Abstract
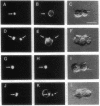
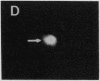
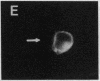
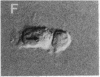
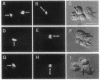
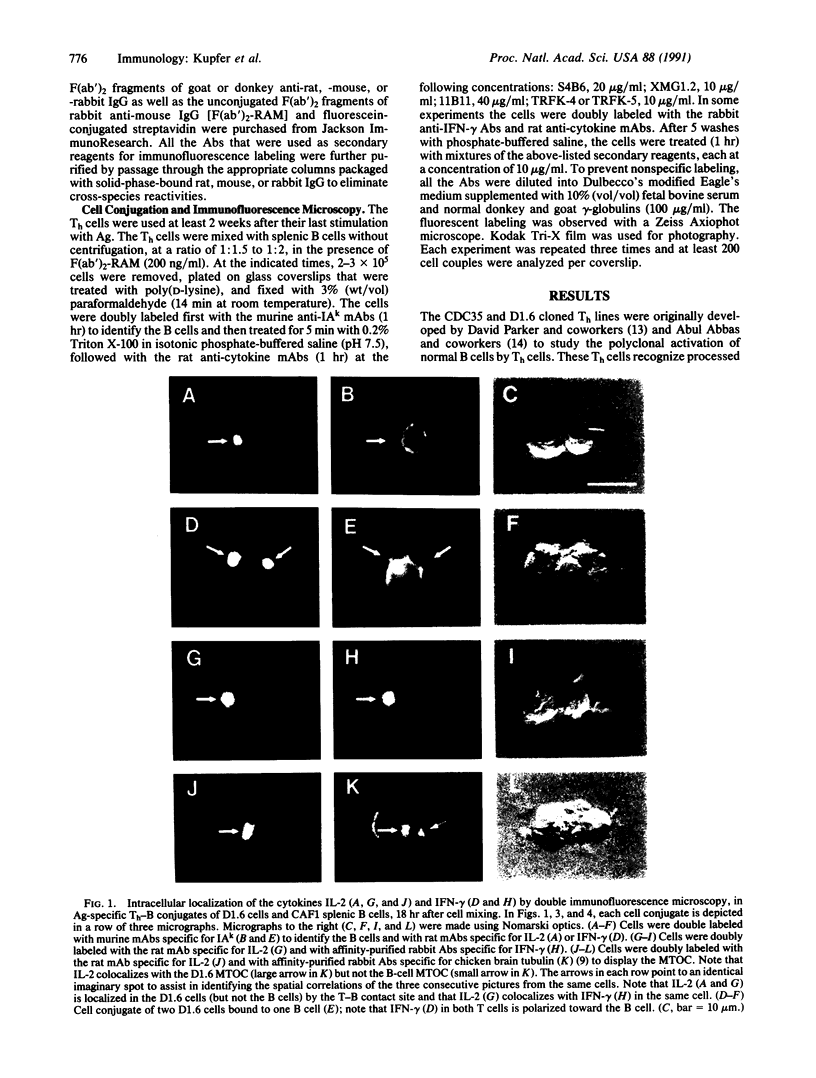
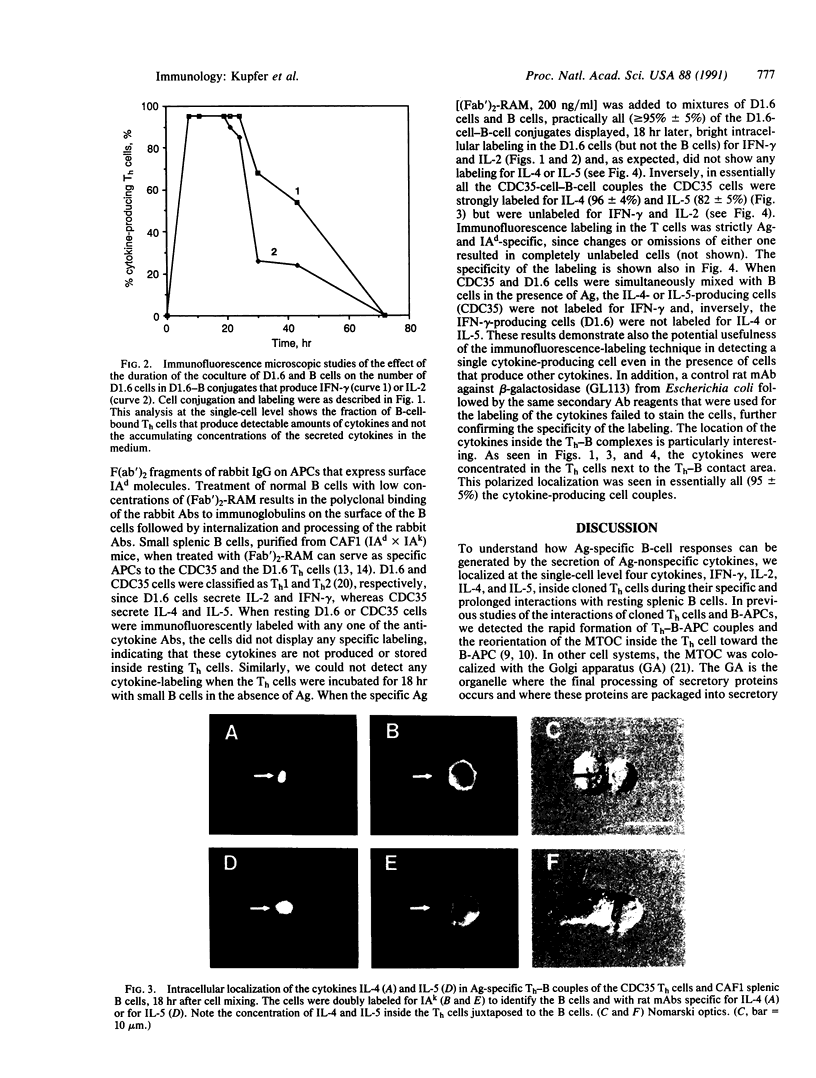
We describe the intracellular localization, by double immunofluorescence microscopy, of four cytokines that were produced during the prolonged interaction of cloned helper T cells with resting splenic B cells. When two rabbit immunoglobulin-specific helper-T-cell clones were mixed, either separately or together, with splenic B cells in the presence of the antigen rabbit anti-mouse immunoglobulin antibodies, stable T-cell-B-cell conjugates were seen up to 29 hr later. Microscopic observations of these cells revealed that interferon gamma and interleukin 2, inside one of the T-cell clones, and interleukins 4 and 5, inside the other T-cell clone, were concentrated very close to the T-cell-B-cell contact area. The cytokines were not seen in the T cells prior to their interaction with the B cells and their production was strictly antigen-specific. These studies show, at the single-cell level, that helper-T-cell clones can remain bound to splenic B cells long enough for the T cells to produce cytokines, which are synthesized near the bound B cells. We propose that the polarized synthesis of the cytokines may result in their directed secretion toward the bound B cells. By locally secreting the cytokines, which are not antigen-specific, at the contacting T-cell-B-cell membranes, where T- and B-cell surface receptors are engaged and clustered, the helper T cells can induce selective and specific B-cell responses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., Justement L. B., Palmer E., Cambier J. C. Induction of c-fos and c-myc expression during B cell activation by IL-4 and immunoglobulin binding ligands. J Immunol. 1989 Aug 1;143(3):1032–1039. [PubMed] [Google Scholar]
- Krusemeier M., Snow E. C. Induction of lymphokine responsiveness of hapten-specific B lymphocytes promoted through an antigen-mediated T helper lymphocyte interaction. J Immunol. 1988 Jan 15;140(2):367–375. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Janeway C. A., Jr, Swain S. L. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Janeway C. A., Jr, Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987 Jun 1;165(6):1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C., Uhr J. W., Vitetta E. S. Activation of antigen-specific B cells: role of T cells, cytokines, and antigen in induction of growth and differentiation. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6628–6631. doi: 10.1073/pnas.80.21.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Owens T. A noncognate interaction with anti-receptor antibody-activated helper T cells induces small resting murine B cells to proliferate and to secrete antibody. Eur J Immunol. 1988 Mar;18(3):395–401. doi: 10.1002/eji.1830180312. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Between two centuries: specificity and regulation in immunology. J Immunol. 1987 Jul 1;139(1):1–6. [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. A single antigen-specific B cell can conjugate to either a type 1 or a type 2 helper T cell. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7724–7728. doi: 10.1073/pnas.85.20.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Tony H. P., Phillips N. E., Parker D. C. Role of membrane immunoglobulin (Ig) crosslinking in membrane Ig-mediated, major histocompatibility-restricted T cell-B cell cooperation. J Exp Med. 1985 Nov 1;162(5):1695–1708. doi: 10.1084/jem.162.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen B. J., Tony H. P., Parker D. C. Characterization of the effector mechanism of help for antigen-presenting and bystander resting B cell growth mediated by Ia-restricted Th2 helper T cell lines. J Immunol. 1988 Oct 1;141(7):2230–2239. [PubMed] [Google Scholar]