Abbreviations
- CSF
cerebrospinal fluid
- CT
computed tomography
- DM
diabetes mellitus
- FSH
follicle‐stimulating hormone
- GH
growth hormone
- HCM
hypertrophic cardiomyopathy
- IGF‐1
insulin‐like growth factor 1
- MRI
magnetic resonance imaging
- MSH
melanocyte‐stimulating hormone
- TSH
thyroid‐stimulating hormone
- USG
urine specific gravity
Case 1
A 16‐year‐old, 6.8 kg, castrated male Domestic Short‐haired cat was presented to Louisiana State University Veterinary Teaching Hospital with a 3‐day history of progressive ataxia, tetraparesis, and altered mentation. The owner did not report having observed evidence of polyuria or polydipsia. The cat had been diagnosed with hypertrophic cardiomyopathy (HCM) 4 years earlier and was receiving atenolol, benazepril, and aspirin. Dull mentation, patchy truncal alopecia, and a grade III of VI systolic heart murmur were noted during the physical examination. The neurologic examination revealed ambulatory tetraparesis that was more severe in the pelvic limbs, plantigrade stance, proprioceptive deficits in all limbs, and positional vertical nystagmus. The CBC, biochemistry profile, and abdominal ultrasound were unremarkable. The blood glucose concentration measured with the chemistry analyzer was 130 mg/dL (7.2 mmol/L). Subsequent blood glucose measurements (n = 6) using a glucometer validated for use in cats1 ranged from 122 mg/dL (6.7 mmol/L) to 159 mg/dL (8.7 mmol/L) during the 5‐day period the cat was hospitalized. These blood glucose concentrations combined with a normal serum fructosamine concentration (228 μmol/L, RI 192–288) ruled out overt diabetes mellitus (DM).
Two‐dimensional echocardiogram revealed severe asymmetric septal hypertrophy (8.7 mm in diastole). The left atrium was normal in size and all valves appeared normal. Continuous wave Doppler through the left ventricular outflow tract revealed dynamic outflow tract obstruction. Color Doppler demonstrated marked turbulence in the left ventricular outflow tract and a narrow eccentric jet of mitral regurgitation associated with systolic anterior motion of the mitral valve.
Magnetic resonance imaging (MRI)2 images of the head were obtained in multiple planes prior to and following contrast3 administration at a dose of 0.1 mmol/kg. In the region of the pituitary gland, there was a T2 hyperintense, T1 hypointense nodule having marked uniform contrast enhancement (Fig 1). This nodule had moderate extension dorsal to the sella turcica and was increased in size compared to a normal pituitary gland: length (0.63 cm), width (0.83 cm), and height (0.85 cm). On T2* weighted (gradient recalled echo) images, some regions of reduced signal and small signal voids were present within the pituitary gland, mostly on the left side, consistent with magnetic susceptibility likely due to regions of hemorrhage within the nodule. Additionally, there was moderate dilation of the entire ventricular system with most severe ventricular enlargement occurring in the fourth ventricle, which caused dorsal elevation and compression of the cerebellum. Findings were most consistent with a pituitary macroadenoma, hydrocephalus, and hydrosyringomyelia. The degree of pituitary enlargement and severity of 4th ventricular dilation did not support obstruction due to the pituitary mass. A cystic accumulation of cerebrospinal fluid (CSF) within the 4th ventricle or obstructive hydrocephalus due to an additional lesion not visible on the MRI could not be ruled out.
Figure 1.
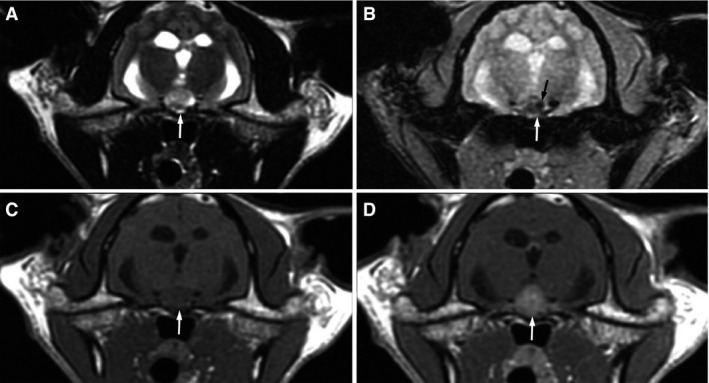
Axial MRI images of the brain show a large nodule that is hyperintense on T2W images (A, white arrow), mildly hypointense on T1W images (C, white arrow), and strongly uniformly contrast enhancing (D, white arrow) in the region of the pituitary gland. A T2* gradient recalled echo sequence shows patchy regions of indistinct hypointensity (B, white arrow) with a small region of marked hypointensity or signal void (B, black arrow).
A clinical diagnosis of hypersomatotropism was supported by the presence of a pituitary mass and a total serum Insulin‐like growth factor 1 (IGF‐1) concentration (radioimmunoassay) consistent with the presence of excess growth hormone (GH) secretion (855 ng/mL, RI <700). The cat was humanely euthanized approximately 8 days after the initial presentation due to a lack of response to medical management, which included corticosteroid treatment, mannitol, furosemide, and a proton‐pump inhibitor.
Histology revealed expansion of the pars distalis by a 0.5 cm diameter, unencapsulated densely cellular neoplasm. The neoplasm was composed of cords and packets of polygonal cells separated by blood filled sinusoids and supported on a fine fibrovascular stroma. Neoplastic cells had oval nuclei with finely stippled chromatin, and a variable amount of finely granular eosinophilic cytoplasm with indistinct cell borders (Fig 2). The cells exhibited mild to moderate anisocytosis and anisokaryosis with a low mitotic index (<1/10 high powered fields), consistent with a pituitary adenoma.
Figure 2.
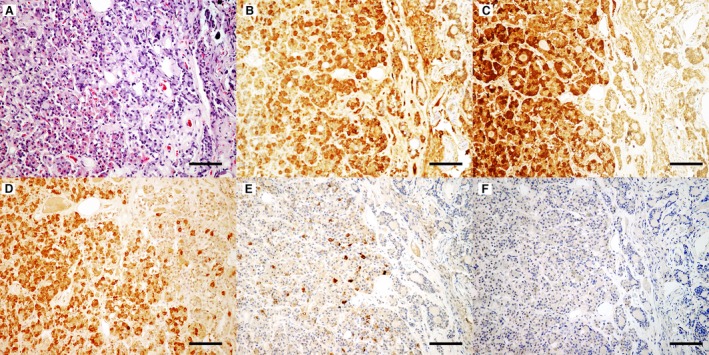
Neoplastic cells vary in their intensity of eosinophilic cytoplasmic staining with many cells resembling acidophils (A). Hematoxylin eosin staining, bar = 200 μm. Neoplastic cells have diffuse cytoplasmic labeling for growth hormone (B), adrenocorticotropic hormone (C), and follicle‐stimulating hormone (D). Small numbers of cells within the neoplasm were positively labeled for melanocyte‐stimulating hormone (E), while labeling for thyroid‐stimulating hormone (F) was not observed. DAB, hematoxylin counterstain, bar = 100 μm.
Immunohistochemistry of the neoplasm (Fig 2) revealed strong positive cytoplasmic labeling for GH, which supports a diagnosis of hypersomatotropism caused by a GH secreting pituitary adenoma. Additionally, there was strong positive labeling for ACTH and follicle‐stimulating hormone (FSH) as well as a small number of cells within the neoplasm that were positively labeled for melanocyte‐stimulating hormone (MSH). Cells with positive labeling for thyroid‐stimulating hormone (TSH) were not identified in the neoplasm. The immunohistochemistry findings are consistent with a plurihormonal adenoma.
Case 2
A 9‐year‐old, 7 kg, castrated male Domestic Short‐haired cat was presented to Queen Mother Hospital for Animals at the Royal Veterinary College with a 2‐month history of weight gain despite a normal appetite. The owner elected to have the cat evaluated for this reason and because a sibling had been previously diagnosed with hypersomatotropism‐induced DM. The physical examination was unremarkable except for broad facial features. The CBC and biochemistry profile were unremarkable. The blood glucose measured at the time of admission using a handheld glucometer1 was 251 mg/dL (13.8 mmol/L). The serum fructosamine concentration measured initially and approximately 3 weeks later were both within reference range (233 and 259 μmol/L, respectively, RI 205–322 μmol/L). A repeat blood glucose concentration measured on a chemistry analyzer4 prior to surgery was 165 mg/dL (9.1 mmol/L). The urinalysis revealed a urine specific gravity (USG) of 1.038 and no detectable glucose. The recheck blood glucose concentration, normal fructosamine concentrations, absence of clinical signs compatible with DM, and lack of glucosuria support stress hyperglycemia as the likely cause of isolated hyperglycemia. Two‐dimensional echocardiogram revealed mild left ventricular free wall hypertrophy (6.5 mm in diastole).
A diagnosis of hypersomatotropism was supported by an increased IGF‐1 concentration (1468 ng/mL, RI <700) and subsequent documentation of increased pituitary length (0.63 cm), width (0.65 cm), and height (0.4 cm) with contrast‐enhanced5 (2 mL/kg by hand injection) computed tomography6 (CT) (Fig 3). Hypophysectomy was performed, and the pituitary was removed in several pieces. The small pieces were submitted for histology, which revealed nervous tissue most consistent with pars nervosa bordered by a densely cellular proliferation of acidophilic cells. The cells had indistinct borders, finely vacuolated eosinophilic cytoplasm, and large round to oval nuclei, with stippled chromatin and variably prominent nucleoli. The cells were arranged in sheets and displayed mild to moderate anisocytosis, anisokaryosis, and cellular pleomorphism. Immunohistochemistry of the pituitary mass revealed positive cytoplasmic labeling for GH as well as strong positive labeling for ACTH, FSH, and MSH consistent with a plurihormonal adenoma. A clinical diagnosis of hypersomatotropism caused by a GH secreting pituitary adenoma was established on the basis of an increased IGF‐1 concentration, pituitary enlargement on CT scan, and the histopathology findings. An inability to control progressive epistaxis that developed post‐hypophysectomy resulted in humane euthanasia. A postmortem examination was declined.
Figure 3.
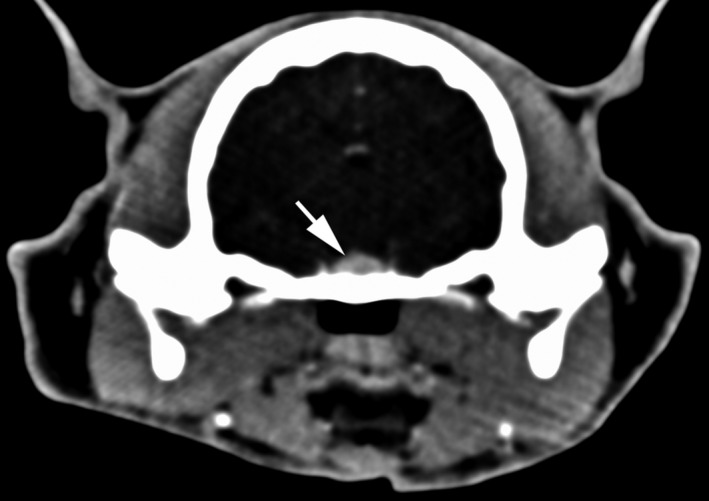
Postcontract CT image shows mild enlargement of the pituitary gland (white arrow).
Case 3
A 16‐year‐old, 7 kg, castrated male Domestic Short‐haired cat was presented to Queen Mother Hospital for Animals at the Royal Veterinary College with a 5‐day history of generalized seizures. The owner had not observed and did not report polyuria or polydipsia. The physical examination revealed mild palmigrade and plantigrade stance and mild pelvic limb muscle atrophy. The neurological examination was unremarkable. The CBC and biochemistry profile were unremarkable with the exception of hyperglycemia 345 mg/dL (19 mmol/L). The urinalysis revealed glucosuria (3+) and a USG of 1.031. Absence of clinical signs suggestive of DM (i.e., weight loss, polyphagia, polyuria, and polydipsia) at the time of presentation and a normal serum fructosamine concentration (253 μmol/L, RI 205–322) make stress the most likely cause of hyperglycemia and resultant glucosuria.
MRI7 images of the head before and after the administration of gadolinium8 at a dose of 28 mg/kg revealed pituitary enlargement: length (0.76 cm), width (0.65 cm), and height (0.59 cm). The region of the pituitary was mildly T2 hyperintense, T1 hyperintense, and strongly contrast enhancing (Fig 4). A round area within the left aspect of the pituitary gland was hypointense to surrounding region of the pituitary on the T1 and T2 weighted images. This same region had reduced contrast enhancement on postcontrast T1 weighted images when compared to the surrounding pituitary. Additionally, a focal extra‐axial intracranial mass overlying the left temporal lobe, demonstrating marked contrast enhancement with a dural tail sign, compatible with a meningioma was seen. Pituitary enlargement combined with an increased IGF‐1 concentration (1902 ng/mL, RI <700) supported a diagnosis of concurrent hypersomatotropism. Approximately 1 month after the initial evaluation, a craniotomy was performed to resect the left temporal lobe tumor. Histology of the tumor was consistent with a fibroblastic meningioma. Phenobarbital was administered postoperatively for approximately 4 weeks. Transition to a high protein and low carbohydrate diet9 was attempted but was not successful due to an unwillingness of the cat to consume the diet. The cat subsequently developed DM 3 months after the initial presentation and insulin treatment was prescribed. The owner declined treatment specifically targeting hypersomatotropism and the cat was humanely euthanized approximately 17 months later. A postmortem examination was declined.
Figure 4.
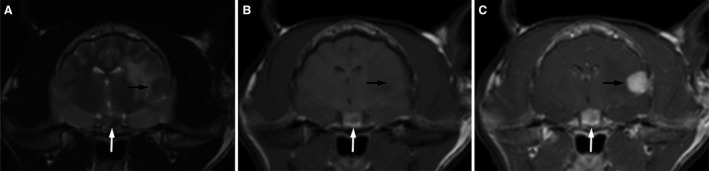
Axial MRI images of the brain show enlargement of the pituitary gland that is mixed hyper and hypo intense on T2W images (A, white arrow), predominantly hyperintense on T1W images (B, white arrow), and strongly contrast enhancing (C, white arrow). A region within the left aspect of the pituitary gland was hypointense to surrounding pituitary on both the T1 and T2 W images. This same region had reduced contrast enhancement on postcontrast T1W images when compared to the surrounding pituitary. A focal, broad based, mildly T2 hypointense, T1 isointense, markedly uniformly contrast enhancing extraaxial mass with regional vasogenic edema is also noted in the left temporal lobe and internal capsule (A–C, black arrow).
This case series presents three cats with GH‐secreting pituitary adenomas that developed hypersomatotropism without concurrent DM. Hypersomatotropism is a state of excessive production and secretion of GH, which in the cat has been described to be the result of a pituitary acidophilic adenoma, carcinoma, or hyperplasia.1, 2, 3, 4 Currently, most cats are diagnosed with hypersomatotropism once they are found to be insulin‐resistant unless the clinician routinely screens all diabetic cats for hypersomatotropism shortly after DM is diagnosed. According to screening studies performed in the UK that measured IGF‐1 concentrations in a large number of diabetic cats, as many as 26–32% were determined to have hypersomatotropism‐induced DM.2, 4, 5 The reason that hypersomatotropism has not been previously reported in cats that are not diabetic is likely because there is a strong and long‐standing clinical association between DM and the recognizable physical characteristics of acromegaly. The non‐diabetic aspects of this clinical image are slow to appear, often subtle, and can go unrecognized.4
It was not possible to directly demonstrate increased GH concentrations in these cases because a validated method for measuring feline GH was not commercially available at the time these cases were evaluated; however, the diagnosis of hypersomatotropism in both people and cats is routinely made by demonstrating an increased IGF‐1 concentration and presence of a pituitary mass or enlargement. In fact, recent practice guidelines for physicians recommend against relying on random GH concentrations to diagnose acromegaly in people.6 Human assays for feline IGF‐1 have been validated, are readily available, and are useful for screening because IGF‐1 reflects 24‐hour GH secretion, parallels changes in GH, and is a sensitive marker for GH excess.4, 7, 8, 9, 10 Unlike GH, IGF‐1 secretion is not pulsatile which allows a single sample collected at any point during the day to have diagnostic utility. It is generally accepted that an IGF‐1 concentration >1000 ng/mL is strongly suggestive of acromegaly in cats, with a positive predictive value of 95%.1, 2, 4 The first cat in this series had immunohistopathologic confirmation of a GH producing pituitary adenoma, but had an IGF‐1 below this cutoff. This case supports recent speculation that the currently used arbitrary IGF‐1 cutoff value of 1000 ng/mL is too high, underestimates the true prevalence of hypersomatotropism, and leads to underdiagnosis of mild or early forms of the disease.1, 7 This has led some authors to recently suggest considering 800–1000 ng/mL as a “gray zone” that warrants further investigation.7 Although the negative predictive value of an IGF‐1 < 1000 ng/mL is low, a case of hypersomatotropism‐induced DM with a lower IGF‐1 was recently reported4 further supporting the existence of cats with hypersomatotropism that have IGF‐1 concentrations below the currently accepted cut‐off.
It could be argued that the cats being reported were not diabetic because they had incidental or nonfunctional pituitary enlargement; however, the elevated serum IGF‐1 concentrations and immunohistochemistry findings argue against such theory, as does the low incidence of incidental pituitary enlargements found in cats. A recent study reported on a cohort of 62 cats undergoing CT imaging for reasons other than pituitary visualization found only one cat (1.6%) with incidental pituitary enlargement.4 Finally, cat 3 ultimately became diabetic.
There were also clinical features that could be compatible with the presence of hypersomatotropism: the weight gain seen in cat 2 and the ventricular myocardial hypertrophy and cardiac changes in cats 1 and 2. Additionally, on retrospective MRI assessment of the head features of cat 3, there was increased parietal bone thickness and increased distance between the lateral aspects of the zygomatic arches. These changes have been described in cats with hypersomatotropism.11
The immunohistochemistry findings of cat 1 and cat 2 were consistent with plurihormonal expression across three lineages of adenohypophysial differentiation (ie, corticotrophs, somatotrophs, gonadotrophs). Although plurihormonal expression in pituitary adenomas has been described in people and other domestic animals, there is only a single report of a somatotroph and corticotroph pituitary double adenoma in a cat with DM and hyperadrenocorticism.12, 13 It has been speculated that plurihormonal expression may be more common, but tumors are rarely analyzed immunohistochemically and instead classified by the hormone that dominates the clinical picture. It is important to note that although cat 1 and cat 2 had strong positive labeling for ACTH, neither cat had characteristic features associated with hyperadrenocorticism or ultrasonographic evidence of adrenomegaly. In addition, adrenomegaly was not identified during the postmortem examination of cat 1.
To our knowledge, the only discussion of a nondiabetic acromegalic cat was an author's personal experience that was included in a textbook chapter.14 This has resulted in the dogma that all hypersomatotropic cats are insulin‐resistant diabetics. It is this dogma and the idea of a “typical” acromegalic phenotype that has likely resulted in very few cats being screened for hypersomatotropism prior to the development of insulin‐resistant DM and an acromegalic phenotype. As a result, the true prevalence in nondiabetic cats remains unknown. As in humans, feline hypersomatotropism likely has a gradual‐onset and a period during which the GH and IGF‐1 concentrations are increased, but DM and signs constituting the syndrome of acromegaly have yet to occur. If the cat does not have dysfunctional pancreatic beta cells, as is suggested to be the case in Type 2 DM, it is likely able to withstand a period of increasing insulin resistance without developing overt DM. This is further substantiated by the fact that most diabetic cats with hypersomatotropism will enter diabetic remission once the somatotrophinoma is removed.5, 15 It is also worth noting that approximately 2/3 of people diagnosed with hypersomatotropism do not become diabetic,16 further suggesting that by focusing only on the diabetic population, we could be missing hypersomatotropic cats prior to the development of overt DM as well as those that may never become diabetic.
With recent evidence of a much higher prevalence of hypersomatotropism in diabetic cats than once realized1, 2, 4, 17, 18, 19 and an unknown prevalence in cats without DM, more work needs to be done to accurately determine the significance of this endocrinopathy in the general cat population. Hopefully, increased awareness and screening will lead to earlier detection and may allow the occurrence of the syndrome of acromegaly, DM, and conditions associated with progressive pituitary enlargement to be delayed or prevented. Finally, recent advances in our ability to treat hypersomatotropism (e.g., increasing availability of hypophysectomy, stereotactic radiation and Gamma Knife technology, and pasireotide) make early diagnosis and intervention more desirable.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
AlphaTrak and AlphaTrak2, Zoetis, Florham Park, NJ
Echelon 1.5T, Hitachi Medical Systems America, Twinsburg, OH
Magnevist, Bayer Healthcare, Wayne, NJ
Stat Profile pHOx Ultra, Nova Biomedical Corporation, Waltham, MA
Omnipaque 350, GE Healthcare Ireland, Cork, Ireland
Mx8000 IDT 16, Philips, Amsterdam, NL
Intera 1.5T, Philips Healthcare, Eindhoven,NL
Dotarem, Guerbet, Milton Keynes, UK
Hill's Prescription Diet m/d Feline, Hill's (registered trademark) Pet Nutrition, Inc., Topeka, KS, USA
References
- 1. Niessen SJM, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Anim Pract 2013;43:319–350. [DOI] [PubMed] [Google Scholar]
- 2. Niessen SJM, Petrie G, Gaudiano F, et al. Feline acromegaly: An underdiagnosed endocrinopathy? J Vet Intern Med 2007;21:899–905. [DOI] [PubMed] [Google Scholar]
- 3. Niessen SJM, Khalid M, Petrie G, Church DB. Validation and application of a radioimmunoassay for ovine growth hormone in the diagnosis of acromegaly in cats. Vet Rec 2007;160:902–907. [DOI] [PubMed] [Google Scholar]
- 4. Niessen SJM, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: Beware of the acromegalic imposter. PLoS ONE 2015;10:e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kenny PJ, Scudder C, Keyte SV, et al. Experiences of a newly established hypophysectomy clinic for treatment of feline hypersomatotropism. J Vet Intern Med 2015;29:423–484 (Abstract). [Google Scholar]
- 6. Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocr Metab 2014;99:3933–3951. [DOI] [PubMed] [Google Scholar]
- 7. Feldman EC, Nelson RW, Reusch C, Scott‐Moncrieff JC. Canine and Feline Endocrinology. St. Louis, MO: Elsevier Saunders; 2014. [Google Scholar]
- 8. Le Roith D, Bondy C, Yakar S, et al. The somatomedin hypothesis: 2001. Endocr Rev 2001;22:53–74. [DOI] [PubMed] [Google Scholar]
- 9. Freda PU. Current concepts in the biochemical assessment of the patient with acromegaly. Growth Horm IGF Res 2003;13:171–184. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro‐Oliveira A Jr, Barkan A. The changing face of acromegaly—advances in diagnosis and treatment. Nat Rev Endocrinol 2012;8:605–611. [DOI] [PubMed] [Google Scholar]
- 11. Lamb CR, Ciasca TC, Mantis P, et al. Computed tomographic signs of acromegaly in 68 diabetic cats with hypersomatotropism. J Feline Med Surg 2014;16:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiupel M, Capen C, Miller M, Smedley R. Histological Classification of Tumors of the Endocrine System of Domestic Animals. Washington, DC: Armed Forces Institute of Pathology in cooperation with the CL Davis DVM Foundation and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology; 2008. [Google Scholar]
- 13. Meij BP, van der Vlugt‐Meijer RH, van den Ingh TSGAM, Rijnberk A. Somatotroph and corticotroph pituitary adenoma (double adenoma) in a cat with diabetes mellitus and hyperadrenocorticism. J Comp Pathol 2004;130:209–215. [DOI] [PubMed] [Google Scholar]
- 14. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. St. Louis, MO: Elsevier Saunders; 2004. [Google Scholar]
- 15. Kenny P, Scudder C, Keyte S, et al. Treatment of feline hypersomatotropism‐ efficacy, morbidity and mortality of hypophysectomy. J Vet Intern Med 2015;29:1257–1283 (Abstract). [Google Scholar]
- 16. Wass JAH, Stewart PM. Oxford Textbook of Endocrinology and Diabetes, 2nd ed Oxford: Oxford University Press; 2011. [Google Scholar]
- 17. Elliott DA, Feldman EC, Koblik PD, et al. Prevalence of pituitary tumors among diabetic cats with insulin resistance. J Am Vet Med Assoc 2000;216:1765–1768. [DOI] [PubMed] [Google Scholar]
- 18. Berg RIM, Nelson RW, Feldman EC, et al. Serum insulin‐like growth factor‐I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007;21:892–898. [DOI] [PubMed] [Google Scholar]
- 19. Niessen SJM, Forcada K, Jensen B, et al. Routine screening of diabetic cats for acromegaly: Overdue or overkill? J Vet Intern Med 2011;25:1470–509 (Abstract). [Google Scholar]


