Abstract
Objectives
Thyroid abnormalities in patients with bipolar disorder (BD) have been linked to lithium treatment for decades, yet other drugs have been less well studied. Our objective was to compare hypothyroidism risk for lithium versus the anticonvulsants and second‐generation antipsychotics commonly prescribed for BD.
Methods
Administrative claims data on 24,574 patients with BD were analyzed with competing risk survival analysis. Inclusion criteria were (i) one year of no prior hypothyroid diagnosis nor BD drug treatment, (ii) followed by at least one thyroid test during BD monotherapy on lithium carbonate, mood‐stabilizing anticonvulsants (lamotrigine, valproate, oxcarbazepine, or carbamazepine) or antipsychotics (aripiprazole, olanzapine, risperidone, or quetiapine). The outcome was cumulative incidence of hypothyroidism per drug, in the presence of the competing risk of ending monotherapy, adjusted for age, sex, physician visits, and thyroid tests.
Results
Adjusting for covariates, the four‐year cumulative risk of hypothyroidism for lithium (8.8%) was 1.39‐fold that of the lowest risk therapy, oxcarbazepine (6.3%). Lithium was non‐statistically significantly different from quetiapine. While lithium conferred a higher risk when compared to all other treatments combined as a group, hypothyroidism risk error bars overlapped for all drugs. Treatment (p = 3.86e‐3), age (p = 6.91e‐10), sex (p = 3.93e‐7), and thyroid testing (p = 2.79e‐87) affected risk. Patients taking lithium were tested for hypothyroidism 2.26–3.05 times more frequently than those on other treatments.
Conclusions
Thyroid abnormalities occur frequently in patients with BD regardless of treatment. Therefore, patients should be regularly tested for clinical or subclinical thyroid abnormalities on all therapies and treated as indicated to prevent adverse effects of hormone imbalances on mood.
Keywords: anticonvulsants, antipsychotics, bipolar disorder, competing risks, hypothyroidism, lithium
Bipolar disorder (BD) is characterized by recurrent episodes of mania, hypomania, and depression 1, occurring in about 2% of the population 2. While acute episodes can be dramatic and require hospitalization, successful treatment rests on the effective prevention of recurrences and of residual symptoms.
Lithium has been a cornerstone of the treatment of acute mania and the prevention of mood episodes during the maintenance phase of the disease 3, 4, 5, 6, 7, 8, 9. Despite its narrow therapeutic range, which requires careful monitoring of drug serum levels, lithium has proven effective in 30–50% of patients, particularly in those with a family history of BD, with non‐rapid cycling BD, and without comorbid substance use disorders 10, 11. Lithium appears to have neuroprotective properties 12, and reduces suicidal behavior 13, 14, 15, 16. However, potentially severe side effects have also been reported, including hypothyroidism 17, 18, particularly in middle‐aged women, for whom rates as high as 37% have been reported 19. The link between hypothyroidism and lithium treatment has been supported by case reports 20, 21, 22, and other observational studies as reflected in a recent meta‐analysis 23. These anticipated adverse effects have led to recommendations for frequent thyroid testing of patients treated with lithium as reflected in recent UpToDate guidelines 24, despite some negative findings 25, 26, 27.
Current guidelines for lithium treatment in patients with BD recommend thyroid function studies prior to treatment, once or twice during the first 6 months of therapy, and every 6–12 months thereafter. Lithium is contraindicated with significant renal impairment, sodium depletion, dehydration, or significant cardiovascular disease. However, neither pretreatment hypothyroidism nor lithium‐induced hypothyroidism contraindicates lithium therapy, as it can be managed by thyroxine treatment 24.
While alternative treatment options have emerged, including mood‐stabilizing anticonvulsants (lamotrigine, valproate, oxcarbazepine, and carbamazepine) and atypical antipsychotics (aripiprazole, olanzapine, risperidone, and quetiapine), less evidence exists about their risk for hypothyroidism, and less emphasis has been placed on thyroid testing in their product labeling (see Discussion).
Large‐scale administrative claims data provide the opportunity to obtain estimates of adverse medication effects in large populations across a range of geographical regions in “real‐world” health care settings, and often over long periods of exposure. Thus, such data capture a substantial amount of heterogeneity in disease severity and treatment response over the disease course, whereas most clinical trials enroll a homogeneous cohort, followed over relatively short time periods. Advantages of claims data, however, can be offset by their observational nature, and inconsistency in collection, coding and recording. As such, treatment biases and confounding factors require specific attention and have been addressed in our analyses 28, 29, 30.
The objective of this study was to determine the comparative risk of hypothyroidism with lithium monotherapy versus eight of the most commonly prescribed anticonvulsants and second‐generation antipsychotics used to treat patients with BD. The study is innovative in that it comprehensively utilizes medical claims data on over a million patients with BD in a competing risk survival analysis framework. It is also the first to compare all of the above‐mentioned therapies head to head in a single study.
Patients and methods
Data source and sample selection
Patient data were obtained from the MarketScan® Commercial Claims and Encounters Databases (Ann Arbor, MI, USA) provided by the Reagan‐Udall Foundation's Innovation in Medical Evidence Development and Surveillance (IMEDS) program 31. The IMEDS data include only de‐identified patient‐level information which fully adheres to Health Insurance Portability and Accountability Act (HIPAA) standards 32, 33. Data have been transformed to the Observational Medical Outcomes Partnership (OMOP) common data model version 4 34, in which different procedures and diagnoses are expressed using the SNOMED‐CT vocabulary. Drugs and treatments are coded based on the OMOP drug vocabulary, which is comprised of RxNorm for drugs and ingredients 35, and includes additional classification systems for higher level concept aggregation (e.g., analgesics). We analyzed data from 141,805,491 commercially insured individuals collected between 2003 and 2013 across the USA, to obtain 1,232,534 patients with BD. Data were collected in primary care and hospital care settings across nearly every US county. From the 1.2 million patients with BD, 24,574 patients (8,517 male and 16,057 female) aged 18–65 years met our inclusion criteria (Fig. 1).
Figure 1.
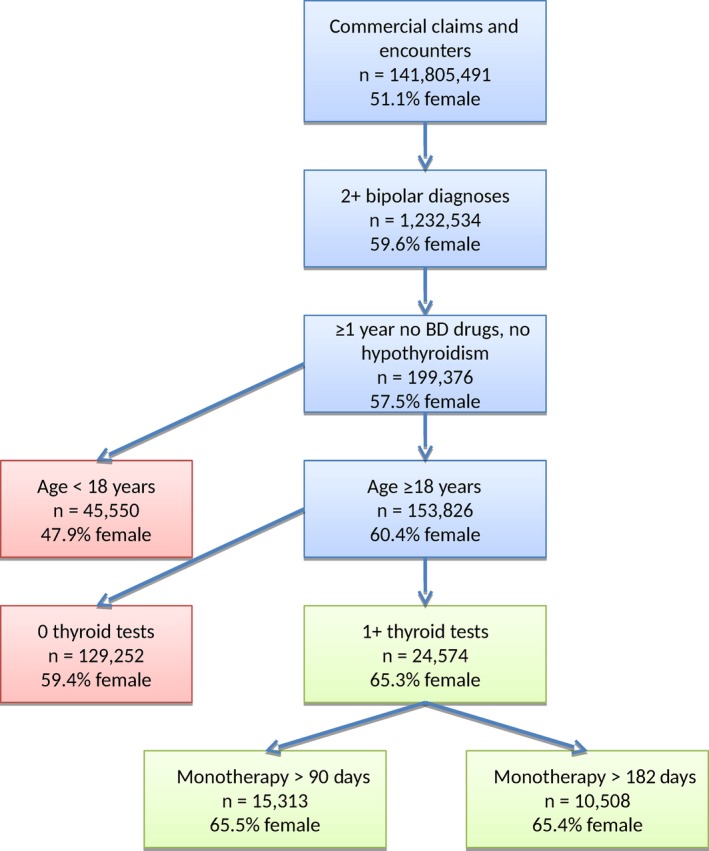
Study design and sample selection. We show the sample size and percentage of female patients at each junction point in our data staging. The final cohort of 24,574 samples is shown in green, along with subsets with >90 days and >182 days of monotherapy. BD = bipolar disorder.
Data staging
The database was first queried to identify the 1.2 million patients who had two or more bipolar diagnoses, followed by a second query to store to a database table each patient's sorted list of events. All records of conditions, visits, procedures, observations, and drug exposures were included. Using internally developed Python code, we then incrementally retrieved the >626 million event records, and, for each patient, two passes over the sorted event data were performed. The first pass verified that inclusion criteria were met, and determined the start of monotherapy, and whether, how, and when the exposure period ended. The second pass calculated drug exposures, pretreatment comorbidities, and thyroid testing information.
Inclusion criteria
Inclusion criteria were: (i) two or more diagnoses of BD at any time in the patient history; (ii) age at start of treatment ≥18 years; (iii) a minimum one year of no prior drug treatment for BD and no hypothyroid diagnosis or treatment; (iv) followed by monotherapy for BD and at least one thyroid test during monotherapy. Results are contrasted with cohort subsets on 3+ (n = 15,430) and 6+ (n = 10,594) months of monotherapy to account for patients with pre‐existing cases of hypothyroidism diagnosed shortly after treatment.
Exposure
Nine monotherapeutic exposures were considered: lithium carbonate (RxNorm ingredient: 42351), aripiprazole (89013), carbamazepine (2002), lamotrigine (28439), olanzapine (61381), oxcarbazepine (32624), quetiapine (51272), risperidone (35636), and valproate (40254). We required that drug exposures had no gaps of 30 days or longer to increase the likelihood that prescriptions were not only filled, but also continuously taken by the patients 36, 37.
Time of follow‐up
The duration of observation ranged from 1 day to 3,255 days (8.9 years), with a mean of 269 days and median of 142 days. We report results up to four years from the start of monotherapy due to the paucity of longer term observations.
Main outcome measures
The outcome was the cumulative incidence of clinically relevant hypothyroidism, as indicated by diagnosis codes (SNOMED: 40930008 and descendants) and filled hypothyroidism prescriptions: thyroxine (RxNorm: 10582 and descendants), desiccated thyroid (10572), triiodothyronine (10814) and thyroglobulin (10565), in the presence of the competing risk of ending monotherapy, adjusted for covariates. Note that the hypothyroidism hierarchy includes subclinical hypothyroidism.
Competing risk survival analysis
Our competing risks were the occurrence of hypothyroidism versus ending monotherapy. Hypothyroidism was captured as the observation of any subcategory of hypothyroidism or prescription of thyroid medications. Ending monotherapy was defined as a ≥30‐day gap in treatment for a given one of our nine therapies, or the addition of one or more of the eight other therapies (polypharmacy). Treatment exposure was considered to start on the day the prescription was filled and continued for as many days as there were days’ supply. When prescriptions overlapped, the refill was added after the previous supply would have run out. Censored observations occurred if the data ended without the competing risks being observed, either because the patient left his/her provider, or the data ended in 2013. The censor time was taken as the last event observed for a given patient.
For a first (potentially biased) look at the data, we employed the counting process‐based non parametric estimation of the cumulative incidence function (CIF). This estimator makes no correction for covariates, but separates out the cumulative risk of hypothyroidism from the cumulative risk of ending monotherapy. For a given competing risk, the estimator is the cumulative sum over each time interval of the probability neither event occurs before time t (the Kaplan−Meier estimate where both competing risks are combined as an event, and the censored observations are treated as censored) multiplied by the fraction experiencing a given event type out of those still at risk at time t 38. Differences in CIFs between lithium and alternate therapies were calculated using Gray's test 39, the log‐rank test 40, and the Pepe and Mori test 41, as implemented in R by Pintilie 42.
To adjust for biases in treatment and observation, we employed the competing risks regression (CRR) approach of Fine and Gray 43, as implemented in the R package cmprsk (version 2.2‐7), taking the following approach to covariates.
Covariates
The following covariates were examined for their effect on the CIF.
Treatment: using lithium as the reference, binary dummy variables were created for the non‐lithium treatments.
Age at treatment: accounts for the higher risk of hypothyroidism among older patients.
Sex: accounts for the higher risk of hypothyroidism among female patients.
Patient visit days in the year preceding monotherapy: a proxy for engagement with the health care system before monotherapy.
Whether thyroid testing was performed in the 14 days preceding monotherapy: accounts for observation bias—patients who tested positive for hypothyroidism were excluded since patients receiving lithium are more likely to be tested pretreatment than patients receiving other therapies.
Nonparametric thyroid testing rank during monotherapy: used to adjust for observation bias—hypothyroidism should not be diagnosed without a thyroid test, and not all treatments have comparable thyroid testing. We used the following SNOMED‐CT procedure concepts and their descendants to count thyroid tests: thyroid‐stimulating hormone measurement (61167004), thyroid hormone measurement (390780008), thyrotropin‐releasing hormone test (252219000), thyroid panel (35650009), and thyroid‐stimulating immunoglobulins measurement (104972000). To account for different observation times, covariates were calculated as follows. For each patient (p), we found the competition rank of p's number of thyroid tests among the smallest time interval centered around p's treatment time that included at least 50 patients, and normalized it to (0,1), with 1 being most frequent. While the post‐treatment thyroid testing rank covariate removes observation time from the covariate, thyroid testing due to standard monitoring for which we want to account remains confounded with thyroid testing ordered as a result of hypothyroidism symptomatology, for which we do not want to account post‐treatment.
Pretreatment prescriptions and comorbidities: in order to account for treatment biases and potential confounders with hypothyroidism risk, we create binary variables to indicate whether at least one prescription was filled for each of the following drug classes in the year before monotherapy, using the First Databank Enhanced Therapeutic Classification (FDB‐ETC) and Anatomical Therapeutic Chemical (ATC) vocabularies: analgesics (FDB‐ETC: 582), diuretics (FDB‐ETC: 248), opioids (ATC: N02A), pain (FDB‐ETC: 3079), and sedatives (FDB‐ETC: 541). We also use binary variables to indicate whether at least one diagnosis of any concept or descendant of the following occurred in the year before monotherapy, using the SNOMED‐CT vocabulary. Mental: attention‐deficit hyperactivity disorder (ADHD) (406506008), anxiety (48694002), BD (13746004), eating disorder (72366004), mental procedure (108310004), personality C (83890006), and psychosis (69322001). Other: autoimmune (85828009), cardiovascular (49601007), central nervous system (23853001), dermatological (95320005), drug dependence (191816009), endocrinopathy (362969004), hypertension (38341003), kidney (90708001), metabolic (75934005), musculoskeletal (928000), nervous system (118940003), pulmonary (19829001), seizure (128613002), or thyroidism (14304000). Note that we excluded hypothyroidism (40930008) and descendant codes from these categories. We also used a high‐level MedDRA vocabulary concept (10040978) to encompass 23 lower level sleep apnea SNOMED‐CT codes. We calculated p‐values using the R chisq.test procedure for the 2 × 9 table over the nine BD therapies to test whether the proportions were the same over all drugs.
Using CRR, a forward stepwise selection procedure was performed on the cohort, starting with a model that included sex, age, treatment, and thyroid testing rank, and incrementally added the next most significant variable in predicting hypothyroidism risk among the aforementioned covariates. To account for multiple testing of 30 covariates, we stopped when no variables achieved a significance of p < 1.67e‐3. A Wald test was employed for testing the overall significance of treatment as an eight‐level factor using the R ‘aod’ package, and Schoenfeld‐type residuals were examined for evidence of time‐varying covariates, as outlined by Scrucca et al. 44. The final resulting model was run a second time, without the thyroid testing rank covariate.
Results
Sample
Out of 141,805,491 patients, we identified 1,232,534 with at least two claims related to a BD diagnosis (Fig. 1). Almost 60% were female. Among the 1.2 million, 199,376 fulfilled our inclusion criteria of no drug treatment for BD and no diagnosis of hypothyroidism for at least one year prior to commencing monotherapy. A total of 45,550 of these patients were under the age of 18 years and were subsequently excluded. Of the remaining 153,826, 30.5% had a thyroid test before monotherapy, 40% ever, and 16% on or after commencing monotherapy. We retained these 16%, excluding 129,252 individuals who were not tested for thyroid abnormalities during monotherapy. In the remaining sample of 24,574 individuals, 65.3% were female, indicating a bias towards female patients being more likely to be tested for thyroid abnormalities.
The average age of the sample was 39.5 years (Table 1). Psychosis was present in 6.1% of the sample, drug dependence in 14.3%, anxiety disorders in 22.5%, and ADHD in 7.3%. At monotherapy commencement, 28.7% of patients received lamotrigine, 15.5% quetiapine, 14.8% lithium, 12.2% valproate, 12.1% aripiprazole, 6.2% risperidone, 5% olanzapine, 3.6% oxcarbazepine, and 2% carbamazepine. There were significant differences between the treatment groups regarding age (p = 1.80e‐4), sex (p = 8.60e‐141), and psychiatric comorbidities, such as psychosis (p = 8.20e‐232) and drug dependence (p = 1.86e‐68).
Table 1.
Cohort demographics by druga
| LITH | ARIP | CBZ | LTG | OLAN | OXC | QUET | RISP | VPA | Total | Treatment bias p‐value | Hypo‐thyroidism CRR p‐value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 3,629 | 2,964 | 492 | 7,056 | 1,230 | 890 | 3,798 | 1,518 | 2,997 | 24,574 | – | – |
| Age, years | 39.5 | 39 | 40.3 | 39 | 40.4 | 39 | 40.1 | 39.4 | 39.7 | 39.5 | 1.80E‐04 | 6.91E‐10 |
| Sex, female, % | 56.4 | 72 | 64.6 | 74 | 55.9 | 70.4 | 65.9 | 60.4 | 53.6 | 65.4 | 8.60E‐141 | 3.93E‐07 |
| Medications (%) | ||||||||||||
| Sedative | 30.8 | 38.2 | 35.2 | 33.5 | 38.5 | 34.4 | 44.4 | 37.1 | 34.8 | 36.0 | 5.09E‐37 | 7.27E‐01 |
| Analgesic | 39.1 | 47.4 | 48.4 | 43.2 | 45.1 | 49.6 | 51.8 | 45.5 | 46.5 | 45.4 | 3.19E‐28 | 4.45E‐01 |
| Opioid | 24.1 | 31.1 | 32.9 | 27 | 30.1 | 31.7 | 35 | 29.6 | 28.9 | 29.1 | 1.04E‐25 | 6.49E‐01 |
| Pain | 24.1 | 31.1 | 32.9 | 27 | 30.1 | 31.7 | 35 | 29.6 | 28.9 | 50.9 | 1.04E‐25 | 2.46E‐01 |
| Diuretic | 9.5 | 12.9 | 14.6 | 11.7 | 12.9 | 13.8 | 13.4 | 13.6 | 11.9 | 12.1 | 1.88E‐06 | 8.55E‐01 |
| Mental (%) | ||||||||||||
| Psychosis | 4.5 | 8 | 3.9 | 2 | 15.5 | 2.9 | 6.4 | 21.6 | 5 | 6.1 | 8.20E‐232 | 5.05E‐01 |
| Bipolar disorder | 71.1 | 58.4 | 67.7 | 67.4 | 53.7 | 66.5 | 54 | 53.4 | 68 | 63.2 | 2.26E‐95 | 3.96E‐01 |
| Mental procedure | 53.2 | 56.9 | 52.2 | 61.9 | 37.2 | 62.6 | 48.8 | 52.7 | 51.5 | 54.7 | 2.23E‐81 | 1.79E‐01 |
| Drug dependence | 12.8 | 13.7 | 14.8 | 9.6 | 16.9 | 13.3 | 22 | 15.5 | 16.5 | 14.3 | 1.86E‐68 | 2.45E‐01 |
| Anxiety | 18.6 | 24.5 | 20.1 | 19.5 | 27.2 | 20.1 | 29 | 26.5 | 21.4 | 22.5 | 1.43E‐39 | 8.06E‐01 |
| Eating disorder | 1 | 2.1 | 0.4 | 1.8 | 2 | 1.3 | 1.6 | 1.8 | 0.4 | 1.5 | 1.37E‐07 | 4.25E‐01 |
| ADHD | 6.1 | 9.2 | 7.1 | 7.7 | 6 | 9 | 6.5 | 7 | 7.2 | 7.3 | 1.58E‐05 | 4.14E‐03 |
| Personality C | 0.2 | 0.3 | 0.4 | 0.1 | 0.3 | 0.1 | 0.2 | 0.3 | 0.2 | 0.2 | 4.93E‐01 | 8.24E‐01 |
| Other (%) | ||||||||||||
| Cardiovascular | 31.5 | 39.9 | 37.4 | 34.2 | 38.7 | 38 | 42.9 | 41 | 40 | 37.4 | 1.42E‐30 | 8.92E‐01 |
| CNS | 13.2 | 16.7 | 19.3 | 14.5 | 16.4 | 20.1 | 18.7 | 19.3 | 21.8 | 16.8 | 3.20E‐26 | 7.73E‐02 |
| Seizure | 0.7 | 0.9 | 4.1 | 1.6 | 1.1 | 4.2 | 1.6 | 2.1 | 3.2 | 1.7 | 1.15E‐22 | 6.32E‐01 |
| Nervous system | 23.3 | 28 | 29.3 | 24.4 | 27.3 | 31.3 | 30.3 | 28.3 | 31.5 | 27.2 | 7.43E‐21 | 3.72E‐01 |
| Pulmonary | 5.3 | 7 | 7.7 | 5 | 8.7 | 7.4 | 9.5 | 8.8 | 7.1 | 6.8 | 3.76E‐20 | 5.24E‐01 |
| Hypertension | 17.9 | 23.9 | 21.1 | 19 | 22.8 | 21.6 | 25 | 25.8 | 22.6 | 21.5 | 4.52E‐20 | 8.36E‐01 |
| Metabolic | 26.1 | 33.8 | 30.9 | 28.5 | 31.4 | 29.6 | 32.5 | 31.4 | 30.9 | 30.1 | 8.63E‐12 | 2.96E‐01 |
| Musculoskeletal | 35.9 | 42 | 43.1 | 40.3 | 39.7 | 39.6 | 44.8 | 40.7 | 41 | 40.7 | 2.28E‐11 | 3.14E‐01 |
| Kidney | 3.2 | 4.9 | 4.7 | 4.1 | 4.9 | 4.3 | 5.7 | 5.4 | 4.9 | 4.5 | 2.15E‐05 | 9.23E‐01 |
| Endrocrinopathy | 11.1 | 15.6 | 12.6 | 12.5 | 11.4 | 12.1 | 13 | 13.6 | 13.1 | 12.8 | 3.51E‐05 | 9.26E‐01 |
| Dermatological | 23.4 | 27.8 | 24.8 | 27.4 | 24.6 | 26.9 | 25.4 | 25.5 | 25.6 | 26.0 | 5.38E‐04 | 8.77E‐01 |
| Apnea | 6.1 | 8.8 | 6.1 | 7.6 | 5.8 | 7.3 | 6.8 | 6.8 | 7.1 | 7.2 | 9.77E‐04 | 1.52E‐01 |
| Autoimmune | 1.2 | 2.1 | 1.6 | 1.5 | 1.5 | 2.4 | 2.2 | 1.4 | 1.7 | 1.7 | 5.78E‐03 | 9.15E‐01 |
| Thyroidism | 2.4 | 3.2 | 4.7 | 2.8 | 3 | 2.6 | 2.9 | 3.9 | 2.4 | 2.9 | 1.72E‐02 | 6.37E‐06 |
LITH = lithium; ARIP = aripiprazole; CBZ = carbamazepine; LTG = lamotrigine; OLAN = olanzapine; OXC = oxcarbazepine; QUET = quetiapine; RISP = risperidone; VPA = valproate; CRR = competing risks regression; ADHD = attention‐deficit hyperactivity disorder; CNS = central nervous system.
For each drug we show the sample size, the age in years, and then the fraction that took various treatments and had various comorbidities in the year prior to monotherapy. Note that we excluded hypothyroidism codes from every category; thus, thyroidism would encompass all other thyroid conditions. The treatment bias p‐values come from chi‐squared tests comparing the proportions of treatment within each binary category, and an ANOVA F‐statistic for age. The hypothyroidism CRR p‐values show the significance of adding the variable in the model along with treatment, sex, pretreatment thyroidism, thyroid testing rank, and 14‐day pretreatment thyroid tested. Only the bold‐type variables in the rightmost column were significant in the full CRR model after multiple testing adjustment (p < 1.67e‐03), and thus were retained in the final model.
Competing risk survival analysis
Hypothyroidism occurred in 7.5% of the sample (n = 1,850) and without bias correction, lithium appeared to have a higher risk for hypothyroidism than other therapies (Fig. 2A). The bias‐uncorrected difference between lithium and other treatment alternatives was significant in all three tests (p < 0.05) for all therapies except quetiapine and carbamazepine (Supplementary Table 1). The competing risk of ending monotherapy occurred in the majority of cases (73.1%, n = 17,954) (Fig. 2B). Censored data were present in 19.4% of the sample, including 38 deaths (n = 4,770). Not all drugs had the same risk of ending monotherapy: olanzapine had the highest risk, whereas lamotrigine had the lowest (Fig. 2B). Biases also existed in the number of thyroid tests administered per treatment group. Based on the moving average of the number of tests from the commencement of monotherapy by drug (Supplementary Fig. 1A), along with a linear fit calculating the slope (Supplementary Fig. 1B), we estimated that patients on lithium were administered thyroid tests 2.26–3.05 times as often as patients on the other drugs. Lithium also had the highest pretreatment thyroid testing frequency, patients on lithium being tested 2.2 times as often as those on the least tested therapy, lamotrigine (Supplementary Fig. 1A caption). We thus employed a regression framework to address these potential biases, along with pretreatment characteristics (Table 1).
Figure 2.
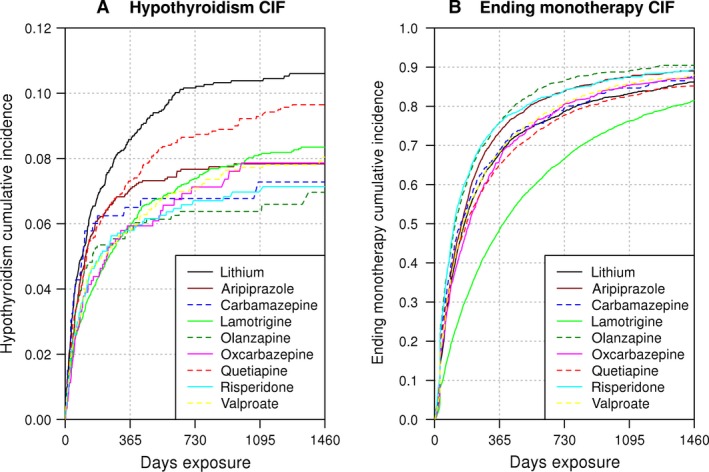
Cumulative incidence of hypothyroidism and ending monotherapy. We show the cumulative incidence function of (A) hypothyroidism and (B) ending monotherapy, calculated with the naïve non‐parametric counting process estimator (analogous to Kaplan−Meier), which separates out the risk of hypothyroidism from ending monotherapy. These curves represent the risk observed as the drugs are currently prescribed, without accounting for treatment and observation biases.
Covariate selection and regression results
Being male (p = 3.93e‐7) and having a thyroid test in the 14 days preceding monotherapy (p = 2.69e‐7) were associated with fewer diagnoses of hypothyroidism. Conversely, hypothyroidism diagnoses increased with age at exposure (p = 6.91e‐10), thyroid testing rank during monotherapy (p = 2.79e‐87), and pretreatment thyroid disorders other than hypothyroidism (p = 6.37e‐6). Except for pretreatment thyroid disorders, these variables remained significant in subcohorts with >90 and >182 days of monotherapy (Table 2). All other covariates were not significant after multiple testing adjustment (p > 1.67E‐3) when they were included in the model (Table 1). Using a Wald test, the combined significance of non‐lithium treatment was p = 3.86e‐3, p = 6.22e‐3, and p = 3.61e‐3 at >0, >90, and >182 days, respectively. Adjusted for covariates, lithium had the highest risk for >0 days of monotherapy, closely followed by quetiapine (Fig. 3). When the patient population was restricted to >90 days of exposure, lithium non‐significantly improved over quetiapine (p = 0.506). The resampled four‐year cumulative risk of hypothyroidism ranged 1.39‐fold from 8.8% for lithium down to 6.3% for oxcarbazepine (Fig. 4), and the 95% confidence limits for the lithium CIFs overlapped with those of all of the other drugs (Supplementary Fig. 2). When adjusted for covariates, the competing risk of ending monotherapy remained biased. Lamotrigine had a significantly lower risk of ending monotherapy (p = 1.89e‐55) than alternative therapies, likely due to the longer time required to titrate lamotrigine to the initial target dose to reduce the risk of Stevens−Johnson syndrome (Supplementary Fig. 3, Supplementary Table 2). Results of the sex‐specific analyses corroborated the known increased risk of hypothyroidism in women (Supplementary Fig. 4). The Schoenfeld‐type residuals for the model covariates showed little evidence that the covariates were time‐varying (Supplementary Fig. 5).
Table 2.
Competing risk regression (CRR) model of hypothyroidism risk at >0 months, >3 months, and >6 months of monotherapya
| p‐value | Beta coefficient | Standard error | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | >0 | >3 | >6 | >0 | >3 | >6 | >0 | >3 | >6 |
| Treatment (Wald) | 3.86E‐03 | 6.22E‐03 | 3.61E‐03 | – | – | – | – | – | – |
| Aripiprazole | 7.39E‐03 | 8.80E‐03 | 2.44E‐03 | −2.37E‐01 | −3.63E‐01 | −5.98E‐01 | 8.85E‐02 | 1.39E‐01 | 1.97E‐01 |
| Carbamazepine | 1.35E‐01 | 6.14E‐02 | 2.20E‐02 | −2.76E‐01 | −6.18E‐01 | −1.34E+00 | 1.85E‐01 | 3.30E‐01 | 5.85E‐01 |
| Lamotrigine | 1.47E‐03 | 2.53E‐02 | 3.48E‐02 | −2.27E‐01 | −2.26E‐01 | −2.70E‐01 | 7.12E‐02 | 1.01E‐01 | 1.28E‐01 |
| Olanzapine | 1.11E‐02 | 5.15E‐02 | 2.47E‐02 | −3.24E‐01 | −4.02E‐01 | −6.84E‐01 | 1.27E‐01 | 2.06E‐01 | 3.05E‐01 |
| Oxcarbazepine | 1.51E‐02 | 2.21E‐01 | 4.50E‐01 | −3.41E‐01 | −2.42E‐01 | −1.79E‐01 | 1.40E‐01 | 1.97E‐01 | 2.37E‐01 |
| Quetiapine | 4.16E‐01 | 5.06E‐01 | 8.51E‐01 | −6.36E‐02 | 7.45E‐02 | 2.68E‐02 | 7.82E‐02 | 1.12E‐01 | 1.43E‐01 |
| Risperidone | 5.90E‐03 | 3.88E‐01 | 6.34E‐01 | −3.19E‐01 | −1.48E‐01 | −1.06E‐01 | 1.16E‐01 | 1.71E‐01 | 2.22E‐01 |
| Valproate | 8.96E‐03 | 3.08E‐01 | 4.41E‐01 | −2.32E‐01 | −1.29E‐01 | −1.25E‐01 | 8.88E‐02 | 1.27E‐01 | 1.62E‐01 |
| Age at exposure | 6.91E‐10 | 2.14E‐05 | 1.12E‐04 | 1.12E‐02 | 1.10E‐02 | 1.32E‐02 | 1.81E‐03 | 2.59E‐03 | 3.41E‐03 |
| Sex, male | 3.93E‐07 | 9.79E‐05 | 2.28E‐05 | −2.65E‐01 | −2.93E‐01 | −4.11E‐01 | 5.23E‐02 | 7.53E‐02 | 9.70E‐02 |
| Pre‐Tx thyroidism | 6.37E‐06 | 8.50E‐02 | 6.71E‐01 | 4.68E‐01 | 2.80E‐01 | 9.73E‐02 | 1.04E‐01 | 1.63E‐01 | 2.29E‐01 |
| Post‐Tx thyroid tests rank | 2.79E‐87 | 3.25E‐53 | 3.65E‐30 | 2.88E+00 | 3.13E+00 | 2.84E+00 | 1.46E‐01 | 2.04E‐01 | 2.49E‐01 |
| Pre‐Tx thyroid tests 14 days | 2.69E‐07 | 2.74E‐03 | 9.58E‐03 | −4.16E‐01 | −4.05E‐01 | −5.06E‐01 | 8.09E‐02 | 1.35E‐01 | 1.95E‐01 |
Tx = treatment.
The final model includes six variables, with treatment coded as eight dummy variables with lithium as reference. The three treatment variable p‐values are the result of Wald tests that calculate the significance of the eight dummy variables combined. Results are contrasted with subcohorts on >3 and >6 months of monotherapy.
Figure 3.
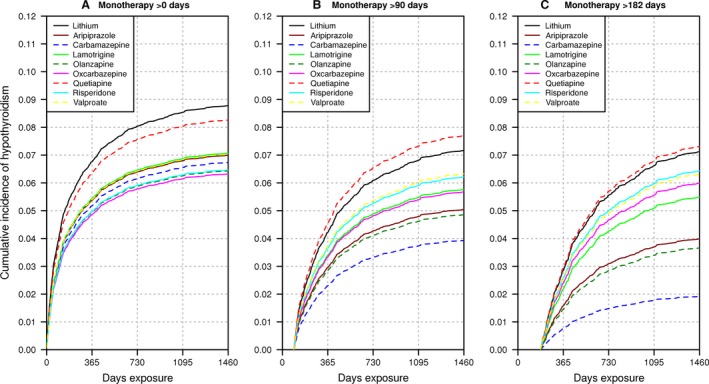
Cumulative incidence of hypothyroidism from competing risks regression (CRR): >0, >90, and >182 days after commencing monotherapy. We show the cumulative incidence function (CIF) of hypothyroidism from CRR at the average value of the covariates for the 24,574 bipolar disorder (BD) patient cohort. (A) includes all 24,574 patients with BD. We then exclude patients who experienced hypothyroidism, ended monotherapy, or were censored: (B) <90 days after commencing monotherapy (n = 15,313); and (C) <6 months after commencing monotherapy (n = 10,508). We set the covariates to the 24,574 patient cohort averages, and switch between setting the treatment covariates to 1 for each of the non‐lithium treatments, and then zero for all covariates to obtain the lithium CIF.
Figure 4.
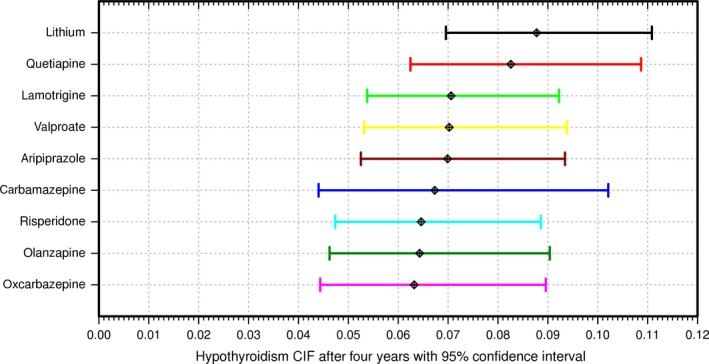
Four‐year estimated risk of hypothyroidism by drug with 95% confidence limits. We contrast the 95% confidence limits of hypothyroidism risk at four years across drugs for >0 days of monotherapy, as estimated by resampling 10,000 times the competing risks regression predictions at the average values of the covariates, with model coefficients set to random normal values defined by the regression model coefficient means and standard errors (as seen in Table 2). The mean four‐year risks are as follows: lithium: 8.78%; quetiapine: 8.26%; lamotrigine: 7.06%; valproate: 7.02%; aripiprazole: 6.99%; carbamazepine: 6.73%; risperidone: 6.46%; olanzapine: 6.43%; oxcarbazepine: 6.32%. CIF = cumulative incidence function.
When the final model was rerun, excluding the thyroid testing rank covariate, the Wald test of overall significance of treatment was, as expected, more significant: p = 2.69e‐6, p = 1.17e‐5, and p = 8.20e‐6 at >0, >90, and >182 days of monotherapy, respectively. This observation‐biased four‐year estimate for hypothyroidism risk ranged 1.54‐fold from 10.7% for lithium down to 7.0% for olanzapine.
Discussion
This is the first large‐scale comparison of all commonly prescribed mood stabilizers and atypical antipsychotics prescribed for the treatment of BD. Although lithium confers a significantly increased risk for hypothyroidism in patients with BD compared with the other therapies as a group, lithium risk was not significantly different from that of quetiapine. The predominant and clinically significant theme of this article is that there is a substantial risk of hypothyroidism developing during BD therapy, regardless of selected pharmacological intervention.
In addition to the longstanding literature on the risk of hypothyroidism under lithium treatment, there is an emerging literature demonstrating the risk of hypothyroidism associated with some, but not all, of the therapies we studied. We next summarize this evidence, first for mood stabilizers, and then for atypical antipsychotics.
A large medical claims study on mood stabilizers in patients with BD found that lithium, carbamazepine and valproate increased hypothyroidism risk, and that a dose−response relationship existed between number of mood stabilizers and risk. Therefore, the authors recommended regular thyroid monitoring for mood stabilizers 45. The product labeling of the US Food and Drug Administration (FDA) for valproate reports altered thyroid function tests associated with valproate, but makes no thyroid testing recommendations 46. Other studies show that lithium and valproate alone or in combination confer additional risk 47, 48. Conversely, for the mood stabilizer lamotrigine, FDA labeling lists hypothyroidism as rare (<1 in 1000 patients) 49, and recent reviews found little to no evidence for an effect of lamotrigine on thyroid hormones 50, 51. Despite the overlapping characteristics, and shared pharmacology of BD and epilepsy 52, 53, 54, 55, we could only find literature on carbamazepine and oxcarbazepine associated with hypothyroidism in epilepsy, a disease in which the pathogenesis may involve thyroid hormones 56. Evidence from epilepsy studies shows adverse thyroid effects from valproate 51, 57, 58, 59, carbamazepine 51, 57, 59, and oxcarbazepine 51, 59. Having similar mechanisms of action, both carbamazepine and oxcarbazepine see a reduction in T4, but little impact on thyroid‐stimulating hormone (TSH), with the effects reversible with discontinuation of therapy 60. Carbamazepine FDA labeling mentions that thyroid function tests “have been reported to show decreased values with Tegretol administered alone” 61, and oxcarbazepine labeling mentions association of the drug with decreases in T4, without changes in T3 or TSH 62, but neither labeling recommends thyroid testing. The effects of oxcarbazepine and carbamazepine seem consistent with central hypothyroidism, via a disruption of the hypothalamic‐pituitary axis 63. We did not differentiate between primary and the rarer central hypothyroidism, whose incidence has been estimated at 1:80:000 to 1:120,000 in the general population 64.
Consistent with our findings, among atypical antipsychotics, evidence of increased hypothyroidism risk has also accumulated for quetiapine 65, 66, 67, 68, 69, 70, 71. However, not all studies agreed. In some studies only changes in T4 were noticed, and not in TSH 72, 73, and in other studies the hormone changes were transient, returning to normal after only 6 weeks of treatment without discontinuation of quetiapine 74. A large, longitudinal, double‐blind clinical trial showed that a combination therapy of quetiapine plus lithium or divalproex had significantly increased hypothyroidism (p = 6.96e‐4) over placebo plus lithium or divalproex 75. Two cases of hypothyroidism with combination quetiapine and valproate were also reported in a Korean study 76. In 2013, FDA product labeling added a recommendation for thyroid hormone testing at baseline and at follow‐up for quetiapine 77. For olanzapine, we could find little information about thyroid effects, and for risperidone 67, 73 and aripiprazole 78, studies concluded that thyroid abnormalities did not occur with treatment, despite some case reports 79. FDA labeling also makes no mention of thyroid issues for these three drugs 80, 81, 82.
Our study suggests that the relative perception of risk for lithium is overestimated by observation bias: lithium‐treated patients received thyroid tests more than twice as frequently as those on other therapies. Moreover, despite the link between hypothyroidism and BD, only 40% of patients received a thyroid test in the year before or during monotherapy. According to our study, hypothyroidism appears underdiagnosed for patients with BD in clinical settings, particularly on non‐lithium therapies, suggesting that increased testing may offer clinical benefits for this treatable disorder. Treatment of even subclinical hypothyroidism may improve outcomes among patients with BD 83. Subclinical hypothyroidism has been associated with treatment resistance and/or rapid cycling 84. Patients with abnormal TSH and/or free thyroxine index spend longer times in the acute treatment phase and have significantly higher mean Hamilton Scale for Depression scores during the maintenance phase 85. Also, elevated TSH has been identified in medication‐naïve patients with mixed mania during the first episode 86. Therefore, we recommend that all patients with BD be periodically assessed for thyroid function, independent of the medication they receive. Currently, only lithium and quetiapine carry testing recommendations in their FDA product labels 77, 87.
Our results support the hypothesis that hypothyroidism occurs with increased frequency in patients with BD. Even in lithium‐naïve bipolar patients, increased hypothyroidism has been found compared to published population averages 88. Importantly, long‐term follow‐up indicates that the risk of hypothyroidism persists beyond the first year after treatment initiation, but does appear to eventually plateau for all treatments. In the case of lithium, this is consistent with a long‐term cross‐sectional study showing that patients with BD with 10–20 years of lithium exposure had the same thyroid function as patients with BD with > 20 years of exposure 89.
Several explanations exist in the literature for the observation of hypothyroidism in patients with BD. While some researchers have proposed a causal link between lithium treatment and hypothyroidism 88, 90, 91, studies on autoimmune thyroiditis (a major cause of hypothyroidism) suggest that a compromised thyroid confers a greater risk of BD 92, and that lithium use confers no risk for autoimmune thyroiditis 27. Increased hypothyroidism in BD could have several explanations. On the one hand, imbalances of the thyroid metabolism could be linked to the pathophysiology of BD, either causally or through linked, potentially parallel mechanisms, influencing each other in a bidirectional way. On the other hand, hypothyroidism could be related to treatment, suggesting that commonly used medications for BD target a common signaling pathway shared with thyroid metabolism. The therapeutic effect of these medications could lead to thyroid abnormalities as an unintended adverse effect. A third explanation would suggest that hypothyroidism occurs independently and is not related to BD risk. It may be detected in patients with BD at a higher rate because of increased testing. Our analysis cannot distinguish between these not necessarily exclusive explanations; however, our observation clearly challenges the assumption of lithium treatment as the only cause of hypothyroidism in patients with BD.
Although the analyses presented here diligently employed statistical methodologies to account for treatment biases and potential confounding factors, such studies face certain challenges. For example, the use of administrative claims data entails a retrospective study design in which patients were neither randomized to treatment nor examined directly during a defined observational period. Consequently, hypothyroidism could be underestimated if patient symptoms went unreported, or their provider did not perform thyroid testing. Because the reported risk estimates are calculated at the average value of the covariates, including thyroid testing, which is low in our population, our risk estimates may be low. While every attempt was made to correct for thyroid testing observation bias with our CRR model, we recognize that we could not disentangle increased thyroid testing due to treatment guidelines from that due to symptoms of hypothyroidism. Nevertheless, even when we exclude the thyroid testing rank covariate, the lithium hypothyroidism risk estimate increases only modestly to 1.54 times the lowest risk therapy, compared to a 1.39‐fold range in the model that corrects for tests. Nevertheless, our corroboration of not only lithium, but also age and sex as known risk factors for hypothyroidism in patients with BD 19, 93, 94 demonstrates that our population is representative of earlier studies of hypothyroidism in BD.
Results presented here demonstrate a modest (statistically significant) effect of lithium on hypothyroidism in BD, compared to alternate therapies. Estimates on the four‐year cumulative hypothyroidism incidence for lithium ranged from 1.06‐fold higher than quetiapine up to 1.39‐fold higher than oxcarbazepine. These results differ from those of a case−control meta‐analysis suggesting a 5.78‐fold increased risk of hypothyroidism over placebo 23. However, the stark differences may be explained by the fact that only lithium treatment was the focus in those and other analyses 94, whereas our results directly compared lithium with other therapies that may also carry hypothyroidism risk. Another study comparing lithium with other therapies did not account for biases in thyroid testing 45.
Since the results presented here were obtained in ‘real world’ settings, and in a sample representing much of the privately insured population of the USA, we propose that our findings are highly representative, are generalizable, and have external validity.
Conclusions
Thyroid abnormalities occur with high frequency in patients with BD regardless of treatment. Therefore, (i) patients should be regularly tested for clinical or subclinical thyroid abnormalities on all therapies and treated as indicated to prevent adverse effects of hormone imbalances on mood; and (ii) since hypothyroidism occurs under all treatments, we suggest that more emphasis should be placed on understanding the role of thyroid disorders in BD. It remains for future work to investigate hypothyroidism risk in untreated patients and patients on polypharmacy.
Author contributions
Study concept and design: CGL, BK, AJM, NGH, DJP, NWH, SSY and RLO. Acquisition, analysis, or interpretation of data: CGL, AJM, NRL, NWH and BK. Drafting of the manuscript: CGL and BK. Critical revision of the manuscript for important intellectual content: CGL, BK, DJP, NGH, SSY, AJM, RLO, NWH, NRL and MT. Statistical analysis: CGL, NWH, NRL and SSY. Obtained funding to cover cloud computing costs: CGL and AJM. Administrative, technical, or material support: DJP.
Disclosures
MT was a full‐time employee at Lilly (1997 to 2008). He has received honoraria from, or consulted for, Abbott, Actavis, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Sunovion, Forest, Gedeon Richter, Roche, Elan, Alkermes, Allergan, Lundbeck, Teva, Pamlab, Wyeth and Wiley Publishing. His spouse was a full‐time employee at Lilly (1998–2013). CGL and AJM received funding from the Reagan‐Udall Foundation for the FDA for cloud computing costs for the project (project RUF‐IMEDS‐SA_0010). The Reagan‐Udall foundation for the FDA provided access to the data used for the study, which is licensed from third parties. They provide a computational framework and resources for methods development and analysis. The funding agency had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
Supporting information
Table S1. Significance of lithium vs. alternate therapies without bias correction.
Table S2. Competing risk regression (CRR) model of risk of ending monotherapy after >0 months, >3 months, and >6 months of monotherapy.
Fig. S1. Moving average of thyroid tests as a function of exposure time and treatment.
Fig. S2. Cumulative incidence of hypothyroidism confidence limits.
Fig. S3. Cumulative incidence of ending monotherapy from CRR: >0, >90, and >182 days after commencing monotherapy.
Fig. S4. Cumulative incidence of hypothyroidism by sex from CRR: >0, >90, and >182 days after commencing monotherapy.
Fig. S5. Schoenfeld‐type residuals for model covariates.
Acknowledgements
We would like to thank two anonymous referees for their helpful suggestions.
Lambert CG, Mazurie AJ, Lauve NR, Hurwitz NG, Young SS, Obenchain RL, Hengartner NW, Perkins DJ, Tohen M, Kerner B. Hypothyroidism risk compared among nine common bipolar disorder therapies in a large US cohort. Bipolar Disord 2016: 18: 247–260. © 2016 The Authors. Bipolar Disorders Published by John Wiley & Sons Ltd.
References
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, (DSM‐5®) [Internet]. American Psychiatric Pub, 2013. Available from: http://dx.doi.org/10.1176/appi.books.9780890425596 [accessed April 2015]. [Google Scholar]
- 2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schou M, Juel‐Nielsen N, Stromgren E, Voldby H. The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry 1954; 17: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baastrup PC, Schou M. Lithium as a prophylactic agents. Its effect against recurrent depressions and manic‐depressive psychosis. Arch Gen Psychiatry 1967; 16: 162–172. [DOI] [PubMed] [Google Scholar]
- 5. Maggs R. Treatment of manic illness with lithium carbonate. Br J Psychiatry. RCP; 1963; 109: 56–65. [Google Scholar]
- 6. Goodwin FK, Murphy DL, Bunney WE Jr. Lithium‐carbonate treatment in depression and mania. A longitudinal double‐blind study. Arch Gen Psychiatry 1969; 21: 486–496. [DOI] [PubMed] [Google Scholar]
- 7. Angst J, Weis P, Grof P, Baastrup PC, Schou M. Lithium prophylaxis in recurrent affective disorders. Br J Psychiatry 1970; 116: 604–614. [DOI] [PubMed] [Google Scholar]
- 8. Baastrup PC, Poulsen JC, Schou M, Thomsen K, Amdisen A. Prophylactic lithium: double blind discontinuation in manic‐depressive and recurrent‐depressive disorders. Lancet 1970; 296: 326–330. [DOI] [PubMed] [Google Scholar]
- 9. Stokes PE, Shamoian CA, Stoll PM, Patton MJ. Efficacy of lithium as acute treatment of manic‐depressive illness. Lancet 1971; 297: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 10. Bowden CL, Calabrese JR, McElroy SL et al. A randomized, placebo‐controlled 12‐month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry. American Medical Association; 2000; 57: 481–489. [DOI] [PubMed] [Google Scholar]
- 11. Keck PEJr, McElroy SL. Pharmacological treatments for bipolar disorder In: Nathan PE, Gorman JM. eds. A Guide to Treatments that Work, fourth edition. New York, NY: Oxford University Press, 2015: 273–306. [Google Scholar]
- 12. Machado‐Vieira R, Manji HK, Zarate CA Jr. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord 2009; 11 (Suppl. 2): 92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coppen A, Standish‐Barry H, Bailey J, Houston G, Silcocks P, Hermon C. Does lithium reduce the mortality of recurrent mood disorders? J Affect Disord 1991; 23: 1–7. [DOI] [PubMed] [Google Scholar]
- 14. Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all‐cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry 2005;162: 1805–1819. [DOI] [PubMed] [Google Scholar]
- 15. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long‐term lithium treatment: a meta‐analytic review. Bipolar Disord 2006; 8: 625–639. [DOI] [PubMed] [Google Scholar]
- 16. Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ. Lithium treatment reduces suicide risk in recurrent major depressive disorder. J Clin Psychiatry 2007; 68: 380–383. [DOI] [PubMed] [Google Scholar]
- 17. Johnston AM, Eagles JM. Lithium‐associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry 1999; 175: 336–339. [DOI] [PubMed] [Google Scholar]
- 18. Kirov G, Tredget J, John R, Owen MJ, Lazarus JH. A cross‐sectional and a prospective study of thyroid disorders in lithium‐treated patients. J Affect Disord 2005; 87: 313–317. [DOI] [PubMed] [Google Scholar]
- 19. Henry C. Lithium side‐effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci 2002; 27: 104–107. [PMC free article] [PubMed] [Google Scholar]
- 20. Schou M, Amdisen A, Eskjaer Jensen S, Olsen T. Occurrence of goitre during lithium treatment. Br Med J 1968; 3: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers MP, Whybrow PC. Clinical hypothyroidism occurring during lithium treatment: two case histories and a review of thyroid function in 19 patients. Am J Psychiatry 1971; 128: 158–163. [DOI] [PubMed] [Google Scholar]
- 22. Candy J. Severe hypothyroidism–an early complication of lithium therapy. Br Med J 1972; 3: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta‐analysis. Lancet 2012; 379: 721–728. [DOI] [PubMed] [Google Scholar]
- 24. UpToDate . Lithium: Drug Information [Internet]. 2015. Available from: http://www.uptodate.com/contents/lithium-drug-information [accessed April 2015].
- 25. Cho JT, Bone S, Dunner DL, Colt E, Fieve RR. The effect of lithium treatment on thyroid function in patients with primary affective disorder. Am J Psychiatry 1979; 136: 115–116. [DOI] [PubMed] [Google Scholar]
- 26. Kuruvilla K, Karunanidhi A, Kanagasabapathy AS. Effects of long‐term lithium carbonate treatment on thyroid function in psychiatric patients. Indian J Psychiatry 1983; 25: 98–101. [PMC free article] [PubMed] [Google Scholar]
- 27. Kupka RW, Nolen WA, Post RM et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biol Psychiatry 2002; 51: 305–311. [DOI] [PubMed] [Google Scholar]
- 28. Hersh WR, Weiner MG, Embi PJ et al. Caveats for the use of operational electronic health record data in comparative effectiveness research. Med Care 2013; 51 (8 Suppl. 3): S30–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiskopf NG, Rusanov A, Weng C. Sick patients have more data: the non‐random completeness of electronic health records. AMIA Annu Symp Proc 2013; 16: 1472–1477. [PMC free article] [PubMed] [Google Scholar]
- 30. Rusanov A, Weiskopf NG, Wang S, Weng C. Hidden in plain sight: bias towards sick patients when sampling patients with sufficient electronic health record data for research. BMC Med Inform Decis Mak 2014; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reagan‐Udall Foundation for the Food and Drug Administration . Innovation in Medical Evidence Development and Surveillance (IMEDS) Program [Internet]. 2015. Available from: http://www.reaganudall.org/our-work/safety-and-better-evidence/imeds-program/ [accessed April 2015].
- 32. Regan‐Udall Foundation for the Food and Drug Administration . Innovation in Medical Evidence Development and Surveillance (IMEDS) Charter [Internet]. 2013. p. 28–9. Available from: http://www.reaganudall.org/wp-content/uploads/2013/04/RUF_IMEDS_IMEDS-Charter_041813-DRAFT2.pdf [accessed September 2015].
- 33. Quint JB. Health Research Data for the Real World: The Marketscan Databases. Truven Health Analytics, 2015. [Google Scholar]
- 34. Voss EA, Makadia R, Matcho A et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015; 22: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu S, Ma W, Moore R, Ganesan V, Nelson S. RxNorm: prescription for electronic drug information exchange. IT Prof 2005; 7: 17–23. [Google Scholar]
- 36. Cantrell CR, Eaddy MT, Shah MB, Regan TS, Sokol MC. Methods for evaluating patient adherence to antidepressant therapy: a real‐world comparison of adherence and economic outcomes. Med Care 2006; 44: 300–303. [DOI] [PubMed] [Google Scholar]
- 37. Cramer JA, Roy A, Burrell A et al. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 38. Satagopan JM, Ben‐Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer 2004; 91: 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 40. Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika 1982; 69: 553–566. [Google Scholar]
- 41. Pepe MS, Mori M. Kaplan–meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 1993; 12: 737–751. [DOI] [PubMed] [Google Scholar]
- 42. Pintilie M. Competing Risks: A Practical Perspective. West Sussex, England: John Wiley & Sons, 2006. [Google Scholar]
- 43. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 44. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010; 45: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 45. Gau C‐S, Chang C‐J, Tsai F‐J, Chao P‐F, Gau SS‐F. Association between mood stabilizers and hypothyroidism in patients with bipolar disorders: a nested, matched case‐control study. Bipolar Disord 2010; 12: 253–263. [DOI] [PubMed] [Google Scholar]
- 46. Depakote® [package insert] [Internet]. AbbVie Inc., 2015. Available from: http://www.rxabbvie.com/pdf/dep3.pdf [accessed January 2016]. [Google Scholar]
- 47. Ezzaher A, Haj Mouhamed D, Mechri A et al. Thyroid function and lipid profile in bipolar I patients. Asian J Psychiatr 2011; 4: 139–143. [DOI] [PubMed] [Google Scholar]
- 48. Tsui KYQ. The impact of Lithium on thyroid function in Chinese psychiatric population. Thyroid Res 2015; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamactil® [package insert] [Internet]. GlaxoSmithKline; Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Lamictal/pdf/LAMICTAL-PI-MG.PDF [accessed January 2016]. [Google Scholar]
- 50. Benedetti MS, Whomsley R, Baltes E, Tonner F. Alteration of thyroid hormone homeostasis by antiepileptic drugs in humans: involvement of glucuronosyltransferase induction. Eur J Clin Pharmacol 2005; 61: 863–872. [DOI] [PubMed] [Google Scholar]
- 51. Hamed SA. The effect of antiepileptic drugs on thyroid hormonal function: causes and implications. Expert Rev Clin Pharmacol 2015; 8: 741–750. [DOI] [PubMed] [Google Scholar]
- 52. Post RM, Weiss SRB, Leverich GS, Smith M, Zhang L‐X. Sensitization and kindling‐like phenomena in bipolar disorder: implications for psychopharmacology. Clin Neurosci Res 2001; 1: 69–81. [Google Scholar]
- 53. Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci Biobehav Rev 2007; 31: 858–873. [DOI] [PubMed] [Google Scholar]
- 54. Wiglusz MS, Landowski J, Cubała WJ, Agius M. Overlapping phenomena of bipolar disorder and epilepsy–a common pharmacological pathway. Psychiatr Danub 2015; 27 (Suppl. 1): S177–S181. [PubMed] [Google Scholar]
- 55. Knott S, Forty L, Craddock N, Thomas RH. Epilepsy and bipolar disorder. Epilepsy Behav. 2015; 52 (Pt A): 267–274. [DOI] [PubMed] [Google Scholar]
- 56. Tamijani SMS, Karimi B, Amini E et al. Thyroid hormones: possible roles in epilepsy pathology. Seizure 2015; 31: 155–164. [DOI] [PubMed] [Google Scholar]
- 57. Aggarwal A, Rastogi N, Mittal H, Chillar N, Patil R. Thyroid hormone levels in children receiving carbamazepine or valproate. Pediatr Neurol 2011; 45: 159–162. [DOI] [PubMed] [Google Scholar]
- 58. Kim SH, Chung HR, Kim SH et al. Subclinical hypothyroidism during valproic acid therapy in children and adolescents with epilepsy. Neuropediatrics 2012; 43: 135–139. [DOI] [PubMed] [Google Scholar]
- 59. Lai EC‐C, Yang Y‐HK, Lin S‐J, Hsieh C‐Y. Use of antiepileptic drugs and risk of hypothyroidism. Pharmacoepidemiol Drug Saf. 2013; 22: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 60. Verrotti A, Scardapane A, Manco R, Chiarelli F. Antiepileptic drugs and thyroid function. J Pediatr Endocrinol Metab 2008; 21: 401–408. [DOI] [PubMed] [Google Scholar]
- 61. Tegretol® [package insert] [Internet]. Novartis Pharmaceuticals Corporation, 2015. Available from: https://www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf [accessed January 2016]. [Google Scholar]
- 62. Trileptal® [package insert] [Internet]. Novartis Pharmaceuticals Corporation, 2014. Available from: https://www.pharma.us.novartis.com/product/pi/pdf/trileptal.pdf [accessed January 2016 ]. [Google Scholar]
- 63. Miller J, Carney P. Central hypothyroidism with oxcarbazepine therapy. Pediatr Neurol 2006; 34: 242–244. [DOI] [PubMed] [Google Scholar]
- 64. Lania A, Persani L, Beck‐Peccoz P. Central hypothyroidism. Pituitary 2008; 11: 181–186. [DOI] [PubMed] [Google Scholar]
- 65. Feret BM, Caley CF. Possible hypothyroidism associated with quetiapine. Ann Pharmacother 2000; 34: 483–486. [DOI] [PubMed] [Google Scholar]
- 66. Ramaswamy S, Siddiqui Z, Saharan S, Gabel TL, Bhatia SC. Quetiapine‐induced hypothyroidism. J Psychiatry Neurosci 2005; 30: 57. [PMC free article] [PubMed] [Google Scholar]
- 67. Greenspan A, Gharabawi G, Kwentus J. Thyroid dysfunction during treatment with atypical antipsychotics. J Clin Psychiatry 2005; 66: 1334–1335. [DOI] [PubMed] [Google Scholar]
- 68. Liappas J, Paparrigopoulos T, Mourikis I, Soldatos C. Hypothyroidism induced by quetiapine: a case report. J Clin Psychopharmacol 2006; 26: 208–209. [DOI] [PubMed] [Google Scholar]
- 69. Kontaxakis V, Karaiskos D, Havaki‐Kontaxaki B et al. P03‐10 Quetiapine and hypothyroidism: a literature review. Eur Psychiatry 2009; 24 (Suppl. 1): S1009. [Google Scholar]
- 70. Poutanen O, Iso‐Koivisto E, Työläjärvi M, Leinonen E. Quetiapine‐associated hypothyroidism in young female patients: a report of three cases. Pharmacopsychiatry 2010; 43: 237–239. [DOI] [PubMed] [Google Scholar]
- 71. Findling RL, Pathak S, Earley WR, Liu S, DelBello MP. Efficacy and safety of extended‐release quetiapine fumarate in youth with bipolar depression: an 8 week, double‐blind, placebo‐controlled trial. J Child Adolesc Psychopharmacol 2014; 24: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Delbello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double‐blind, randomized, placebo‐controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002; 41: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 73. Kelly DL, Conley RR. Thyroid function in treatment‐resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. J Clin Psychiatry 2005; 66: 80–84. [DOI] [PubMed] [Google Scholar]
- 74. Kontaxakis VP, Karaiskos D, Havaki‐Kontaxaki BJ, Ferentinos P, Papadimitriou GN. Can quetiapine‐induced hypothyroidism be reversible without quetiapine discontinuation? Clin Neuropharmacol 2009; 32: 295–296. [DOI] [PubMed] [Google Scholar]
- 75. Suppes T, Vieta E, Liu S, Brecher M, Paulsson B; Trial 127 Investigators . Maintenance treatment for patients with bipolar I disorder: results from a north american study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry 2009; 166: 476–488. [DOI] [PubMed] [Google Scholar]
- 76. Park Y‐M, Kang S‐G, Lee B‐H, Lee H‐J. Decreased thyroid function in Korean women with bipolar disorder receiving valproic acid. Gen Hosp Psychiatry. 2011; 33: 200.e13–200.e15. [DOI] [PubMed] [Google Scholar]
- 77. Seroquel XR® [package insert] [Internet]. AstraZeneca Pharmaceuticals LP, 2013. Available from: http://www.azpicentral.com/seroquel-xr/seroquelxr.pdf [accessed January 2016]. [Google Scholar]
- 78. Stip E, Tourjman V. Aripiprazole in schizophrenia and schizoaffective disorder: a review. Clin Ther 2010; 32 (Suppl. 1): S3–S20. [DOI] [PubMed] [Google Scholar]
- 79. Church CO, Callen EC. Myxedema coma associated with combination aripiprazole and sertraline therapy. Ann Pharmacother 2009; 43: 2113–2116. [DOI] [PubMed] [Google Scholar]
- 80. Zyprexa® [package insert] [Internet]. Eli Lilly and Company, 2015. Available from: http://pi.lilly.com/us/zyprexa-pi.pdf [accessed January 2016]. [Google Scholar]
- 81. Risperdal® [package insert] [Internet]. Janssen Pharmaceuticals, Inc., 2014. Available from: http://www.janssen.com/us/sites/www_janssen_com_usa/files/products-documents/risperdal.pdf [accessed January 2016]. [Google Scholar]
- 82. Abilify® [package insert] [Internet]. Bristol‐Myers Squibb Company, 2014. Available from: http://packageinserts.bms.com/pi/pi_abilify.pdf [accessed January 2016]. [Google Scholar]
- 83. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, Alpert JE. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs 2009; 23: 1003–1021. [DOI] [PubMed] [Google Scholar]
- 84. Kleiner J, Altshuler L, Hendrick V, Hershman JM. Lithium‐induced subclinical hypothyroidism: review of the literature and guidelines for treatment. J Clin Psychiatry 1999; 60: 249–255. [PubMed] [Google Scholar]
- 85. Fagiolini A, Kupfer DJ, Scott J et al. Hypothyroidism in patients with bipolar I disorder treated primarily with lithium. Epidemiol Psichiatr Soc 2006; 15: 123–127. [DOI] [PubMed] [Google Scholar]
- 86. Zarate CA, Tohen M, Zarate SB. Thyroid function tests in first‐episode bipolar disorder manic and mixed types. Biol Psychiatry 1997; 42: 302–304. [DOI] [PubMed] [Google Scholar]
- 87. Lithobid® [package insert] [Internet]. Noven Therapeutics, LLC, 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018027s056lbl.pdf [accessed January 2016]. [Google Scholar]
- 88. Valle J, Ayuso‐Gutierrez JL, Abril A, Ayuso‐Mateos JL. Evaluation of thyroid function in lithium‐naive bipolar patients. Eur Psychiatry 1999; 14: 341–345. [DOI] [PubMed] [Google Scholar]
- 89. Kraszewska A, Chlopocka‐Wozniak M, Abramowicz M, Sowinski J, Rybakowski JK. A cross‐sectional study of thyroid function in 66 patients with bipolar disorder receiving lithium for 10–44 years. Bipolar Disord 2015; 17: 375–380. [DOI] [PubMed] [Google Scholar]
- 90. Lazarus JH. The effects of lithium therapy on thyroid and thyrotropin‐releasing hormone. Thyroid 1998; 8: 909–913. [DOI] [PubMed] [Google Scholar]
- 91. Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab 2009; 23: 723–733. [DOI] [PubMed] [Google Scholar]
- 92. Vonk R, van der Schot AC, Kahn RS, Nolen WA, Drexhage HA. Is autoimmune thyroiditis part of the genetic vulnerability (or an endophenotype) for bipolar disorder? Biol Psychiatry 2007; 62: 135–140. [DOI] [PubMed] [Google Scholar]
- 93. Bauer M, Glenn T, Pilhatsch M, Pfennig A, Whybrow PC. Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord 2014; 16: 58–71. [DOI] [PubMed] [Google Scholar]
- 94. Shine B, McKnight RF, Leaver L, Geddes JR. Long‐term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 2015; 386: 461–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Significance of lithium vs. alternate therapies without bias correction.
Table S2. Competing risk regression (CRR) model of risk of ending monotherapy after >0 months, >3 months, and >6 months of monotherapy.
Fig. S1. Moving average of thyroid tests as a function of exposure time and treatment.
Fig. S2. Cumulative incidence of hypothyroidism confidence limits.
Fig. S3. Cumulative incidence of ending monotherapy from CRR: >0, >90, and >182 days after commencing monotherapy.
Fig. S4. Cumulative incidence of hypothyroidism by sex from CRR: >0, >90, and >182 days after commencing monotherapy.
Fig. S5. Schoenfeld‐type residuals for model covariates.


