Abstract
Background
The (R)‐enantiomer of racemic albuterol (levalbuterol) has bronchodilatory properties whereas the (S)‐enantiomer causes adverse effects in human airways, animal models, and isolated equine bronchi. Levalbuterol is commercially available and improves pulmonary function of asthmatic patients with a longer duration of effect than albuterol.
Objective
To determine the dose at which inhaled levalbuterol produces maximal bronchodilatory effect (EDmax) and determine its duration of action in recurrent airway obstruction (RAO)‐affected horses in comparison to racemic albuterol.
Animals
Nine horses with inducible and reversible RAO.
Methods
Randomized, crossover trial. Horses were challenged with moldy hay to induce airway obstruction. Horses were treated with nebulized albuterol or levalbuterol chosen randomly. Pulmonary function testing (PFT) was measured before and for up to 3 hours after bronchodilatation challenge. Maximum change in transpulmonary pressure (DP max) was measured to assess the dose effect and duration of action of each drug. After a 24 hours washout period, the bronchodilatation challenge was repeated with the second bronchodilator.
Results
The duration of effect was 60 minutes for albuterol and 120 minutes for levalbuterol. The dose of bronchodilator EDmax was not significantly different between albuterol and levalbuterol (EDmax = 125.0 [125–125 μg] and EDmax = 188 [125–188 μg] respectively; P = .068). The magnitude of bronchodilatation was not significantly different between the 2 treatments (61.1 and 59.9% decrease in DP max for albuterol and levalbuterol respectively; P = .86).
Conclusions and clinical importance
Levalbuterol is as effective a bronchodilator as albuterol; although levalbuterol lasts twice as long as albuterol, its duration of action is still too short to make it practical for RAO treatment.
Keywords: Aerosol treatment, Bronchodilator, Heaves
Abbreviations
- Cdyn
dynamic lung compliance
- DPmax
maximum change in transpulmonary pressure
- ED50%
dose producing 50% decrease in DPmax
- EDmax
dose producing maximum bronchodilation
- IL‐4
interleukin‐4
- IQR
interquartile range
- PFT
pulmonary function testing
- RAO
recurrent airway obstruction
- RL
lung resistance
Recurrent airway obstruction (RAO), also known has “heaves”, is an inducible and reversible chronic inflammatory airway disease commonly encountered in older horses in the northern hemisphere. An episode of acute RAO can be triggered by inhalation of dust from hay and the disease in horses has many similarities to asthma in humans.1, 2 Functional changes include increases in maximum transpulmonary pressure change (DPmax) and pulmonary resistance (R L) and a decrease in dynamic lung compliance (C dyn).3
Equine RAO is a disease that cannot be cured but can be managed with medical treatment (systemic or inhaled corticosteroids and bronchodilators) and environmental changes (decreasing dust exposure by modifying housing and diet). Some horses need continuous medical management because of the challenge of achieving appropriate environmental control. Aerosol treatment is considered the best approach because of its rapid onset of action and deposition of high concentration of medication in the peripheral airways while minimizing systemic adverse effects.
Bronchodilators are used commonly in horses with acute exacerbation of RAO as a rescue medication, as treatment in combination with corticosteroids or as a diagnostic tool.4 Albuterol, a short acting β2‐adrenergic receptor agonist, is an effective bronchodilator that relieves acute airway obstruction by relaxing airway smooth muscle.5 Within 5 minutes after administration, pulmonary function is improved maximally. The effect, however, lasts only 30–60 minutes on average, making long‐term control of RAO impractical.4, 5 Albuterol is a racemic mixture containing equal concentrations of (R) – and (S)‐enantiomers. The (R)‐enantiomer, called levalbuterol, is responsible for the rapid bronchodilatory effect, decrease in wall edema, inhibition of mast cells, and decrease in eosinophilic migration to airways.6 The (S)‐enantiomer has been shown to have adverse effects in the human respiratory tract such as inducing airway hyper‐responsiveness, increasing production of histamine and interleukin‐4 (IL‐4) in mast cells and promoting activation of eosinophils.6, 7, 8 The (S)‐ enantiomer also causes airway hyper‐responsiveness and bronchoconstriction in isolated equine bronchi in vitro9, but, there is no report regarding the clinical effect of levalbuterol in horses. Levalbuterol is commonly used in human medicine because it improves pulmonary function and provides up to 6 hours of bronchodilatation.6, 10, 11, 12 However, recent evidence has shown no clinical benefit of levalbuterol versus albuterol in adult patients with asthma and chronic obstructive pulmonary disease (COPD).13 The purpose of our study was to compare the bronchodilatory effect as well as the duration of action of levalbuterol and albuterol in horses with an acute episode of RAO.
Materials and Methods
Study Design
All methods were approved by the Purdue University Animal Care and Use Committee.
Nine horses (3 geldings, 6 mares; age range, 9–24 years) owned by Purdue University and with a history of inducible and reversible RAO were involved in this randomized, crossover trial. All of the horses remained on pasture on a complete pelleted feed for at least 2 months before the trial to achieve clinical remission. The animals were transported to the research facilities and resided there for the duration of the trial. On arrival, a physical examination and pulmonary function testing (PFT) were performed.
The horses were housed in individual stalls bedded with wood shavings and fed complete pelleted feed as well as free choice hay placed in a hay net. In addition, they were exposed to moldy hay which was shaken 2 times a day for 3 minutes next to the horse's nose to increase dust exposure.4 A daily physical examination was performed, and a clinical score was assigned to every horse based on a scale that ranges from 0 to 21 as previously described.14 When the clinical score reached ≥10, PFT was performed. The horse was enrolled in the study if DPmax ≥ 15 cm H2O. The PFT was performed at baseline followed by administration of the bronchodilator challenge, randomly chosen (racemic albuterol or levalbuterol). The bronchodilator was given in a stepwise fashion until maximum dilatation was achieved. The PFT was performed 5 minutes after each dose. The horse then was returned to the stall for a washout period of at least 24 hours. The second treatment was administered the next day if the same inclusion criteria were achieved and testing was repeated.
Pulmonary Function Test (PFT)
An esophageal balloon catheter1 was placed to obtain measurements analogous to pleural pressure. The position of the catheter was estimated by measuring the distance between the horse's nostrils to mid thorax. The horse was placed in stocks with no sedation and an air‐tight mask2 was fitted around the horse’ muzzle with the port of the mask holding the pneumotachometer3 to measure airflow. Esophageal pressure and airflow output signals from both devices were recorded simultaneously by computer software4 for 2 minutes to average the measurement of DPmax and calculation of pulmonary R L and C dyn over 10 adequate breaths.4, 15
Bronchodilator Challenge
A first dose (62.5 μg) of albuterol sulfate5 or levalbuterol hydrochloride6 solution was chosen randomly by flipping a coin. This low dose corresponded to 0.15 mL of the bronchodilator solution as measured with a 1‐mL syringe and given nondiluted. The bronchodilator was administered using an ultrasonic nebulizer7 and DPmax measured 5 minutes later. The doses were repeated and the DPmax was measured 5 minutes after each dose to evaluate the effects of cumulative bronchodilator administration and the dose at which maximal bronchodilatation was achieved. If the DPmax reached a plateau consisting of a difference ≤10% between 2 successive doses, the air‐tight mask was repositioned and PFT repeated 10, 20, 30, 40, 60, 90, 120, 180 minutes after the last dose of bronchodilator to determine the duration of the treatment effect, as previously described.4 After a washout period of 24 hours, physical examination and clinical scores were obtained to ensure that enrollment criteria were met again, and measurements were repeated after switching treatment drug.
The dose of bronchodilator that resulted in a 50% decrease in DPmax (ED50%) was calculated by linear interpolation and the dose resulting in maximum bronchodilatation (EDmax) was determined as the first dose of the plateau effect.
Statistical Analysis
Clinical scores and PFT data were compared between treatments using the Wilcoxon Matched Paired Test.8 The dose that resulted in a 50% and maximum decrease in DPmax from baseline was compared between both drugs administered. Changes in PFT variables between baseline and subsequent time points were compared using Friedman ANOVA. Post‐hoc tests were conducted using Wilcoxon matched pairs test when appropriate. Association between DPmax at baseline and EDmax was assessed by Spearman's rank correlation. Data were summarized as median and interquartile range (IQR) and significance was defined as P < .05.
Results
All 9 horses met the inclusion criteria and responded to the moldy hay during 2 weeks of exposure. None of them developed anorexia or clinically relevant weight loss throughout the study. Results of baseline PFT (DPmax, R L and C dyn) before administration of bronchodilators were not statistically different between the 2 treatment groups (P = .76).
The mean duration of bronchodilator effect was 60 minutes for albuterol and 120 minutes for levalbuterol (Fig 1). A comparable response was observed with R L data whereby albuterol effect lasted 40 minutes and levalbuterol effect was significant up to 120 minutes (Fig 2). Bronchodilator effect on C dyn followed a similar trend but was only statistically significant for 10–40 minutes. Two of the 9 horses continued to experience decreased DPmax 3 hours after levalbuterol administration. The effects on PFT were noted after the first dose (63 μg) of either albuterol or levalbuterol and resulted in average decreases of 50 and 56% in DPmax respectively. The calculated ED50% and EDmax of the doses were not significantly different between albuterol (ED50% = 43.6 [38.2–47.3 μg] and EDmax = 125.0 [125–125 μg]) and levalbuterol (ED50% = 39.7 [35.0–52.5 μg; P = .40] and EDmax = 188 [125–188 μg; P = .068]; Fig 3). Similarly, the relative magnitude of bronchodilatation was not significantly different between the 2 treatments (61.1 [43.1–67.9] and 59.9 [52.2–63.6] % decrease in DPmax for albuterol and levalbuterol respectively; P = .86). There was no significant correlation between DPmax at baseline and EDmax.
Figure 1.

Mean ± standard deviation of the maximum change in transpulmonary pressure (DP max) recorded with the mask before (0) and after completion of the bronchodilator challenge with albuterol (solid squares) and levalbuterol (open diamonds). *: significantly different from baseline (P < .05).
Figure 2.
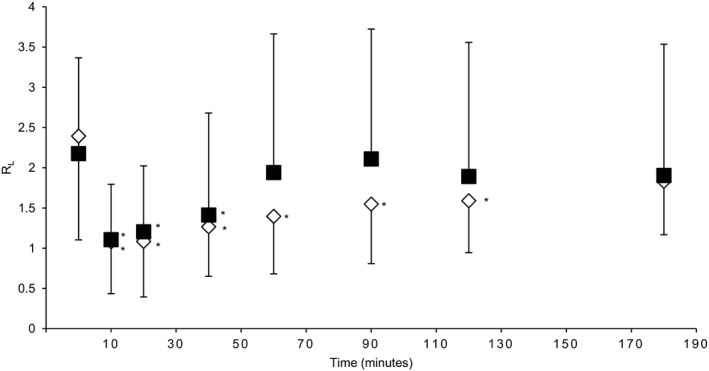
Mean ± standard deviation of the lung resistance (R L) recorded with the mask before (0) and after completion of the bronchodilator challenge with albuterol (solid squares) and levalbuterol (open diamonds). *: significantly different from baseline (P < .05).
Figure 3.

Mean ± standard deviation of the maximum change in transpulmonary pressure (DP max) recorded without the mask before (0) and following each dose of nebulized albuterol (solid squares) and levalbuterol (open diamonds). *: significantly different from baseline (P < .05).
Discussion
Levalbuterol, a β‐agonist containing only the (R)‐enantiomer of racemic albuterol, has been developed for human asthmatic patients to avoid the adverse effects of the (S)‐enantiomer.6, 11, 12, 16, 17 To our knowledge, no previous studies have compared the use of levalbuterol and racemic albuterol for the treatment of horses with an acute episode of RAO.
Our results suggest that there are no significant differences in the magnitude of improvement in pulmonary function achieved by both bronchodilators, although the effects of levalbuterol lasted twice as long as those of albuterol (2 versus 1 hour respectively). No adverse effects were noted with this single short‐term treatment with either bronchodilatator, but future studies using daily treatment over several days would be needed to further assess this possibility. Despite this longer duration of action, the return of pulmonary pressures to baseline values by 180 minutes limits the use of levalbuterol to a rescue medication for horses with an acute RAO crisis or as a diagnostic method to confirm reversibility of airway obstruction.
Before the bronchodilator challenge, there was no significant difference in pulmonary function (DPmax, R L, and C dyn) between each arm of the study, demonstrating that a washout period of 24 hours was adequate, as previously reported.4
The average albuterol dose that induced a maximum bronchodilatory effect (125 μg) is markedly lower than the dose reported in previous studies (360–540 μg).4, 5 This difference suggests that a higher proportion of drug was deposited in the lung using the ultrasonic nebulizer, presumably because of the smaller particle size delivered with the nebulizer compared to the hand‐held device used in the previous studies.4 The nebulizer was easy to place and it took approximately 1 minute to administer each dose. The horses showed no adverse behavior throughout the bronchodilator challenge, similar to reports in previous studies.18 Bronchodilatation was significant after the first dose (63 μg) of either albuterol or levalbuterol, which corresponded to 0.15 mL of solution. We did not attempt to start the bronchodilator challenge with a lower dose because of the difficulty in accurate measurement of small volumes.
The deposition of the aerosol reaching the lungs in horses with ultrasonic nebulizers (5.09 ± 0.66%) is lower than the average 10% of aerosol deposition achieved in human patients.18 This difference is likely attributed to horses being nasal breathers, causing higher filtration. In addition, the deposition of small‐sized aerosol particles in the human lung is augmented by deep inhalation, followed by a period of breath‐holding at total lung capacity. This maneuver is not possible in horses.19, 20, 21 Horses experiencing an acute RAO episode tend to have rapid and shallow breaths that would be expected to decrease lung deposition of the bronchodilator. However, the doses of albuterol and levalbuterol resulting in EDmax in horses (125 and 188 μg, respectively) were very small compared to EDmax reported in human patients with severe asthma exacerbation (7.5 and 3.25 mg respectively).22 An additional effect from oral absorption of drugs deposited in the nasopharynx is unlikely because it would be expected to result in a delayed therapeutic effect, but this phenomenon was not observed in our study. Furthermore, there is evidence that oral albuterol is poorly absorbed in the horse9 and a closely related compound, terbutaline, also is not bioavailable orally in horses.23 Therefore, nebulized albuterol and levalbuterol are highly potent bronchodilators in horses with acute RAO exacerbation.
The effect of levalbuterol lasted 2 hours in RAO horses compared to 6 hours in human asthmatic patients.12, 24 This decreased duration of action of levalbuterol in the horse is similar to that observed with another bronchodilator, salmeterol xinafoate.25 Salmeterol in human asthmatic patients has a duration of action of 12 hours and only 6 hours in horses with RAO. We hypothesized that the difference might be attributed to rapid drug clearance from the lung because of enhanced alveolar and bronchial absorption secondary to increased airway epithelial permeability25 or a species‐specific pharmacokinetic difference in disposition of the drug. There are no validated explanations of why the duration of action of these bronchodilators is shorter in horses than in people.
Our results suggest that levalbuterol is effective to treat acute RAO in horses but, the only benefit of levalbuterol is the slightly longer duration of bronchodilatory effect compared to albuterol. There was no statistically significant difference in PFT after EDmax using either bronchodilator. These results suggest that both bronchodilators achieve a similar degree of bronchodilatation in horses, as opposed to humans in whom levalbuterol is more efficient at improving pulmonary function in addition to having a longer acting bronchodilatory effect than albuterol.6
In conclusion, unlike findings in human asthmatic patients, administration of levalbuterol to horses with RAO did not result in greater bronchodilatation compared to albuterol. Levalbuterol is an effective bronchodilator, but the short duration of action makes this drug nonpractical for treatment of RAO.
Funding
The United States Equestrian Federation and the state of Indiana, the Purdue University College of Veterinary Medicine Research account funded by the Total Wager Tax.
Acknowledgment
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at Purdue University.
Footnotes
Tygon lab catheter, ¼ “ID × 3/8” OD, Cole Parmer, Vernon Hills, IL
Equine AeroMask, Trudell Medical International, London, Ontario, Canada
No. 4 Fleisch, EMKA Technologies, Paris, France
Pulmonary mechanics analyzer, XA version, Buxco Electronics Inc, Sharon, CT
1.25 mg of albuterol sulfate in 3‐mL unit‐dose vial, Nephron Pharmaceuticals Corporation, Orlando, FL
Xopenex®, 1.25 mg of levalbuterol in 3‐mL unit‐dose vial, Sunovion Pharmaceuticals Inc., Marlborough, MA
SaHoMa™ – II, NEBU‐TEC International, Elsenfeld, Germany
Statistica, Statsoft Inc., Tulsa, OK
Ball MA, Pharmacokinetics of orally administered albuterol in the horse. World Equine Airway Symposium proceedings, 1998
References
- 1. Ghio AJ, Mazan MR, Hoffman AM, Robinson NE. Correlates between human lung injury after particle exposure and recurrent airway obstruction in the horse. Equine Vet J 2006;38:362–367. [DOI] [PubMed] [Google Scholar]
- 2. Leclere M, Lavoie‐Lamoureux A, Lavoie J‐P. Heaves, an asthma‐like disease of horses. Respirology 2011;16:1027–1046. [DOI] [PubMed] [Google Scholar]
- 3. Couetil LL, Rosenthal FS, DeNicola DB, Chilcoat CD. Clinical signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary function in horses with inflammatory respiratory disease. Am J Vet Res 2001;62:538–546. [DOI] [PubMed] [Google Scholar]
- 4. Bertin FR, Ivester KM, Couëtil LL. Comparative efficacy of inhaled albuterol between two hand‐held delivery devices in horses with recurrent airway obstruction. Equine Vet J 2011;43:393–398. [DOI] [PubMed] [Google Scholar]
- 5. Derksen FJ, Olszewski MA, Robinson NE, et al. Aerosolized albuterol sulfate used as a bronchodilator in horses with recurrent airway obstruction. Am J Vet Res 1999;60:689–693. [PubMed] [Google Scholar]
- 6. Nelson HS, Bensch G, Pleskow WW, et al. Improved bronchodilation with levalbuterol compared with racemic albuterol in patients with asthma. J Allergy Clin Immunol 1998;102:943–952. [DOI] [PubMed] [Google Scholar]
- 7. Henderson WR Jr, Banerjee ER, Chi EY. Differential effects of (S)‐ and (R)‐enantiomers of albuterol in a mouse asthma model. J Allergy Clin Immunol 2005;116:332–340. [DOI] [PubMed] [Google Scholar]
- 8. Page CP, Morley J. Contrasting properties of albuterol stereoisomers. J Allergy Clin Immunol 1999;104(Pt 2):S31–S41. [DOI] [PubMed] [Google Scholar]
- 9. Matera MG, Calzetta L, Rogliani P, et al. Evaluation of the effects of the R‐ and S‐enantiomers of salbutamol on equine isolated bronchi. Pulm Pharmacol Ther 2011;24:221–226. [DOI] [PubMed] [Google Scholar]
- 10. Cho SH, Hartleroad JY, Oh CK. (S)‐albuterol increases the production of histamine and IL‐4 in mast cells. Int Arch Allergy Immunol 2001;124:478–484. [DOI] [PubMed] [Google Scholar]
- 11. Gupta MK, Singh M. Evidence based review on levosalbutamol. Indian J Pediatr 2007;74:161–167. [DOI] [PubMed] [Google Scholar]
- 12. Schreck DM, Babin S. Comparison of racemic albuterol and levalbuterol in the treatment of acute asthma in the ED. Am J Emerg Med 2005;23:842–847. [DOI] [PubMed] [Google Scholar]
- 13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm 2015;72:1026–1035. [DOI] [PubMed] [Google Scholar]
- 14. Tesarowski DB, Viel L, McDonell WN. Pulmonary function measurements during repeated environmental challenge of horses with recurrent airway obstruction (heaves). Am J Vet Res 1996;57:1214–1219. [PubMed] [Google Scholar]
- 15. Couëtil LL, Rosenthal FS, Simpson CM. Forced expiration: a test for airflow obstruction in horses. J Appl Physiol 2000;88:1870–1879. [DOI] [PubMed] [Google Scholar]
- 16. Handley D. The asthma‐like pharmacology and toxicology of (S)‐isomers of beta agonists. J Allergy Clin Immunol 1999;104(Pt 2):S69–S76. [DOI] [PubMed] [Google Scholar]
- 17. Perrin‐Fayolle M, Blum PS, Morley J, et al. Differential responses of asthmatic airways to enantiomers of albuterol. Clin Rev Allergy Immunol 1996;14:139–147. [DOI] [PubMed] [Google Scholar]
- 18. Votion D, Ghafir Y, Munsters K, et al. Aerosol deposition in equine lungs following ultrasonic nebulisation versus jet aerosol delivery system. Equine Vet J 1997;29:388–393. [DOI] [PubMed] [Google Scholar]
- 19. Chamberlain MJ, Morgan WK, Vinitski S. Factors influencing the regional deposition of inhaled particles in man. Clin Sci (Lond) 1983;64:69–78. [DOI] [PubMed] [Google Scholar]
- 20. Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med 1980;37:337–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newman SP. AErosol deposition considerations in inhalation therapy. Chest 1985;88(Suppl):152S–160S. [DOI] [PubMed] [Google Scholar]
- 22. Nowak R, Emerman C, Hanrahan JP, et al. A comparison of levalbuterol with racemic albuterol in the treatment of acute severe asthma exacerbations in adults. Am J Emerg Med 2006;24:259–267. [DOI] [PubMed] [Google Scholar]
- 23. Törneke MK, Ingvast‐Larsson JC, Johansson JM, Appelgren LE. Pharmacokinetics and pharmacodynamics of terbutaline in healthy horses. Am J Vet Res 2000;61:761–765. [DOI] [PubMed] [Google Scholar]
- 24. Gawchik SM, Saccar CL, Noonan M, et al. The safety and efficacy of nebulized levalbuterol compared with racemic albuterol and placebo in the treatment of asthma in pediatric patients. J Allergy Clin Immunol 1999;103:615–621. [DOI] [PubMed] [Google Scholar]
- 25. Henrikson SL, Rush BR. Efficacy of salmeterol xinafoate in horses with recurrent airway obstruction. J Am Vet Med Assoc 2001;218:1961–1965. [DOI] [PubMed] [Google Scholar]


