Abstract
A new reaction for the rhodium‐catalyzed geminal‐difunctionalization‐based fluorination is presented. The substrates are aromatic and aliphatic diazocarbonyl compounds. As the fluorine source a stable and easily accessible benziodoxole reagent was used. A variety of alcohol, phenol, and carboxylic acid reagents were employed to introduce the second functionality. The reaction was extended to trifluoromethylation using a benziodoxolon reagent. The fluorination and trifluoromethylation reactions probably proceed by a rhodium‐containing onium ylide type intermediate, which is trapped by either the F or CF3 electrophiles.
Keywords: diazo compounds, fluorine, hypervalent compounds, reaction mechanisms, rhodium catalyst
Organofluorine compounds have widespread use as pharmaceuticals,1 agrochemicals,2 and as ligands for medicinal imaging.3 This widespread use is based on the advantageous pharmacokinetic and metabolic properties of organofluorine species, as well as the nuclear properties of the artificial 18F isotopes incorporated into bioactive substances. However, in most cases fluorine is just one of the important functionalities in bioactive molecules. In bioactive organofluorines other functional groups interacting with the macromolecules of the leaving organisms are also necessary. Therefore, development of difunctionalization (or polyfunctionalization) based fluorination/fluoroalkylation methods are very important.4
The group of Sodeoka5 and our group6 independently published the first catalytic vicinal (Cα‐β) oxytrifluoromethylation method using the Togni reagent.4a This study was followed by a large number of excellent studies on oxytrifluoromethylation,7 aminotrifluoromethylation,8 carbotrifluoromethylation,9 and related difunctionalizations.10 Similarly, development of oxyfluorination,11 aminofluorination,11a,11d,11e, 12 carbofluorination,11a, 13 and similar methodologies14 have attracted a lot of recent interest. Most of the methods are based on vicinal fluoro functionalization of π‐systems, such as alkenes. Recently, several difunctionalization methods appeared to introduce fluorine together with another functional group at the geminal (Cα‐α) carbon atom.15 However, trifluoromethylation‐based geminal difunctionalization is a largely unexplored area in organic synthesis.16
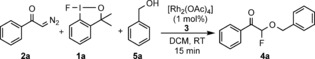
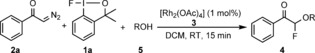
An interesting approach for geminal‐difunctionalization‐based fluorination15c–15g involves use of diazocarbonyl compounds.17 The first studies on halofluorination of diazocarbonyl compounds were reported by Olah and co‐workers,18 and was followed by several recent studies,15c–15e in which either selectfluor or hypervalent iodine reagents were used for the introduction of fluorine. Geminal oxyfluorination is a much less explored area than halofluorination. Wakselman and Leroy reported19 a method for electrophilic oxyfluorination of diazocarbonyl compounds with trifluoro (fluoro‐oxy)methane (CF3OF). The synthetic scope was limited by application of CF3OF, which contained both the alkoxy and fluorine component in the same reagent. We have now found an alternative approach for oxyfluorination of diazocarbonyl compounds (such as 2 a) by using the fluoro‐benziodoxole reagent 1 a 20 and various alcohols (see Table 1).
Table 1.
Oxyfluorination of 2 a under various reaction conditions.[a]
| Entry | Deviation from the optimal reaction conditions | Yield [%][b] |
|---|---|---|
| 1 | none | 62 |
| 2 | 1.2 equiv of 2 a | 50 |
| 3 | 5 mol % of 3 | 65 |
| 4 | [Rh(PPh3)3Cl] | 5[c] |
| 5 | [{Rh(COD)Cl}2] | 6[c] |
| 6 | [Rh2(esp)2] | 56 |
| 7 | without 3 | NR |
| 8 | −20 °C instead of RT | 60 |
| 9 | THF | 32 |
| 10 | toluene | 47 |
| 11 | acetonitrile | 44 |
| 12 | Selectfluor instead of 1 a | 0 |
| 13 | NFSI instead of 1 a | 60 |
[a] Optimal reaction conditions: 1 a (0.1 mmol), 2 a (0.2 mmol), 5 a (0.3 mmol), [Rh2(OAc)4] (3; 0.001 mmol) was reacted in DCM (2.0 mL) for 15 minutes at RT. [b] Yield of isolated product. [c] 19F NMR yield using trifluoromethyl benzene as internal standard. COD=1,5‐cyclooctadiene, DCM=dichloromethane, NFSI=N‐fluorobenzenesulfonimide, THF=tetrahydrofuran.
The attractive feature of this method is the separation of the fluorine and alkoxide sources into two reagents (1 a and the alcohol component), thus potentially resulting in broad synthetic scope. In addition, the electrophilic fluorination reagent 1 a is very stable11b and easily accessible from nucleophilic fluorinating sources, and is potentially important for the development of late‐stage fluorination methods.21 Very recent synthetic applications demonstrated that 1 a is an excellent fluorinating reagent22 in copper‐, zinc‐, palladium, and acid‐catalyzed reactions.11b,11f, 14a, 15a, 20c,20d However, as far as we know this reaction is the first rhodium‐catalyzed fluorination with 1 a. After extensive optimization, we found (Table 1) that the best reaction conditions comprised 2.0 equivalents of 2 a and 3.0 equivalents of the alcohol, and 1 mol % of the rhodium catalyst 3. The reaction in DCM was complete within 15 minutes at room temperature (entry 1). Use of less than 2.0 equivalents of 2 a afforded an acceptable, but lower yield (entry 2). Probably the reaction intermediate formed from 2 a is unstable, thus using excess 2 a with respect to 1 a is beneficial for the yield. Increase of the catalyst loading (entry 3) did not lead to significantly higher yield. [Rh2(OAc)4] proved to be the best catalyst, as [Rh(PPh3)3Cl] and [{Rh(COD)Cl}2] gave only trace amounts of the 4 a (entries 4 and 5). The reaction catalyzed with [Rh2(esp)2] proceeds with 56 % yield (entry 6). We could not observe any formation of the oxyfluorinated product in the absence of the rhodium catalyst (entry 7). Using −20 °C instead of room temperature did not change the yield significantly (entry 8). The solvent screening showed that DCM was the best solvent (entries 9–11). Selectfluor was not a suitable fluorine source (entry 12). Huang and co‐workers15g have shown that diazoacetates react with NFSI to give geminal amino fluorination products under metal‐free conditions. We found that NFSI with 2 a and 5 a also gave 4 a in the rhodium‐catalyzed reaction (entry 13). In this process, we did not find the aminofluorination product. Formation of 4 a was not observed from a reaction of 2 a, 5 a and NFSI under metal‐free conditions.
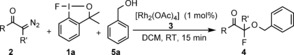
With the optimal reaction conditions in hand, we studied the synthetic scope of the reaction. First we investigated the reactivity of various diazo carbonyl substrates. Methyl‐substituted diazocarbonyl compounds (2 b–d) reacted to give a slightly lower product yield than 2 a (Table 2, entries 2–4). However, in the presence of an electron‐withdrawing fluoro substituent (2 e) the yield was higher than with 2 a (entry 5). The diazoketone 2 f, having an electron‐donating para‐methoxy substituent, reacted with a significantly lower yield than 2 e (entry 6). Heteroaryls, such as the furyl‐substituted diazoketone 2 g, reacted to give a similar yield to that obtained with 2 a (entry 7). Not only aromatic but also aliphatic diazoketones could be employed. The reaction with 2 h and 2 i proceeded smoothly (entries 8 and 9). In case of 2 i the reaction gave the product 4 i with a fluorine substituent at the quaternary carbon center. In the presence of a carbethoxy substituent in the diazo compound 2 j the reaction was slower than for the parent compound 2 a (entry 10). The reaction of the diazoester 2 k and 1 a gave a complex reaction mixture from which 4 k could be isolated in only 35 % yield (entry 11). Apparently diazoketones are more reactive substrates in the geminal oxyfluorination reaction than diazoesters. NFSI proved to be useful for oxyfluorination of 2 h but was inefficient for the synthesis of quaternary fluorines 4 i–j (entries 9 and 10). The presented oxyfluorination method could easily be scaled up to five times the original scale without a significant change in yield (entry 1).
Table 2.
Reaction of various diazocarbonyl compounds.[a]
|
[a] For reaction conditions see caption [a] in Table 1. Yield is that of the isolated product. [b] Reaction performed on 0.5 mmol scale. [c] NFSI (0.1 mmol) was used as a fluorine source. [d] The reaction time was 2 h. [e] Yield determined by 19F NMR analysis using trifluoromethylbenzene as an internal standard.
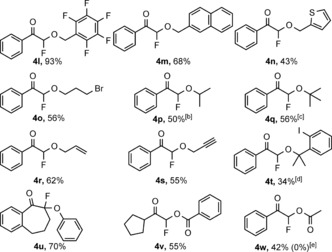
A variety of alcohols could be employed in the rhodium‐catalyzed geminal oxyfluorination reaction (Table 3). Electron‐deficient benzyl alcohol reacted with high yield to give 4 l. The substrate scope could be extended to naphthyl (4 m) and heteroaromatic (4 n) alcohols. The product 4 m was crystalline, so we were able to obtain an X‐ray structure for this compound. Linear or branched aliphatic alcohols including tBuOH also reacted, albeit with moderate yields (4 o–q). For the synthesis of oxyfluorinated products with iPr (4 p) and tBu (4 q) groups the amount of the alcohol used had to be increased to obtain acceptable yields. The reaction easily could be carried out with allyl (4 r) and propargylic alcohols (4 s). Interestingly, fluorination11a,11b, 14a, 15a of the alkene and alkyne functionalities was avoided. In the absence of any external alcohol the product 4 t incorporated the alcohol component of 1 a. The substrate scope of the geminal oxyfluorination was extended to phenol and carboxylic acids. Phenol reacted with the cyclic diazo compound 2 i under the standard optimized reaction conditions, thus affording the quaternary phenoxyfluorination product 4 u. The reaction of benzoic and acetic acid afforded the acyloxyfluorinated products 4 v and 4 w, respectively, with moderate yields. NFSI was inefficient for acyloxyfluorination.
Table 3.
Variation of the different oxygen nucleophiles.[a]
|
[a] For reaction conditions see caption [a] of Table 1. Yield is that of the isolated product. [b] Used 6.0 equiv of iPrOH. [c] Used 12.0 equiv of tBuOH. [d] Without added alcohol. [e] NFSI (0.1 mmol) was used as fluorine source.
In the presented oxyfluorination method fairly complex bioactive alcohols can also be used for transformation of the hydroxy functionality. For example, cholesterol reacted with 2 a in the presence of 1 a and the catalyst 3 in a rapid process (15 min) to afford 4 x in 68 % yield with a diasteromeric ratio of 1:1 (Figure 1).
Figure 1.
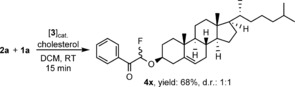
Oxyfluorination of cholesterol.
As a part of our fluorine chemistry program,6, 9a, 11a, 14a, 15a, 23 we compared the reactivity and selectivity features of 1 a and its CF3 counterpart 1 b (Figure 2). To our delight 1 b reacted with 2 a and 5 a under basically the same reaction conditions as 1 a, thus resulting in 6 a, the CF3 analogue of 4 a. However, the yield of 6 a was poor (30 %), therefore we used the benziodoxolon analogue 1 c in the presence of 5 equivalents of benzyl alcohol (5 a) to improve the yield to 85 %. The Umemoto reagent24 1 d could also be used albeit with low yield (11 %).
Figure 2.
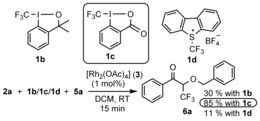
Rhodium‐catalyzed oxytrifluoromethylation of 2 a.
It is remarkable that under basically the same catalytic conditions 1 a and 1 b/1 c gave the same type of geminal difunctionalized products, 4 a and 6 a, respectively. According to our experience with copper‐ and palladium‐catalyzed reactions the F and CF3 benziodoxole(on) reagents give different types of products.6, 9a, 11a, 14a, 15a, 23c The same outcome for the rhodium‐catalyzed geminal difunctionalization of 2 a with either 1 a or 1 b/1 c indicates a similar mechanism is operative. This assumption is a noteworthy, because in the previously reported studies substantially different mechanisms have been postulated for the F and CF3 group transfer from benziodoxole(on) systems.5, 6, 8a, 9a, 11a,11b,11f, 14a, 15a, 20d, 23c
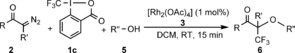
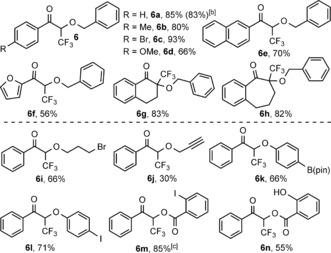
As 1 c gave the highest yield in the oxytrifluoromethylation reactions, we used this reagent in further studies. Similar to the fluorination (Tables 2 and 3), the synthetic scope of the trifluoromethylation reaction is also very broad (Table 4). Variation of the diazoketone components gave a pattern similar to that we observed for the fluorination reaction (upper part of Table 4). The reaction with different substituents at the para‐position of the diazoketone gave the products 6 a–d with good to high yields. Similar to the fluorination reaction (Table 2, entry 6), the para‐methoxy product 6 d (Table 4) was formed with lower yield than the other analogues (6 a–c). The yield is somewhat decreased, when the aryl group is replaced with naphthyl (6 e) or furyl (6 f) substituents. However, cyclic diazoketones reacted smoothly with high yield to give the quaternary CF3 products 6 g–h. The hydroxy component could also be successfully varied (lower part of Table 4). An aliphatic alcohol with a Br handle reacted smoothly to give 6 i. However, the product 6 j, from 2 a and propargylic alcohol, was formed with low yield. The possible reason is a lower tolerance of the propargyl group in trifluoromethylation than in the analogous fluorination reaction (Table 3, 4 s). Similarly to the analogous fluorination reactions, phenoxytrifluoromethylation and acyloxytrifluoromethylation reactions were easily performed and resulted in 6 k–n (Table 4). The iodo and Bpin handles in 6 k and 6 l, respectively, were nicely tolerated. When 2‐iodobenzoic acid was added, 6 m was formed in high yield, apparently involving the rest part of 1 c (see Table 3, 4 t). Apparently, the acyloxy functionality reacts faster than the phenoxy one, as the internal competition reaction resulted in solely 6 n (Table 4). This reaction could also be scaled up to five times the original scale without a significant change in yield.
Table 4.
Oxytrifluoromethylation of diazo compounds.[a]
|
[a] The reaction conditions are the same as for fluorination (see caption [a] in Table 1) except that 5 equivalents of ROH were used. [b] The reaction was performed on a 0.5 mmol scale. [c] With addition of 1.5 equiv of 2‐iodobenzoic acid.
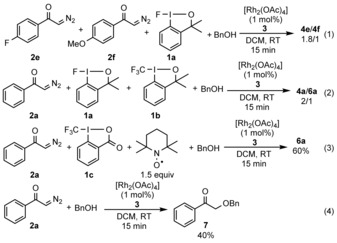
To obtain more insight into the electron demand of the process we reacted 2 e and 2 f with 1 a (one equivalent of each) under our standard reaction conditions. This competitive reaction resulted in 4 e and 4 f in a 1.8 to 1 ratio [Eq. (1)], thus indicating that an electron‐deficient diazoketone (2 e) reacts faster than an electron‐rich one (2 f). The fluorination with 1 a proceeded about twice as fast as the trifluoromethylation with 1 b [Eq. (2)]. In many cases TEMPO inhibits the trifluoromethylation reactions with 1 c, and often leads to postulation of a radical CF3 transfer mechanism.4a Yet, the rhodium‐catalyzed oxytrifluoromethylation of 2 a is not affected at all by the addition of TEMPO [Eq. (3); Table 4, 6 a]. When 2 a was reacted with BnOH (5 a) in the absence of 1 a, the reaction resulted in 7 [Eq. (4)]. This product suggests that the C−O bond formation is very easy and it probably occurs before the fluorination step.
Based on the above experiments and literature data17a,17b,17g we postulate a mechanism for the fluorination process (Figure 3). Considering the reported analogous rhodium‐catalyzed reactions of diazoketones, the first step is probably formation of the rhodium carbenoid25 8 from 2 a and 3. The insertion of the hydroxy group into the carbenoid gives onium ylide 9. Formation of onium ylides are known for OH insertion into rhodium carbenes formed from diazocarbonyl precursors.17a,17b,17g The onium ion is probably formed more easily from electron‐deficient rhodium carbenoids than from electron‐rich ones. This reactivity may explain the faster reaction of 2 e versus 2 f in the competitive experiment [Eq. (1)]. The next step is electrophilic trapping of the onium ylide 9 with fluorine (1 a) to give 11. Similar trapping of aldehydes, imines, and other electrophiles with onium ylides has been suggested for related reactions.17a,17b,17g In the present fluorination reaction the electrophilic trapping may take place by oxidative addition of rhodium followed by reductive elimination of the fluoride with the oxygen‐substituted carbon atom. Alternatively, the fluorine transfer from 1 a to 9 may occur by σ‐bond metathesis. Proton transfer from 11 to 10 affords the final product 4 with regeneration of the catalyst. Considering this mechanism, other amine or sulfide nucleophiles could also be used for the ylide formation (such as 9). However, 1 a is sensitive to nucleophilic displacement of the fluorine. Thus, when we used nitrogen or sulfur nucleophiles 1 a decomposed, and the reactions did not result in fluorinated products (such as 4).
Figure 3.
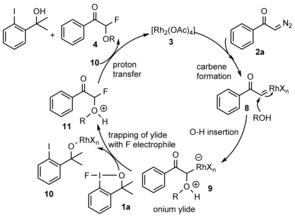
Plausible mechanism for rhodium‐catalyzed 1,1‐difunctionalization.
The reaction with the CF3 reagents 1 b/1 c probably follows the same mechanism as 1 a. The 2 a→9 steps could obviously be the same, while in the trifluoromethylation with 1 b/1 c undergo the electrophilic trapping. The competitive experiments [Eq. (2)] indicate that the oxidation power/electrophilicity of the benziodoxole reagent is important in the trapping of the onium ylide 9, as 1 a reacts about twice as fast as 1 b.
Based on the above observations for fluorination and trifluoromethylation reactions, as well as on recently published studies,26 we hypothesize that, the onium ylide formation/ylide trapping mechanism can be generalized for a broad variety of metal‐catalyzed difunctionalizations of diazocarbonyls with benziodoxole(on) reagents.
In summary, we have shown that the benziodoxole reagents 1 a and 1 c undergo efficient oxyfluorination and oxytrifluoromethylation reactions, respectively, with alcohols, phenols, and carboxylic acids. In oxyfluorination NFSI can also be used as a fluorine source in some processes but 1 a has a much broader synthetic scope, including cyclic substrates (such as 2 i) and carboxylic acids, as coupling components. As far as we know this is the first study on rhodium‐catalyzed geminal‐difunctionalization‐based fluorination and trifluoromethylation reactions. The reactions are fast (15 minutes) at room temperature (even with fairly complex alcohol substrate, such as cholesterol). Therefore, these processes are suitable for rapid late‐stage fluorination and trifluoromethylation reactions. In addition, the above methods open new routes for the synthesis of organofluorine compounds for drug design and new fertilizers.1, 2 Further studies including the exploration of new difunctionalization reactions with various nucleophiles and benziodoxole(on) reagents, as well as mechanistic/modelling studies are ongoing in our laboratory.
Experimental Section
Typical procedure: The fluoroiodane reagent 1 a (28 mg, 0.1 mmol), [Rh2(OAc)4] (3; 0.4 mg, 0.001 mmol), and the corresponding hydroxy compound (0.3 mmol) are mixed in DCM (1 mL). Then the diazo compound 2 (0.2 mmol), dissolved in DCM (1 mL), was added dropwise within 5 minutes. After a 15 min reaction time at RT the reaction mixture was concentrated and the residue purified by silica gel chromatography.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Support from the Swedish Research Council and the Knut och Alice Wallenbergs Foundation are greatly acknowledged.
W. Yuan, L. Eriksson, K. J. Szabó, Angew. Chem. Int. Ed. 2016, 55, 8410.
Contributor Information
Dr. Weiming Yuan, http://www.organ.su.se/ks/
Prof. Kálmán J. Szabó, Email: kalman@organ.su.se
References
- 1.
- 1a. Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Aceña J. L., Soloshonok V. A., Izawa K., Liu H., Chem. Rev. 2016, 116, 422; [DOI] [PubMed] [Google Scholar]
- 1b. Wang J., Sánchez-Roselló M., Aceña J. L., del Pozo C., Sorochinsky A. E., Fustero S., Soloshonok V. A., Liu H., Chem. Rev. 2014, 114, 2432; [DOI] [PubMed] [Google Scholar]
- 1c. Müller K., Faeh C., Diederich F., Science 2007, 317, 1881; [DOI] [PubMed] [Google Scholar]
- 1d. Purser S., Moore P. R., Swallow S., Gouverneur V., Chem. Soc. Rev. 2008, 37, 320. [DOI] [PubMed] [Google Scholar]
- 2. Jeschke P., ChemBioChem 2004, 5, 570. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Preshlock S., Tredwell M., Gouverneur V., Chem. Rev. 2016, 116, 719; [DOI] [PubMed] [Google Scholar]
- 3b. Tredwell M., Preshlock S. M., Taylor N. J., Gruber S., Huiban M., Passchier J., Mercier J., Génicot C., Gouverneur V., Angew. Chem. Int. Ed. 2014, 53, 7751; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 7885; [Google Scholar]
- 3c. Zeng J.-L., Wang J., Ma J.-A., Bioconjugate Chem. 2015, 26, 1000. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Charpentier J., Früh N., Togni A., Chem. Rev. 2015, 115, 650; [DOI] [PubMed] [Google Scholar]
- 4b. Yang X., Wu T., Phipps R. J., Toste F. D., Chem. Rev. 2015, 115, 826; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Kong W., Merino E., Nevado C., Chimia 2014, 68, 430; [DOI] [PubMed] [Google Scholar]
- 4d. Wolstenhulme J. R., Gouverneur V., Acc. Chem. Res. 2014, 47, 3560; [DOI] [PubMed] [Google Scholar]
- 4e. Liang T., Neumann C. N., Ritter T., Angew. Chem. Int. Ed. 2013, 52, 8214; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 8372; [Google Scholar]
- 4f. Alonso C., Martínez de Marigorta E., Rubiales G., Palacios F., Chem. Rev. 2015, 115, 1847; [DOI] [PubMed] [Google Scholar]
- 4g. Chemler S. R., Bovino M. T., ACS Catal. 2013, 3, 1076; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4h. Chen P., Liu G., Synthesis 2013, 2919; [Google Scholar]
- 4i. Prieto A., Baudoin O., Bouyssi D., Monteiro N., Chem. Commun. 2016, 52, 869; [DOI] [PubMed] [Google Scholar]
- 4j. Chu L., Qing F.-L., Acc. Chem. Res. 2014, 47, 1513. [DOI] [PubMed] [Google Scholar]
- 5. Egami H., Shimizu R., Sodeoka M., Tetrahedron Lett. 2012, 53, 5503. [Google Scholar]
- 6. Janson P. G., Ghoneim I., Ilchenko N. O., Szabó K. J., Org. Lett. 2012, 14, 2882. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Li Y., Studer A., Angew. Chem. Int. Ed. 2012, 51, 8221; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 8345; [Google Scholar]
- 7b. Zhu R., Buchwald S. L., Angew. Chem. Int. Ed. 2013, 52, 12655; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 12887; [Google Scholar]
- 7c. Jiang X.-Y., Qing F.-L., Angew. Chem. Int. Ed. 2013, 52, 14177; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 14427. [Google Scholar]
- 8.
- 8a. Kawamura S., Egami H., Sodeoka M., J. Am. Chem. Soc. 2015, 137, 4865; [DOI] [PubMed] [Google Scholar]
- 8b. Wang F., Zhu N., Chen P., Ye J., Liu G., Angew. Chem. Int. Ed. 2015, 54, 9356; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 9488; [Google Scholar]
- 8c. Wang F., Qi X., Liang Z., Chen P., Liu G., Angew. Chem. Int. Ed. 2014, 53, 1881; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 1912. [Google Scholar]
- 9.
- 9a. Ilchenko N. O., Janson P. G., Szabo K. J., J. Org. Chem. 2013, 78, 11087; [DOI] [PubMed] [Google Scholar]
- 9b. Wang F., Wang D., Mu X., Chen P., Liu G., J. Am. Chem. Soc. 2014, 136, 10202; [DOI] [PubMed] [Google Scholar]
- 9c. Li Z., García-Domínguez A., Nevado C., J. Am. Chem. Soc. 2015, 137, 11610. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Yu W., Xu X.-H., Qing F.-L., Adv. Synth. Catal. 2015, 357, 2039; [Google Scholar]
- 10b. He Z., Tan P., Hu J., Org. Lett. 2016, 18, 72. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Yuan W., Szabó K. J., Angew. Chem. Int. Ed. 2015, 54, 8533; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 8653; [Google Scholar]
- 11b. Geary G. C., Hope E. G., Stuart A. M., Angew. Chem. Int. Ed. 2015, 54, 14911; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 15124; [Google Scholar]
- 11c. Rauniyar V., Lackner A. D., Hamilton G. L., Toste F. D., Science 2011, 334, 1681; [DOI] [PubMed] [Google Scholar]
- 11d. Lozano O., Blessley G., Martinez del Campo T., Thompson A. L., Giuffredi G. T., Bettati M., Walker M., Borman R., Gouverneur V., Angew. Chem. Int. Ed. 2011, 50, 8105; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8255; [Google Scholar]
- 11e. Parmar D., Rueping M., Chem. Commun. 2014, 50, 13928; [DOI] [PubMed] [Google Scholar]
- 11f. Ulmer A., Brunner C., Arnold A. M., Pöthig A., Gulder T., Chem. Eur. J. 2016, 22, 3660. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Wu T., Yin G., Liu G., J. Am. Chem. Soc. 2009, 131, 16354; [DOI] [PubMed] [Google Scholar]
- 12b. Shunatona H. P., Früh N., Wang Y.-M., Rauniyar V., Toste F. D., Angew. Chem. Int. Ed. 2013, 52, 7724; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 7878; [Google Scholar]
- 12c. Kong W., Feige P., de Haro T., Nevado C., Angew. Chem. Int. Ed. 2013, 52, 2469; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 2529. [Google Scholar]
- 13.
- 13a. Kindt S., Heinrich M. R., Chem. Eur. J. 2014, 20, 15344; [DOI] [PubMed] [Google Scholar]
- 13b. Wolstenhulme J. R., Rosenqvist J., Lozano O., Ilupeju J., Wurz N., Engle K. M., Pidgeon G. W., Moore P. R., Sandford G., Gouverneur V., Angew. Chem. Int. Ed. 2013, 52, 9796; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 9978; [Google Scholar]
- 13c. Braun M.-G., Katcher M. H., Doyle A. G., Chem. Sci. 2013, 4, 1216; [Google Scholar]
- 13d. Nie J., Zhu H.-W., Cui H.-F., Hua M.-Q., Ma J.-A., Org. Lett. 2007, 9, 3053; [DOI] [PubMed] [Google Scholar]
- 13e. Wang L., Meng W., Zhu C.-L., Zheng Y., Nie J., Ma J.-A., Angew. Chem. Int. Ed. 2011, 50, 9442; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 9614. [Google Scholar]
- 14.
- 14a. Ilchenko N. O., Cortés M. A., Szabo K. J., ACS Catal. 2016, 6, 447; [Google Scholar]
- 14b. Barluenga J., González J. M., Campos P. J., Asensio G., Angew. Chem. Int. Ed. Engl. 1985, 24, 319; [Google Scholar]; Angew. Chem. 1985, 97, 341; [Google Scholar]
- 14c. Yuan Z., Wang H.-Y., Mu X., Chen P., Guo Y.-L., Liu G., J. Am. Chem. Soc. 2015, 137, 2468. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Ilchenko N. O., Tasch B. O. A., Szabó K. J., Angew. Chem. Int. Ed. 2014, 53, 12897; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 13111; [Google Scholar]
- 15b. Kitamura T., Muta K., Oyamada J., J. Org. Chem. 2015, 80, 10431; [DOI] [PubMed] [Google Scholar]
- 15c. Tao J., Tran R., Murphy G. K., J. Am. Chem. Soc. 2013, 135, 16312; [DOI] [PubMed] [Google Scholar]
- 15d. Emer E., Twilton J., Tredwell M., Calderwood S., Collier T. L., Liégault B., Taillefer M., Gouverneur V., Org. Lett. 2014, 16, 6004; [DOI] [PubMed] [Google Scholar]
- 15e. Yasui N., Mayne C. G., Katzenellenbogen J. A., Org. Lett. 2015, 17, 5540; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15f. Hu M., Ni C., Li L., Han Y., Hu J., J. Am. Chem. Soc. 2015, 137, 14496; [DOI] [PubMed] [Google Scholar]
- 15g. Chen G., Song J., Yu Y., Luo X., Li C., Huang X., Chem. Sci. 2016, 7, 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Hu M., Ni C., Hu J., J. Am. Chem. Soc. 2012, 134, 15257; [DOI] [PubMed] [Google Scholar]
- 16b. Ramanjaneyulu B. T., Mahesh S., Vijaya Anand R., Org. Lett. 2015, 17, 6. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Ford A., Miel H., Ring A., Slattery C. N., Maguire A. R., McKervey M. A., Chem. Rev. 2015, 115, 9981; [DOI] [PubMed] [Google Scholar]
- 17b. Guo X., Hu W., Acc. Chem. Res. 2013, 46, 2427; [DOI] [PubMed] [Google Scholar]
- 17c. Murphy G. K., Stewart C., West F. G., Tetrahedron 2013, 69, 2667; [Google Scholar]
- 17d. Davies H. M. L., Denton J. R., Chem. Soc. Rev. 2009, 38, 3061; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17e. Xiao Q., Zhang Y., Wang J., Acc. Chem. Res. 2013, 46, 236; [DOI] [PubMed] [Google Scholar]
- 17f. Doyle M. P., Duffy R., Ratnikov M., Zhou L., Chem. Rev. 2010, 110, 704; [DOI] [PubMed] [Google Scholar]
- 17g. DeAngelis A., Panish R., Fox J. M., Acc. Chem. Res. 2016, 49, 115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17h. Xia Y., Zhang Y., Wang J., ACS Catal. 2013, 3, 2586. [Google Scholar]
- 18.
- 18a. Olah G. A., Welch J., Synthesis 1974, 896; [Google Scholar]
- 18b. Olah G. A., Welch J. T., Vankar Y. D., Nojima M., Kerekes I., Olah J. A., J. Org. Chem. 1979, 44, 3872. [Google Scholar]
- 19. Leroy J., Wakselman C., J. Chem. Soc. Perkin Trans. 1 1978, 1224. [Google Scholar]
- 20.
- 20a. Legault C. Y., Prévost J., Acta Crystallogr. Sect. E 2012, 68, o1238; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b. Matoušek V., Pietrasiak E., Schwenk R., Togni A., J. Org. Chem. 2013, 78, 6763; [DOI] [PubMed] [Google Scholar]
- 20c. Geary G. C., Hope E. G., Singh K., Stuart A. M., Chem. Commun. 2013, 49, 9263; [DOI] [PubMed] [Google Scholar]
- 20d. Geary G. C., Hope E. G., Singh K., Stuart A. M., RSC Adv. 2015, 5, 16501. [Google Scholar]
- 21. Lee E., Kamlet A. S., Powers D. C., Neumann C. N., Boursalian G. B., Furuya T., Choi D. C., Hooker J. M., Ritter T., Science 2011, 334, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y., Hari D. P., Vita M. V., Waser J., Angew. Chem. Int. Ed. 2016, 55, 4436; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 4512. [Google Scholar]
- 23.
- 23a. Larsson J. M., Pathipati S. R., Szabo K. J., J. Org. Chem. 2013, 78, 7330; [DOI] [PubMed] [Google Scholar]
- 23b. Janson P. G., Ilchenko N. O., Diez-Varga A., Szabó K. J., Tetrahedron 2015, 71, 922; [Google Scholar]
- 23c. Ilchenko N. O., Janson P. G., Szabó K. J., Chem. Commun. 2013, 49, 6614; [DOI] [PubMed] [Google Scholar]
- 23d. Zhao T. S. N., Szabó K. J., Org. Lett. 2012, 14, 3966. [DOI] [PubMed] [Google Scholar]
- 24. Umemoto T., Chem. Rev. 1996, 96, 1757. [DOI] [PubMed] [Google Scholar]
- 25. Werlé C., Goddard R., Philipps P., Farès C., Fürstner A., J. Am. Chem. Soc. 2016, 138, 3797. [DOI] [PubMed] [Google Scholar]
- 26. Hari D. P., Waser J., J. Am. Chem. Soc. 2016, 138, 2190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


