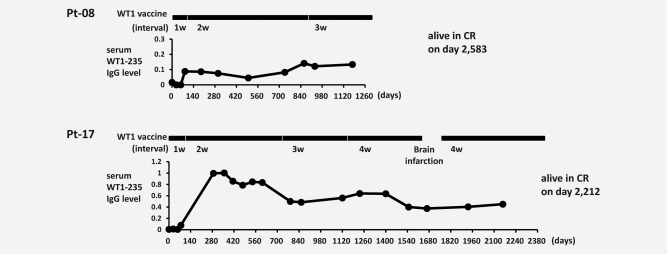
Persistent production of WT1‐235 IgG antibody during long‐term WT1 vaccination. Serum WT1‐235 IgG antibody was measured at the indicated time points in two GBM patients in whom tumor was controlled for more than 1 year. Intervals of WT1 peptide vaccination were 1 week in 3 months after the start of WT1 peptide vaccination, which was then gradually extended to 2, 3, and 4 weeks.
Discussion
In this study, we present three important findings based on the analysis of WT1‐specific immune responses in GBM patients who were vaccinated with WT1 peptide. First, humoral immune responses against WT1‐235 peptide (aa 235–243) were elicited in the majority of the GBM patients, and IgG‐isotype WT1‐235 antibody response was detected in 50.8%. Second, WT1‐235 IgG antibody production was significantly associated with prolonged PFS and OS times, suggesting that it might be a useful predictive marker for WT1 peptide vaccine‐treated GBM patients. Third, combination of WT1‐235 IgG antibody production and positive DTH to WT1 peptide was a better predictor of long‐term survivors.
The production of WT1‐235 IgG antibody relies on the survival and proliferation of B cells that recognize WT1‐235, their differentiation into plasma cells, and class switching from IgM to IgG;43 thus, WT1‐235 IgG antibody production observed in our patients was likely associated with the activation of WT1‐235 specific helper T (Th) cells. In a Th‐dependent response to antigenic challenge, CD4+ Th cells that recognize the antigenic peptide in complex with an HLA class II molecule aid B cells in cytokine secretion via the CD40–CD40L system.44 Therefore, WT1‐235 IgG antibody production detected in our GBM patients indicated that HLA‐A*24:02 (class I)‐restricted, 9‐mer WT1 peptide induced WT1‐specific CD4+ Th cells. However, stimulation of antigen‐presenting cells (APCs) by HLA class II‐restricted, WT1 helper epitopes (peptides) is needed to induce WT1‐specific CD4+ Th cells. Thus, immunoglobulin class switching from IgM to IgG by WT1‐specific CD4+ Th cells that were induced by the class I‐restricted WT1 peptide in our patients was an important and intriguing finding. The most plausible explanation for this outcome is the repeated immunization with WT1‐235 peptide, which could in turn activate APCs to induce WT1‐235‐specific CTLs, subsequently leading to overall induction of immune responses. This could subsequently promote the presentation of HLA class II‐restricted, WT1 helper peptides endogenously and abundantly expressed in patients with WT1‐expressing GBM, with the resultant induction of WT1‐specific CD4+ Th cells. We previously reported that the clinical efficacy of WT1 vaccination with the WT1 peptide used in this study was significantly correlated with the spontaneous induction of WT1‐332 helper peptide (KRYFKLSHLQMHSR)‐specific CD4+ Th cells at 4 weeks post‐WT1 vaccination,45 an outcome that lends support to the abovementioned mechanism. Thus, we propose that repeated immunization with HLA‐A*24:02 (class I)‐restricted, 9‐mer WT1‐235 peptide activates WT1‐specific Th cells that can support the production of WT1‐235 IgG antibody in GBM patients.
Our results also indicated that WT1‐235 IgG antibody was produced in the majority of vaccinated GBM patients and that both IgG1 and IgG3 subclasses were dominant. Based on previous reports showing that class switching from IgM to IgG1 and IgG3 was promoted by immunity‐enhancing cytokines such as IL‐21,46 WT1‐235 IgG1 and IgG3 antibody production observed in this study indicated a similar mechanism as a contributor to the favorable clinical outcomes observed in GBM patients vaccinated with WT1 peptide.
The association of WT1‐235 IgG antibody production with favorable outcomes could alternatively be attributable to its inhibitory effect on tumor growth during vaccination. However, transport of IgG molecules across the blood‐brain barrier is limited.47 In addition, WT1 protein was not detected on the cell surface of glioblastoma cells in our study, and binding of WT1‐235 IgG antibody to the WT1 peptide‐MHC class I complex was undetectable. Therefore, WT1‐235 IgG antibody was unlikely to directly inhibit tumor growth. It is possible that WT1‐235 IgG could bind to WT1‐235 peptide used for vaccination, acting indirectly as an opsonin. Reportedly, opsonization of cancer antigen MAGE‐A3 with an antibody promoted its uptake by DCs. The induction of CD8+ T‐cell response rather than CD4+ T‐cell response was favored by naïve T cells stimulated with antibody‐opsonized MAGE‐A3 protein, indicating the existence of an uptake route‐dependent mechanism by which the subsequent immune responses were modulated.48 In addition, the efficacy of uptake could vary according to the subclass of opsonizing IgG antibody. Goh et al. generated humanized antibodies that shared identical antigen‐binding V‐regions with constant regions from different IgG subclasses and analyzed opsonizing activities of these IgG antibodies on the Salmonella enterica OmpA surface protein. Opsonization with all tested IgG subclasses increased Salmonella uptake by human phagocytes; however, the efficacy of uptake varied across different subclasses.49 In our study, the dominant WT1‐235 peptide IgG subclasses were IgG1 and IgG3 that act as ligands for the major activating FCγ receptor I on APCs.50 Thus, based on our and previously reported findings, we propose that the opsonizing effects of WT1 peptide IgG antibody may lead to promotion of peptide uptake by DCs with more effective induction of WT1‐specific cellular immune responses.
As discussed above, WT1‐235 IgG antibody production with WT1‐specific CD4+ Th cell induction and subsequent enhancement of WT1‐specific CTLs by Th cells likely resulted in improved clinical outcomes, as reflected in prolonged PFS and OS times. Interestingly, the combination of WT1‐235 IgG antibody production and positive DTH to WT1 peptide was a better predictor of prolonged PFS and OS times, compared to either alone. These findings indicated that immune responses as determined by either WT1‐235 IgG antibody production or positive DTH to WT1 peptide were distinct. Whereas WT1‐235 IgG antibody production suggested WT1‐specific CD4+ Th induction, the determination of immune mechanisms underlying the association between positive DTH to WT1 peptide and improved outcomes are more challenging. However, positive DTH response to WT1 peptide was previously reported to correlate with higher frequencies of WT1‐specific CTLs in patients with advanced pancreatic cancers vaccinated with the same WT1 peptide.37 Thus, the improved utility of the combination of these markers for predicting clinical outcomes might be partially explained by simultaneous assessment of the induction of both CTLs and Th cells.
WT1 IgM and IgG antibodies against WT1 protein fragments have been previously reported in hematological malignancies20, 21, 51 and nonsmall cell lung cancer (NSCLC).22 In myelodysplastic syndrome (MDS), in which transformed malignant cells expressed WT1 at high levels, the majority of patients spontaneously produced WT1 IgM and IgG antibodies against WT1 protein fragments. Intriguingly, in these patients, immunoglobulin isotype class switching from IgM to IgG occurred along with progression of MDS from refractory anemia to preleukemia, suggesting that spontaneously induced WT1‐specific CD4+ Th cells drove this class switching. In NSCLC patients, higher titers of WT1 IgG antibody against WT1 protein fragment were a predictable marker for long PFS after complete surgical tumor resection, suggesting that spontaneous induction of WT1‐specific CD4+ Th cells contributed to improved prognosis. Interestingly, antibodies against WT1‐235 peptide were not spontaneously produced regardless of spontaneous production of WT1 antibodies against WT1 protein fragments. These findings suggested that WT1‐235 peptide was originally a hidden epitope within WT1 protein that was not recognized by the immune system, resulting in the lack of spontaneous production of antibodies against it. As WT1‐235 IgG antibody was undetectable prior to WT1 peptide vaccination in our patients, detection of WT1‐235 IgG antibody was a direct evidence of immune responses to vaccination. Thus, measurement of WT1‐235 IgG antibody was an easy and efficient method to determine whether WT1 peptide vaccination induced immune responses.
To obtain long‐term control of tumor, not only the induction but also the maintenance of antitumor immune responses is required. Serum WT1‐235 IgG antibody levels were maintained at higher levels during repeated WT1 vaccination despite gradual extension of WT1 vaccination intervals over time; both GBM patients, 08 and 17, were in CR on days 2,583 and 2,212, respectively. These results suggested that WT1‐specific CD4+ Th cell induction was sustained during WT1‐235 peptide vaccination and was contributing to the achievement and maintenance of CR in cooperation with WT1‐specific CTLs.
In conclusion, induction of humoral immune responses with 9‐mer WT1 peptide vaccination was associated with prolonged survival in recurrent GBM patients. In addition, serum WT1‐235 IgG antibody may be a useful immune monitoring marker, with better predictive performance in combination with DTH to WT1 peptide, of WT1 peptide vaccine immunotherapy.
Acknowledgements
We thank Tomoe Umeda (Osaka University) for her sincere contribution to the practice of the clinical trial. We also thank Keiko Udaka (Kochi University) for valuable discussion of this work. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1. Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 2000;60:509–20. [DOI] [PubMed] [Google Scholar]
- 3. Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 1994;84:3071–9. [PubMed] [Google Scholar]
- 4. Saito Y, Kitamura H, Hijikata A, et al. Identification of therapeutic targets for quiescent, chemotherapy‐resistant human leukemia stem cells. Sci Transl Med 2010;2:17ra9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oji Y, Miyoshi S, Maeda H, et al. Overexpression of the Wilms' tumor gene WT1 in de novo lung cancers. Int J Cancer 2002;100:297–303. [DOI] [PubMed] [Google Scholar]
- 6. Oji Y, Yamamoto H, Nomura M, et al. Overexpression of the Wilms' tumor gene WT1 in colorectal adenocarcinoma. Cancer Sci 2003;94:712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loeb DM, Evron E, Patel CB, et al. Wilms' tumor suppressor gene (WT1) is expressed in primary breast tumors despite tumor‐specific promoter methylation. Cancer Res 2001;61:921–5. [PubMed] [Google Scholar]
- 8. Miyoshi Y, Ando A, Egawa C, et al. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res 2002;8:1167–71. [PubMed] [Google Scholar]
- 9. Oji Y, Nakamori S, Fujikawa M, et al. Overexpression of the Wilms' tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci 2004;95:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oji Y, Suzuki T, Nakano Y, et al. Overexpression of the Wilms' tumor gene WT1 in primary astrocytic tumors. Cancer Sci 2004;95:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Algar EM, Khromykh T, Smith SI, et al. A WT1 antisense oligonucleotide inhibits proliferation and induces apoptosis in myeloid leukaemia cell lines. Oncogene 1996;12:1005–14. [PubMed] [Google Scholar]
- 12. Yamagami T, Ogawa H, Tamaki H, et al. Suppression of Wilms' tumor gene (WT1) expression induces G2/M arrest in leukemic cells. Leuk Res 1998;22:383–4. [DOI] [PubMed] [Google Scholar]
- 13. Ito K, Oji Y, Tatsumi N, et al. Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene 2006;25:4217–29. [DOI] [PubMed] [Google Scholar]
- 14. Oji Y, Tatsumi N, Kobayashi J, et al. Wilms' tumor gene WT1 promotes homologous recombination‐mediated DNA damage repair. Mol Carcinog 2015;54:1758–71. [DOI] [PubMed] [Google Scholar]
- 15. Inoue K, Tamaki H, Ogawa H, et al. Wilms' tumor gene (WT1) competes with differentiation‐inducing signal in hematopoietic progenitor cells. Blood 1998;91:2969–76. [PubMed] [Google Scholar]
- 16. Svedberg H, Chylicki K, Baldetorp B, et al. Constitutive expression of the Wilms' tumor gene (WT1) in the leukemic cell line U937 blocks parts of the differentiation program. Oncogene 1998;16:925–32. [DOI] [PubMed] [Google Scholar]
- 17. Jomgeow T, Oji Y, Tsuji N, et al. Wilms' tumor gene WT1 17AA(−)/KTS(−) isoform induces morphological changes and promotes cell migration and invasion in vitro . Cancer Sci 2006;97:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wagner N, Michiels JF, Schedl A, et al. The Wilms' tumour suppressor WT1 is involved in endothelial cell proliferation and migration: expression in tumour vessels in vivo . Oncogene 2008;27:3662–72. [DOI] [PubMed] [Google Scholar]
- 19. Wagner KD, Cherfils‐Vicini J, Hosen N, et al. The Wilms' tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun 2014;5:5852. [DOI] [PubMed] [Google Scholar]
- 20. Elisseeva OA, Oka Y, Tsuboi A, et al. Humoral immune responses against Wilms’ tumor gene WT1 product in patients with hematopoietic malignancies. Blood 2002;99:3272–9. [DOI] [PubMed] [Google Scholar]
- 21. Wu F, Oka Y, Tsuboi A, et al. Th1‐biased humoral immune responses against Wilms tumor gene WT1 product in the patients with hematopoietic malignancies. Leukemia 2005;19:268–74. [DOI] [PubMed] [Google Scholar]
- 22. Oji Y, Kitamura Y, Kamino E, et al. WT1 IgG antibody for early detection of nonsmall cell lung cancer and as its prognostic factor. Int J Cancer 2009;125:381–7. [DOI] [PubMed] [Google Scholar]
- 23. Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic T lymphocyte responses specific for peptides of wild‐type Wilms’ tumor gene WT1 product. Immunogenetics 2000;51:99–107. [DOI] [PubMed] [Google Scholar]
- 24. Gao L, Bellantuono I, Elsässer A, et al. Selective elimination of leukemic CD34+ progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood 2000;95:2198–203. [PubMed] [Google Scholar]
- 25. Ohminami H, Yasukawa M, Fujita S. HLA class I‐restricted lysis of leukemia cells by a CD8+ cytotoxic T‐lymphocyte clone specific for WT1 peptide. Blood 2000;95:286–93. [PubMed] [Google Scholar]
- 26. Oka Y, Udaka K, Tsuboi A, et al. Cancer Immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 2000;164:1873–80. [DOI] [PubMed] [Google Scholar]
- 27. Oka Y, Tsuboi A, Murakami M, et al. WT1 peptide‐based immunotherapy for patients with overt leukemia from myelodysplastic syndome (MDS) or MDS with myelofibrosis. Int J Hematol 2003;78:56–61. [DOI] [PubMed] [Google Scholar]
- 28. Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)‐specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA 2004;101:13885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mailänder V, Scheibenbogen C, Thiel E, et al. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia 2004;18:165–6. [DOI] [PubMed] [Google Scholar]
- 30. Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen‐targeted dendritic cell vaccination. Proc Natl Acad Sci USA 2010;107:13824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi H, Okamoto M, Shimodaira S, et al. Impact of dendritic cell vaccines pulsed with Wilms' tumour‐1 peptide antigen on the survival of patients with advanced non‐small cell lung cancers. DC‐vaccine study group at the Japan Society of Innovative Cell Therapy (J‐SICT). Eur J Cancer 2013;49:852–9. [DOI] [PubMed] [Google Scholar]
- 32. Stupp R, Mason WP van den Bent MJ. National Cancer Institute of Canada Clinical Trials Group , et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 33. Gilbert MR, Wang M, Aldape KD, et al. Dose‐dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 2013;31:4085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jhanwar‐Uniyal M, Labagnara M, Friedman M, et al. Glioblastoma: molecular pathways, stem cells and therapeutic targets. Cancer (Basel) 2015;7:538–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 2008;108:963–71. [DOI] [PubMed] [Google Scholar]
- 36. Clay TM, Hobeika AC, Mosca PJ, et al. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res 2001;7:1127–35. [PubMed] [Google Scholar]
- 37. Nishida S, Koido S, Takeda Y, et al. Wilms Tumor Gene (WT1) peptide‐based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother 2014;37:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oji Y, Oka Y, Nishida S, et al. WT1 peptide vaccine induces reduction in minimal residual disease in an imatinib‐treated CML patient. Eur J Haematol 2010;85:358–60. [DOI] [PubMed] [Google Scholar]
- 39. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 40. Ichinohasama R, Oji Y, Yokoyama H, et al. Sensitive immunohistochemical detection of WT1 protein in tumors with anti‐WT1 antibody against WT1 235 peptide. Cancer Sci 2010;101:1089–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuzushima K, Hayashi N, Kimura H, et al. Efficient identification of HLA‐A*2402‐restricted cytomegalovirus‐specific CD8+ T‐cell epitopes by a computer algorithm and an enzyme‐linked immunospot assay. Blood 2001;98:1872–81. [DOI] [PubMed] [Google Scholar]
- 42. Dao T, Yan S, Veomett N, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med 2013;5:176ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodnow CC, Vinuesa CG, Randall KL, et al. Control systems and decision making for antibody production. Nat Immunol 2010;11:681–8. [DOI] [PubMed] [Google Scholar]
- 44. Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol 2015;15:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fujiki F, Oka Y, Kawakatsu M, et al. A clear correlation between WT1‐specific Th response and clinical response in WT1 CTL epitope vaccination. Anticancer Res 2010;30:2247–54. [PubMed] [Google Scholar]
- 46. Pène J, Gauchat JF, Lécart S, et al. Cutting edge: IL‐21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol 2004;172:5154–7. [DOI] [PubMed] [Google Scholar]
- 47. Triguero D, Buciak JB, Yang J, et al. Blood–brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci USA 1989;86:4761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moeller I, Spagnoli GC, Finke J, et al. Uptake routes of tumor‐antigen MAGE‐A3 by dendritic cells determine priming of naïve T‐cell subtypes. Cancer Immunol Immunother 2012;61:2079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goh YS, Grant AJ, Restif O, et al. Human IgG isotypes and activating Fcγ receptors in the interaction of Salmonella enterica serovar Typhimurium with phagocytic cells. Immunology 2011;133:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levin D, Golding B, Strome SE, et al. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol 2015;33:27–34. [DOI] [PubMed] [Google Scholar]
- 51. Nicoli P, Defilippi I, Carturan S, et al. Detection of humoral immune responses against WT1 antigen in patients affected by different hematological malignancies. Acta Haematol 2008;120:47–50. [DOI] [PubMed] [Google Scholar]


