Abstract
Background
Serum thymidine kinase type 1 (TK1) and canine C‐Reactive Protein (cCRP) might be useful in detecting dogs with cancer. Algorithms combining biomarkers are sometimes more accurate than results of individual tests.
Objectives
The aim of this study was to compare serum TK1 and cCRP and Neoplasia Index (NI) in healthy and tumor‐bearing dogs.
Animals
Client‐owned dogs with (n = 253) and without (n = 156) cancer.
Methods
Retrospective case–control study. Dogs with cancer were identified after submission of samples for commercial assay and case details were retrospectively collected. Healthy dogs (control) were identified through breed groups and health status was confirmed by health questionnaire for a minimum of 6 months. Serum TK1 activity was measured using a quantitative chemiluminescent assay and serum cCRP was measured using a quantitative ELISA assay.
Results
TK1 activity in the cancer (n = 253) and control group (n = 156) were 7.0 μ/L (median, range <0.5 to >100) and 1.8 μ/L (median, range 0.4 to 55.3), respectively (P < .001). cCRP concentrations in the cancer and control group were 6.0 mg/L (median, range <0.5 to >50) and 1.6 mg/L (median, range 0.09 to >50), respectively (P < .001). The NI in the cancer and control group were 6.4 (median, range 0–9.9) and 0.9 (median, range 0–7.6), respectively (P < .001). ROC AUCs of the NI and TK1 for all cancers were greater than 0.8, highest for lymphoma and histiocytic sarcoma.
Conclusions and Clinical Importance
Increased concentrations of TK1 and cCRP, when present in dogs with cancer, might be useful in confirming a diagnosis and monitoring response to treatment.
Keywords: Biomarker, Inflammation, Monitoring, Screening
Abbreviations
- cCRP
canine C‐reactive protein
- NI
neoplasia index
- TK1
thymidine kinase
Interest in TK1 as a tumor marker began in the 1970s and centers around the increased serum activity or concentration of this compound during dysregulated proliferation. Cancer cell division is frequently incomplete resulting in necrosis and release of DNA. The salvage pathway, using TK1, recaptures exogenous thymidine from the re‐utilization of DNA. As a result of active cancer proliferation and a dysregulated cell cycle, TK1 activity increases substantially and is released into the bloodstream. Furthermore, the level of TK1 activity correlates with the tumor grade and treatment. The progression of the disease increases TK1 activity and TK1 activity can decrease or return to normal with successful treatment.1, 2, 3, 4
There is high TK1 activity in non‐Hodgkins lymphoma and leukemia in people.4, 5 Increases in TK1 activity occur in a wide range of hematologic and solid tumors including lymphoma, leukemia, multiple myeloma, breast cancer, prostate cancer, and small cell lung cancer in people.4, 5, 6, 7, 8, 9, 10
Increased plasma and serum TK1 activity occurs in dogs with lymphoma and higher activity correlates with advanced stage of disease as well as poorer prognosis.11, 12, 13, 14, 15, 16 TK1 has also been investigated in dogs with hemoabdomen and correlates with the presence of hemangiosarcoma.17 TK1, in conjunction with measurement of serum canine‐specific C‐reactive protein (cCRP), has been useful when screening clinically healthy dogs for occult cancer.18 Although these studies refer to enzymatic activity, recent studies have also evaluated protein concentration.19, 20 It is not known which test is more accurate as protein concentration and enzymatic activity have not been directly compared. TK1 enzymatic activity reflects only functional intact enzyme which might be relevant to certain cancers. Protein concentration allows for measurement of TK1 as a dimer or fragment, which might be more relevant to certain solid tumors, and these assays might have less impact from variations in sample handling compared to assays of activity.21, 22 It remains to be determined what constitutes a true positive and true negative test, and if these definitions for statistics are contextual.
cCRP is an acute phase protein used as a marker of inflammation.21, 23 Increases in cCRP have been associated with cancer, pancreatitis, infections, IMHA, parvovirus infection, SIRS, polyarthritis, renal disease, and other inflammatory diseases in dogs,21, 22, 23, 24, 25, 26, 27, 28, 29 and in a recent study correlated with all‐cause mortality.18 TK1 and cCRP were combined in an algorithm to generate a Neoplasia Index (NI) which was higher in clinically normal dogs that were diagnosed with cancer within 3–6 months of sampling.18 This parallels similar findings in 2 large‐scale human trials in which residents participating in a cancer screening programs were tested for TK1 and those with increased concentrations had a higher chance of being diagnosed with cancer.14, 30
The complex relationship between inflammation and cancer underscores the rationale for combining these 2 biomarkers. Our goal was to explore TK1 and cCRP concentrations in dogs with a wide variety of cancers, such that future investigations can focus on both diagnostic applications for early detection or to support other definitive diagnostics and the use of serial monitoring to improve therapy and treatment decisions for relapsed disease.
Materials and Methods
Dogs presenting for evaluation for newly diagnosed cancer, and undergoing routine evaluation and staging, had serum collected at the time of evaluation. Any dog with a confirmed diagnosis of cancer was eligible, and included cases were deemed to have been sampled before treatment based upon the relative dates of sampling and reported treatment. After a large number of samples had been submitted, information regarding signalment and diagnosis was collected retrospectively from submission forms. In 117 cases, the submission information was not clear as to whether treatment was started before or after the sample so statistical analyses were performed considering those cases both with and without treatment. Control samples were collected and banked from dogs deemed healthy based on physical examination and history. Bloodwork or other analysis was not required for inclusion. Dogs' health was followed by owner questionnaire for a minimum of 6 months to ensure that dogs remained healthy. To determine whether differences between groups were attributable to age‐related changes in general health (such as a greater incidence of chronic inflammatory conditions), a subgroup was analyzed within the control group comparing an older subgroup with the same median age and sex/neuter status to the dogs with cancer.
Specimen Handling
All specimens were drawn using a serum separator tube, separated within 1 hour and then frozen at −20°C. Specimens were then transported with an ice pack to the laboratory by express/overnight service and run within 1 hour of thawing. This procedure was validated for both TK1 and cCRP by examining tube type, time to centrifuge, and impact of storage temperature. Both red top plain and serum separator tubes were evaluated to determine how both time and proximity to clot affect results, using random samples from dogs separate from this study that were prospectively handled in various conditions.
TK1 Assay
The TK1 assay is an indirect, modified 2‐step, competitive chemiluminesence immunoassay (CLIA)1 for the quantitative determination of TK1 in serum, previously validated for use in dogs.18, 31, 32, 33 The assay utilizes AZT as substrate and an isoluminol‐AZTMP (3′‐azido‐3′‐deoxythymidine monophosphate) conjugate.
cCRP Assay
The cCRP assay is a canine‐specific sandwich enzyme linked immunosorbent assay2 (ELISA) for the quantitative determination of CRP in canine serum, previously validated for use in dogs.18
Neoplasia Index
The Neoplasia Index was calculated using a proprietary algorithm for which the 2 biomarkers were combined using weighting factors that prevent either marker from having an inappropriately dominant effect on the result. This preserves the ability to integrate both proliferation and inflammation into a laboratory value that represents a complex physiologic process. A previously reported cohort was used to group TK1 and CRP into ranges that optimized separation between normal, benign, and cancer and then categorized by unitless discrete values (0, 1, 2, etc.) to prevent high values of 1 biomarker overly influencing the value of another.23 Receiver operating characteristic (ROC) analysis was used to determine limits that optimize specificity. This resulted in discrete cut points for both TK1 and CRP. Because TK1 is viewed as the primary biomarker in this algorithm, no weighting was applied to cCRP when TK1 was below 1.8 μ/L. The goal was to improve overall specificity. Logistic regression was then performed on the discretized data with resulting weighting coefficients for each biomarker. As a result, the Neoplasia Index is a unitless number ranging from 0 to 9.9; 0 being a healthy dog, and 9.9 the best model fit for a dog with cancer.
Statistics
Statistical analysis was performed by 1 co‐author (RR) using commercially available software.3 A Kruskal‐Wallis one‐way ANOVA on ranks or a Mann‐Whitney rank‐sum test was used to compare continuous data. A receiver operating characteristic (ROC) curve was used to determine the area under the curve (AUC) and select the optimum cutoff value that maximized the Yuden's J statistic (sensitivity +specificity−1) for sensitivity and specificity reporting. Significance was set at P < .05.
To ensure an unbiased assessment, statistical analysis was repeated by a statistician unfamiliar with the study, study subjects, and commercial company (PP). Descriptive statistics were calculated by cross‐tabulations (for categorical variables), and ANOVA (for continuous and categorical variable comparisons). Chi‐square, Fishers exact, F‐ and t‐tests were used to assess differences between the distribution of categorical and continuous measures. Multiple pairwise comparisons of the means across categorical groups were performed using Tukey's honest significance test.
To determine the power of TK, CRP, and NI for discriminating any cancer (yes/no), ROC curves based on percentile value (PV) calculations were generated as described.34 The null hypothesis that TK, CRP, and NI were no different in their predictive ability with respect to their areas under the ROC curve (AUC) was also assessed and bootstrap standard errors and bias corrected confidence intervals for AUC and marker differences were calculated.34
Results
There were 156 control and 253 tumor‐bearing dogs (cancer group). Signalment data are summarized in Table 1. Because at‐risk breeds contributed the greatest number of samples for the control group and because these were collected through breed groups, there were significantly more sexually intact dogs in the control group (P < .001). Male castrated dogs (n = 133 cancer, n = 32 control) and female spayed dogs (n = 104 cancer, n = 47 control) constituted the largest group with male intact (n = 8 cancer, n = 41 control) and female intact (n = 8 cancer, n = 35 control) occurring less frequently. Large breed dogs such as Golden Retriever, German Shepherds, and Labradors, represented the largest group. The mean age of the control group (6.5 years) was significantly less than the cancer group (9.5 years, P < .001). Signalment data and comparisons were similar with both statistical analyses. When the age‐ and sex‐matched subgroup was compared (normal dogs with the same median age and sex distribution compared to rest of control population), there were no clinically relevant differences in TK1 or cCRP and there were 5 outlying values. Overall, using pairwise comparisons of means with equal variances, TK1 increased by 0.88 units with each year of age (P = .03). However, using least squares regression and ANOVA, TK1 was not significant (0.3 units/year, P = .13).
Table 1.
Signalment data for control and cancer groups. Although both included a wide range in age, the dogs in the control group were significantly younger (P < .001), and there were more sexually intact dogs (P < .001) than in the test group which reflects a selection bias based on targeting at‐risk breeds
| Control | Cancer | |
|---|---|---|
| N | 156 | 253 |
| Median age in years (range) | 6.5 (0.4–10.9) | 9.5 (1.8–16) |
| Sex (n) | F(35), FS(47), M(42), MN(32) | F(8), FS(104), M(8), MN(133) |
| Breed (n) | Giant (5) | |
| Large (156) | Large (137) | |
| [GSD n = 58] | [Lab/mix n = 47] | |
| [Golden Retriever n = 97] | [Golden Ret/mix n = 29] | |
| Medium (31) | ||
| Small (61) | ||
| [Beagle n = 13] | ||
| Unknown (19) |
The cancer group was categorized into tumor type as depicted in Table 2 and also grouped as hematopoietic or solid tumors. The largest tumor group was lymphoma (n = 83) for which cell type was known for 13 patients (small cell n = 3, large cell n = 10). Immunophenotype was not recorded for most cases. Other hematopoietic tumors were multiple myeloma (n = 1), plasmacytoma (n = 1), and lymphocytic leukemia (n = 2). Solid tumors included carcinoma (n = 53), sarcoma (n = 36), mast cell tumor (n = 28), histiocytic sarcoma (n = 9), melanoma (n = 8), and hemangiosarcoma (n = 7).
Table 2.
Tumor type and number of dogs within the group of dogs with cancer
| Tumor Type | Number (n) |
|---|---|
| Carcinoma | 53 |
| Histiocytic sarcoma | 9 |
| Hemangiosarcoma | 7 |
| Lymphoma | 83 |
| Mast cell | 28 |
| Melanoma | 8 |
| Osteosarcoma | 16 |
| Others | 13 |
| Sarcoma | 36 |
| Hematopoeitic | 87 |
| Solid | 166 |
The use of standard red top tubes increased TK1 from 5 to 16.4 μ/L when serum was allowed to remain in contact with the clot for periods greater than 1 hour. When a serum separator tube was used and the serum was removed within 2.5 hours, there was no increase in TK1 values. Regardless of tube type, there was no effect on cCRP results (Table 3). Serum was tested at room temperature, 4°C, and −20°C. Room temperature and 4°C storage resulted in an average loss of TK1 activity within 24 hours of 18% and 12%, respectively. There was no significant loss of TK1 activity when specimens were frozen at −20°C. cCRP was unaffected at all storage conditions. Up to 2 freeze/thaw had negligible effect on both TK1 and cCRP (Table 4).
Table 3.
Effect of tube type and time on clot on TK1 (µ/L) and cCRP (mg/L)
| Specimen 1 | Specimen 2 | Specimen 3 | Specimen 4 | Specimen 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TK1 | cCRP | TK1 | cCRP | TK1 | cCRP | TK1 | cCRP | TK1 | cCRP | |
| SST1 | 4.2 | 4.3 | 7.4 | 13.6 | 3.4 | 9.6 | 1.4 | 1.2 | 3.2 | 5.6 |
| SST2 | 3.4 | 5.5 | 8.0 | 13.8 | 3.0 | 9.3 | 1.6 | 1.3 | 3.4 | 5.6 |
| RT1 | 4.4 | 4.4 | 8.8 | 14.6 | 3.0 | 8.8 | 1.2 | 1.1 | 5.0 | 5.6 |
| RT2 | 3.8 | 4.4 | 11.0 | 13.9 | 12.2 | 9.7 | 6.2 | 1.2 | 18.2 | 6.0 |
| RT3 | 13.4 | 4.4 | 20.4 | 14.5 | 6.2 | 9.8 | 14.0 | 1.2 | 21.4 | 5.6 |
All samples were allowed to clot for 20 minutes before processing. SST1 = serum separator tube; immediately centrifuged; serum removed within 1 hour. SST2 = serum separator tube; immediately centrifuged; serum removed within 2.5 hours. RT1 = red top tube; immediately centrifuged; serum separated within 1 hour. RT2 = red top tube; immediately centrifuged; serum separated within 2.5 hours. RT3 = red top tube; sat for 1 hour; centrifuged and serum separated. As a group, specimens processed without a gel barrier and delayed clot separation (RT2 and RT3 – shaded) had significantly higher TK1 concentrations than SST1 processed samples (P < .01). This difference in processing resulted in an average increase in TK1 of 4 times. Processing had no significant effect on cCRP concentrations.
Table 4.
Effect of temperature on TK1 activity and cCRP concentration in serum
| Initial | 24 hours | 48 hours | −20°C | ||||
|---|---|---|---|---|---|---|---|
| RT | 4C | RT | 4C | 1× FT | 2× FT | ||
| TK1 (U/L) | |||||||
| Specimen 1 | 16.0 | 14.6 | 15.9 | 11.9 | 12.0 | 16.8 | 15.7 |
| Specimen 2 | 31.5 | 23.5 | 31.9 | 19.2 | 24.4 | 32.4 | 31.1 |
| Specimen 3 | 7.3 | 6.3 | 6.8 | 4.4 | 5.8 | 7.1 | 7.6 |
| cCRP (mg/L) | |||||||
| Specimen 1 | 5.6 | 5.4 | 5.7 | 5.5 | 5.6 | 5.3 | 5.7 |
| Specimen 2 | 12.9 | 13.3 | 12.8 | 12.5 | 12.7 | 12.7 | 13.2 |
| Specimen 3 | 37.8 | 37.1 | 38.0 | 36.9 | 37.5 | 37.4 | 38.3 |
RT, room temperature; 4°C, 4° celcius refigeration; −20°C, minus 20° celcius; FT, freeze/thaw.
Data were not normally distributed so nonparametric statistical methods were used for data analysis. The overall median, mean and range of TK1 activity in the cancer group versus the control group were 7.0 μ/L, 20.1 μ/L (range <0.5 to >100) and 1.8 μ/L, 3.2 μ/L (range 0.4 to 55.3), respectively. Median values, 25–75% percentile, and range by tumor type are shown in the box‐and‐whisker plot in Figure 1A. The overall median, mean and range of cCRP concentrations in the cancer group versus the control group was 6.0 mg/L, 15.3 mg/L (range <0.5 to >50) and 1.6 mg/L, 3.3 mg/L (range 0.09 to >50). Median values, 25–75% percentile, and range by tumor type are shown in the box‐and‐whisker plot in Figure 1B. The overall median, mean and range of Neoplasia Index in the cancer group versus the control group was 6.4, 6.3 (range 0–9.9) and 0.9, 1.6 (range 0–7.6). Median values, 25–75% percentile, and range by tumor type are shown in the box‐and‐whisker plot in Figure 1C.
Figure 1.
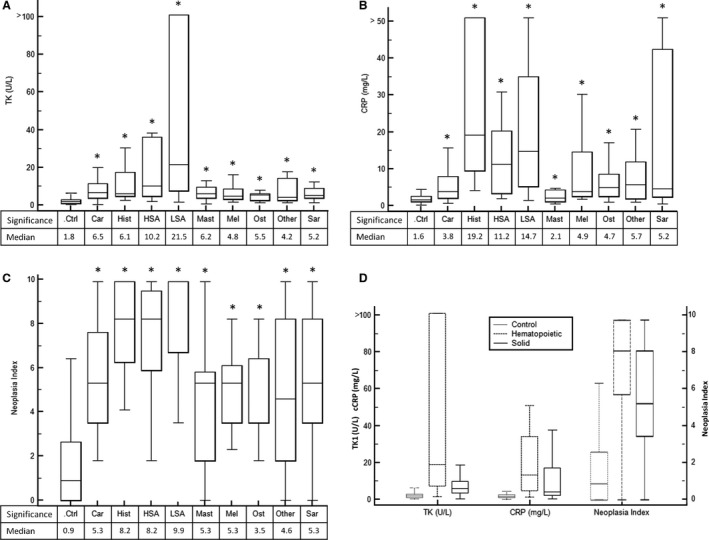
(A) Box and whiskers plot of serum TK1 activity for dogs in control and cancer groups. Groups that were statistically different (P < .05) than the control group are indicated by *. (B) Box and whiskers plot of serum cCRP for dogs in control and cancer groups. Groups that were statistically different (P < .05) than the control group are indicated by *. (C) Box and whiskers plot of Neoplasia Index for dogs in control and cancer groups. Groups that were statistically different (P < .05) than the control group are indicated by *. (D) Box and whiskers plot of TK1, cCRP, and Neoplasia Index for control, hematopoietic, and solid tumors.
When tumors were grouped as hematopoietic or solid, TK1 median, mean and range was 19.1 μ/L, 40.3 μ/L (range 1.5 to >100), and 5.9 μ/L, 9.3 (range 0.4 to >100), respectively; cCRP median, mean and range was 13.3 mg/L, 20.7 mg/L (range 1.2 to >50), and 4.1 mg/L, 12.5 mg/L (range 0.4 to >50), respectively; Neoplasia Index median, mean, and range was 8.2, 7.7 (range 0–9.9) and 5.3, 5.5 (range 0–9.9), respectively. Median values, 25–75% percentile, and range by tumor type are shown in the box‐and‐whisker plot in Figure 1D.
TK1, cCRP, and Neoplasia Index were evaluated by tumor grade. When available, across all diagnoses, tumor grade was recorded as low/grade 1, moderate/grade 2, or high/grade 3. Biomarker values and their variability both increased with grade as depicted in Figure 2. Despite the overlap of values, the difference in TK1 between high and low or moderate grade tumors, was statistically significant (P < .001). The difference in NI among grades was significant only between high and low tumors (P = .003).
Figure 2.
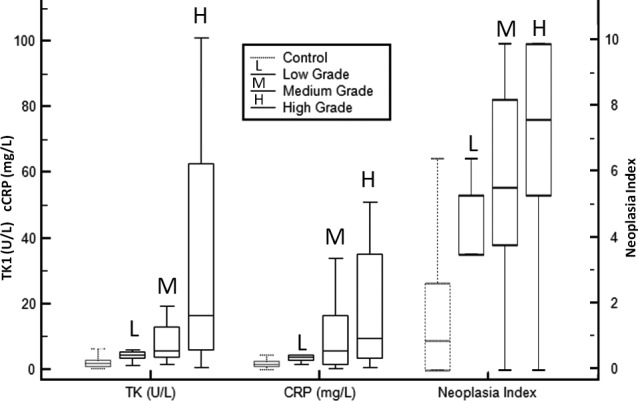
Box‐and‐whisker plot of TK1, cCRP, and Neoplasia Index for control, and tumor grades.
Using ROC analysis, Table 5 evaluates the area under the curve (AUC) for each tumor type for both the Neoplasia Index and TK1. After MedCalc analysis, in all cases except for mast cell tumor, the ROC AUC was greater using the Neoplasia Index than TK1 alone. The difference was not significant except for histiocytic sarcoma (P = .004). When grouped by solid versus hematopoietic there was no statistical difference, however, if mast cell tumors were removed from the solid group the difference became significant (P = .02). Using nonparametric analysis, the ROC AUC for TK1 was greater than for cCRP or NI (Table 6). Statistical analysis for individual tumor types was not repeated with pairwise comparisons because of small numbers in some groups. Statistical findings in Table 5 were reviewed by both statisticians and assessed as an appropriate univariate analysis.
Table 5.
TK1 and Neoplasia Index interval likelihood ratios based upon number of dogs within each group
| Interval TK1 (U/L) | Car | Hist | HSA | LSA | Mast | Mel | Ost | Othr | Sar | Ctrl | Total Cancer | Likelihood Ratio | Sens. | Spec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1.9 | 1 | 0 | 0 | 1 | 2 | 1 | 3 | 3 | 3 | 87 | 14 | 0.10 | 1.00 | 0.00 |
| 2.0–5.9 | 20 | 4 | 3 | 11 | 11 | 5 | 8 | 4 | 19 | 56 | 85 | 0.94 | 0.95 | 0.56 |
| 6.0–9.0 | 14 | 2 | 0 | 10 | 6 | 0 | 5 | 2 | 5 | 5 | 44 | 5.43 | 0.61 | 0.92 |
| 9.1–19.9 | 12 | 1 | 1 | 18 | 8 | 2 | 0 | 3 | 6 | 4 | 51 | 7.86 | 0.44 | 0.95 |
| ≥20 | 6 | 2 | 3 | 43 | 1 | 0 | 0 | 1 | 3 | 4 | 59 | 9.10 | 0.27 | 0.98 |
| Total | 53 | 9 | 7 | 83 | 28 | 8 | 16 | 13 | 36 | 156 | 253 | |||
| ROC AUC | 0.866 | 0.887 | 0.891 | 0.948 | 0.837 | 0.813 | 0.783 | 0.775 | 0.841 | 0.876 | ||||
| Neoplasia index | ||||||||||||||
| 0–1.7 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 1 | 78 | 6 | 0.05 | 1.00 | 0.00 |
| 1.8–4.1 | 21 | 1 | 1 | 9 | 11 | 3 | 10 | 4 | 13 | 62 | 73 | 0.73 | 0.87 | 0.75 |
| 4.2–5.3 | 11 | 0 | 1 | 7 | 7 | 2 | 0 | 2 | 6 | 8 | 36 | 2.78 | 0.69 | 0.90 |
| 5.4–7.5 | 4 | 2 | 0 | 4 | 3 | 2 | 3 | 0 | 4 | 7 | 22 | 1.94 | 0.56 | 0.95 |
| 7.6–10 | 17 | 6 | 5 | 62 | 5 | 1 | 3 | 5 | 12 | 1 | 116 | 71.53 | 0.46 | 0.99 |
| Total | 53 | 9 | 7 | 83 | 28 | 8 | 16 | 13 | 36 | 156 | 253 | |||
| ROC AUC | 0.886 | 0.980 | 0.935 | 0.954 | 0.805 | 0.895 | 0.862 | 0.789 | 0.873 | 0.896 | ||||
Car, carcinoma; Hist, histiocytic sarcoma; HSA, hemangiosarcoma; LSA, lymphoma; Mast, mast cell; Mel, melanoma; Ost, osteosarcoma; Othr, other; Sar, sarcoma. Likelihood ratios based on total cancer group. Numbers in boxes represent number of dogs in each stratum (except for ROC, Ratio, Sensitivity, and Specificity).
Table 6.
Summary of receiver operating characteristics (ROCs) for TK1, cCRP, and NI to discriminate cancer from healthy dogs (all tumor types combined). In this analysis (PP), TK1 performed better than cCRP (P = .038) and than NI (P = .032). There was no difference between cCRP and NI
| Variable | AUC | Standard Error | 95% Confidence Interval |
|---|---|---|---|
| TK1 | 0.873 | 0.018 | 0.838–0.908 |
| cCRP | 0.818 | 0.021 | 0.776–0.860 |
| NI | 0.844 | 0.019 | 0.807–0.882 |
Table 5 also evaluates likelihood ratios at different values for TK1 and the Neoplasia Index. In all cases, there was a significant improvement in likelihood ratios when NI was ≥7.6, when compared to TK1 as a sole biomarker. Table 7 lists tumor types with NI ≤3.0, tumor types that significantly overlap the control group (ie, false negatives). Although a few represent cancers that are typically aggressive with rapid growth or metastasis (melanoma, hemangiosarcoma, osteosarcoma), included are tumors that typically follow a more indolent course and therefore would be less likely to have a large proliferating fraction (cutaneous lymphoma, sarcomas, and anal sac adenocarcinoma). Tumor types with very high likelihood ratios for the Neoplasia Index to be increased (≥7.6) included those that produced significant increases in both TK1 and cCRP. Common aggressive tumors predominate such as carcinomas and sarcomas of the viscera, histiocytic sarcoma, most of the osteosarcoma cases in this series, lymphoma, and hemangiosarcoma (Table 8).
Table 7.
Tumor types with Neoplasia Index ≤3.0 (false negative)
| Tumor Type | Location |
|---|---|
| Carcinoma | Prostate |
| Anal gland (4) | |
| Dorsal neck | |
| Salivary | |
| Shoulder | |
| Hemangiosarcoma | Spleen |
| Lymphoma | Cutaneous (4) |
| Lymph node | |
| Mast cell | Cutaneous (9) |
| Melanoma | Oral |
| Osteosarcoma | Forelimb (3) |
| Other | Thyroid |
| Multiple myeloma | |
| Plasmacytoma cutaneous (3) | |
| Sarcoma | Fibrosarcoma mandible |
| Neurofibrosarcoma medistinal | |
| Spindle cell oral | |
| Sarcoma elbow (2) | |
| Sarcoma abdominal | |
| Chondrosarcoma chest |
Table 8.
Tumor types with Neoplasia Index ≥7.6 (high likelihood ratio)
| Tumor Type | Location |
|---|---|
| Carcinoma | Bladder |
| Stomach | |
| Lung (2) | |
| Liver (2) | |
| Upperjaw | |
| Kidney | |
| Spleen | |
| Inguinal | |
| Anal sac (3) | |
| Submandibular | |
| Sinus | |
| Jugular furrow | |
| Hist Sar | Mediastinum |
| Distal humerus | |
| Chest (2) | |
| Liver | |
| Pulmonary | |
| Hemangiosarcoma | Tongue |
| Pelvic canal | |
| Spleen | |
| Liver (2) | |
| Lymphoma | Lymph node (53) |
| Liver (2) | |
| Periorbital | |
| Bone marrow | |
| Epitheliotropic | |
| Spleen (3) | |
| Mast cell | Cutaneous (5) |
| Melanoma | Oral |
| Osteosarcoma | Forelimb (2) |
| Ilium | |
| Other | Gastric |
| Thyoma (2) | |
| Blood (leukemia) | |
| Spleen | |
| Sarcoma | Spleen (3) |
| Heart | |
| Shoulder | |
| Axilla | |
| Leg | |
| Lung | |
| Liver | |
| Pelvis | |
| Prostate | |
| Forelimb |
Using pairwise comparisons, round cell tumors had significantly higher TK1 activity (P < .001) and NI (P < .04) than epithelial and mesenchymal tumors. There was no difference in TK1 activity, cCRP concentration, or NI when the effect of possible treatment was considered for cases (n = 117) for which it was unclear whether treatment had been started before the blood draw (P > .05 for all).
Discussion
These data demonstrate that TK1 is significantly higher in dogs with a wide range of hematopoietic and solid tumors than in healthy dogs. Dogs with multicentric lymphoma have a median of 6.2 μ/L with 47% above the reference interval compared to our study median of 21.5 μ/L and 86% above the reference interval, which included cases that generate less TK1 activity such as cutaneous and indolent lymphoma.16 There is no significant TK1 increase in dogs with solid tumors unlike what has been demonstrated in our study. Results will differ between plasma and serum, making comparisons problematic. Also, sample handling and shipment in a cooled container without being frozen until the following day upon arrival at the laboratory, could affect results.11 We found that the handling of specimens for TK1 testing is important. This could explain the difference in findings between our and other studies. Also with regard to hematopoietic tumors, lymphoma of T cell immunophenotype produces lesser increases in TK1.16 Unfortunately, most dogs with lymphoma in our series did not have their lymphoma immunophenotyped, which is a limitation of the interpretation of our results. A subset of T‐cell lymphoma cases could explain the variability in our results, and could hinder understanding the value of TK1 in monitoring B‐cell lymphoma. However, given the usual distribution of cases, it is likely that most cases were of B‐cell origin and the highest values of TK1 were from among the lymphoma cases, most of which were very high. Therefore, although low values (primarily from cutaneous lymphoma) might lower the overall value, the greater concern is perhaps an inability to identify how many of the dogs with high TK1 might have had T‐cell lymphoma, thus suggesting some utility in that disease.
Marked increases in serum TK1 were seen in dogs with histiocytic sarcoma, and this tumor type can be very unpredictable in its behavior and difficult to treat. The use of TK1 for monitoring dogs with this disease should be explored.
The higher TK1, cCRP, and NI in dogs with increasing grade of tumor is unsurprising, given that higher grade tumors have consistently been associated with higher markers of proliferation on immunohistochemistry (Ki67, AgNORs, PCNA).22, 35 These biomarkers could be useful in identifying dogs with a higher risk of failure from their disease, and those that could be more likely to benefit from more aggressive local and systemic treatment. Although TK1 was not evaluated in the findings presented here as a prognostic factor, its association with outcome in hematopoietic tumors is well‐documented, as is the association of grade with outcome across most tumor types. The use of TK1 to monitor response to treatment should also be explored.
Consistent with previous work by the authors,18 the Neoplasia Index is able to achieve high likelihood ratios when increased (high specificity [or strong rule‐in]) and conversely very low likelihood ratios when decreased (high sensitivity [or strong rule‐out]). Therefore, different cutoffs would be used for screening (higher sensitivity) than for monitoring response to treatment (higher specificity). The data presented here are largely descriptive in nature and create a baseline for future studies.
Acknowledgments
Conflict of Interest Declaration: Dr Selting is a paid consultant to Veterinary Diagnostics Institute (VDI), Inc., and Randy Ringold is an employee of VDI. Neither had direct contact with or knowledge of the cases included in this series and all information on disease diagnosis and treatment status was collected after samples were assayed. Dr Husbands had no role in interpretation of the data and contributed only samples, requested case information, and assisted with preparation of the manuscript. Dr Pithua had no prior knowledge of the study or subjects at the time of statistical analysis, and is not affiliated with VDI in any way.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Samples were submitted from 11 practices nationwide with 126 cases from Dr Husbands's practice in Minnesota, all assays and initial statistics were run at VDI in California, and all data was collated at the University of Missouri. This research was supported by the Veterinary Diagnostics Institute, Inc. and was presented at the Annual Conference of the Veterinary Cancer Society in Minneapolis, MN, October 2013.
Footnotes
LIAISON, DiaSorin, Stillwater, MN
TECO Medical, Sissach, Switzerland
MedCalc version 15.8, Ostend, Belgium
References
- 1. Taylor A Jr, Hewitt EG, Jones OW. Tumor‐associated thymidine kinase in the sera of rats with transplanted hepatomas. Cancer Res 1976;36:2070–2072. [PubMed] [Google Scholar]
- 2. Ellims PH, Van der Weyden MB, Medley G. Thymidine kinase isoenzymes in human malignant lymphoma. Cancer Res 1981;41:691–695. [PubMed] [Google Scholar]
- 3. Gronowitz JS, Hagberg H, Kallander CF, et al. The use of serum deoxythymidine kinase as a prognostic marker, and in the monitoring of patients with non‐Hodgkin's lymphoma. Br J Cancer 1983;47:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rehn S, Gronowitz JS, Kallander C, et al. Deoxythymidine kinase in the tumour cells and serum of patients with non‐Hodgkin lymphomas. Br J Cancer 1995;71:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadamori N, Ichiba M, Mine M, et al. Clinical significance of serum thymidine kinase in adult T‐cell leukaemia and acute myeloid leukaemia. Br J Haematol 1995;90:100–105. [DOI] [PubMed] [Google Scholar]
- 6. McKenna PG, O'Neill KL, Abram WP, et al. Thymidine kinase activities in mononuclear leukocytes and serum from breast cancer patients. Br J Cancer 1988;57:619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Gaast A, van Putten WL, Oosterom R, et al. Prognostic value of serum thymidine kinase, tissue polypeptide antigen and neuron specific enolase in patients with small cell lung cancer. Br J Cancer 1991;64:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robertson JF, O'Neill KL, Thomas MW, et al. Thymidine kinase in breast cancer. Br J Cancer 1990;62:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poley S, Stieber P, Nussler V, et al. Serum thymidine kinase in non‐Hodgkin lymphomas with special regard to multiple myeloma. Anticancer Res 1997;17:3025–3029. [PubMed] [Google Scholar]
- 10. Zhou J, He E, Skog S. The proliferation marker thymidine kinase 1 in clinical use. Mol Clin Oncol 2013;1:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura N, Momoi Y, Watari T, et al. Plasma thymidine kinase activity in dogs with lymphoma and leukemia. J Vet Med Sci 1997;59:957–960. [DOI] [PubMed] [Google Scholar]
- 12. von Euler H, Einarsson R, Olsson U, et al. Serum thymidine kinase activity in dogs with malignant lymphoma: a potent marker for prognosis and monitoring the disease. J Vet Intern Med 2004;18:696–702. [DOI] [PubMed] [Google Scholar]
- 13. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med 2004;18:595–596. [PubMed] [Google Scholar]
- 14. von Euler H, Eriksson S. Comparative aspects of the proliferation marker thymidine kinase 1 in human and canine tumour diseases. Vet Comp Oncol 2011;9:1–15. [DOI] [PubMed] [Google Scholar]
- 15. Von Euler HP, Rivera P, Aronsson AC, et al. Monitoring therapy in canine malignant lymphoma and leukemia with serum thymidine kinase 1 activity–evaluation of a new, fully automated non‐radiometric assay. Int J Oncol 2009;34:505–510. [PubMed] [Google Scholar]
- 16. Elliott JW, Cripps P, Blackwood L. Thymidine kinase assay in canine lymphoma. Vet Comp Oncol 2013;11:1–13. [DOI] [PubMed] [Google Scholar]
- 17. Thamm DH, Kamstock DA, Sharp CR, et al. Elevated serum thymidine kinase activity in canine splenic hemangiosarcoma. Vet Comp Oncol 2012;10:292–302. [DOI] [PubMed] [Google Scholar]
- 18. Selting KA, Sharp CR, Ringold R, et al. Serum thymidine kinase 1 and C‐reactive protein as biomarkers for screening clinically healthy dogs for occult disease. Vet Comp Oncol 2015;13:373–384. [DOI] [PubMed] [Google Scholar]
- 19. Jagarlamudi KK, Westberg S, Ronnberg H, et al. Properties of cellular and serum forms of thymidine kinase 1 (TK1) in dogs with acute lymphocytic leukemia (ALL) and canine mammary tumors (CMTs): implications for TK1 as a proliferation biomarker. BMC Vet Res 2014;10:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiran KJ, Sharif H, Westberg S, et al. High levels of inactive thymidine kinase 1 polypeptide detected in sera from dogs with solid tumours by immunoaffinity methods: implications for in vitro diagnostics. Vet J 2013;197:854–860. [DOI] [PubMed] [Google Scholar]
- 21. Eckersall PD, Bell R. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 22. Mansfield CS, James FE, Robertson ID. Development of a clinical severity index for dogs with acute pancreatitis. J Am Vet Med Assoc 2008;233:936–944. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura M, Takahashi M, Ohno K, et al. C‐reactive protein concentration in dogs with various diseases. J Vet Med Sci 2008;70:127–131. [DOI] [PubMed] [Google Scholar]
- 24. Nielsen L, Toft N, Eckersall PD, et al. Serum C‐reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J Vet Intern Med 2007;21:1231–1236. [DOI] [PubMed] [Google Scholar]
- 25. Griebsch C, Arndt G, Raila J, et al. C‐reactive protein concentration in dogs with primary immune‐mediated hemolytic anemia. Vet Clin Pathol 2009;38:421–425. [DOI] [PubMed] [Google Scholar]
- 26. Raila J, Schweigert FJ, Kohn B. C‐reactive protein concentrations in serum of dogs with naturally occurring renal disease. J Vet Diagn Invest 2011;23:710–715. [DOI] [PubMed] [Google Scholar]
- 27. Kocaturk M, Martinez S, Eralp O, et al. Prognostic value of serum acute‐phase proteins in dogs with parvoviral enteritis. J Small Anim Pract 2010;51:478–483. [DOI] [PubMed] [Google Scholar]
- 28. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care 2009;19:450–458. [DOI] [PubMed] [Google Scholar]
- 29. Foster JD, Sample S, Kohler R, et al. Serum biomarkers of clinical and cytologic response in dogs with idiopathic immune‐mediated polyarthropathy. J Vet Intern Med 2014;28:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao X, Wang Y, Yang P, et al. Application of serum thymidine kinase 1 of 26 055 cases in health screening for early detection of premalignant/early malignant tumors. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1029–1034. [DOI] [PubMed] [Google Scholar]
- 31. Ohrvik A, Lindh M, Einarsson R, et al. Sensitive nonradiometric method for determining thymidine kinase 1 activity. Clin Chem 2004;50:1597–1606. [DOI] [PubMed] [Google Scholar]
- 32. Konoplev SN, Fritsche HA, O'Brien S, et al. High serum thymidine kinase 1 level predicts poorer survival in patients with chronic lymphocytic leukemia. Am J Clin Pathol 2010;134:472–477. [DOI] [PubMed] [Google Scholar]
- 33. von Euler HP, Ohrvik AB, Eriksson SK. A non‐radiometric method for measuring serum thymidine kinase activity in malignant lymphoma in dogs. Res Vet Sci 2006;80:17–24. [DOI] [PubMed] [Google Scholar]
- 34. Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J 2009;9:1. [PMC free article] [PubMed] [Google Scholar]
- 35. Madewell BR. Cellular proliferation in tumors: a review of methods, interpretation, and clinical applications. J Vet Intern Med 2001;15:334–340. [PubMed] [Google Scholar]


