Abstract
Background
The dynamic component of disc‐associated cervical spondylomyelopathy (DA‐CSM) currently is evaluated using traction magnetic resonance imaging (MRI), which does not assess changes in flexion and extension of the cervical vertebral column. In humans with cervical spondylotic myelopathy, kinematic MRI is used to identify dynamic compressions.
Hypothesis/Objectives
To evaluate the feasibility and utility of kMRI in Doberman Pinschers with DA‐CSM using a novel positioning device. We hypothesized that kMRI would identify compressive lesions not observed with neutral positioning and change the dimensions of the spinal cord and cervical vertebral canal.
Animals
Nine client‐owned Doberman Pinschers with DA‐CSM.
Methods
Prospective study. After standard MR imaging of the cervical spine confirmed DA‐CSM, dogs were placed on a positioning device to allow imaging in flexion and extension. Morphologic and morphometric assessments were compared between neutral, flexion, and extension images.
Results
Flexion was associated with improvement or resolution of spinal cord compression in 4/9 patients, whereas extension caused worsening of compressions in 6/9 patients. Extension identified 6 new compressive lesions and was significantly associated with dorsal and ventral compression at C5‐C6 (P = .021) and C6‐C7 (P = .031). A significant decrease in spinal cord height occurred at C6‐C7 from neutral to extension (P = .003) and in vertebral canal height at C5‐C6 and C6‐C7 from neutral to extension (P = .011 and .017, respectively).
Conclusions and clinical importance
Our results suggest that kMRI is feasible and provides additional information beyond what is observed with neutral imaging, primarily when using extension views, in dogs with DA‐CSM.
Keywords: Dynamic, Extension, Flexion, Wobbler
Abbreviations
- DA‐CSM
disc‐associated cervical spondylomyelopathy
- DW
disc width
- human CSM
cervical spondylotic myelopathy in humans
- kMRI
kinematic magnetic resonance imaging
- MRI
magnetic resonance imaging
- sag
sagittal
- SCA
spinal cord area
- SCH
spinal cord height
- SCW
spinal cord width
- trans
transverse
- VCH
vertebral canal height
Disc‐associated cervical spondylomyelopathy (DA‐CSM) is a common condition in middle‐aged to older Doberman Pinschers.1, 2 Spinal cord compression in patients with DA‐CSM is secondary to both static and dynamic factors.3 For this reason, DA‐CSM is similar to cervical spondylotic myelopathy in human patients (human CSM). Flexion and extension of the cervical vertebral column are 2 dynamic factors that contribute to progression of disease in both DA‐CSM3 and human CSM.4, 5 In ex vivo and magnetic resonance imaging (MRI) studies, flexion has been shown to cause an increase in the diameter of the vertebral canal6, 7 and intervertebral foramina in dogs and humans.8, 9 Flexion of the cervical vertebral column also creates a tethering effect which may lead to stretch injury of the spinal cord.5 Extension creates a decrease in the diameter of the vertebral canal6, 7, 10 and intervertebral foramina in humans and dogs,8, 9 which has been shown to worsen both spinal cord11 and nerve root compression.8
Despite similarities in pathogenesis, current diagnostic evaluation differs between DA‐CSM and human CSM. In dogs with DA‐CSM, MRI of the cervical region typically is performed with the vertebral column in neutral position. Traction MRI of the cervical vertebral column has been described as a method to evaluate dynamic spinal cord compression in patients with DA‐CSM.12, 13 Myelography in flexion and extension has been used in dogs,14 but it is not performed routinely because of a risk of neurologic worsening.14 In human patients, kinematic MRI (kMRI) of the cervical vertebral column is used to assess the dynamic component of human CSM. For this technique, an MRI‐compatible positioning device is used to provide controlled flexion and extension of the cervical vertebral column.11 15, 16, 17, 18, 19, 20, 21, 22 Results of kMRI have been shown to direct therapy in 26% of human patients,15 and to change the surgical approach over what is predicted by neutral position alone in 40% of patients with human CSM.15 Prospective studies investigating kMRI in dogs with CSM are lacking.
The purposes of this prospective study were to evaluate feasibility of a novel, MRI‐compatible positioning device that allows controlled flexion and extension of the canine cervical vertebral column, and to characterize the changes that occur with flexion and extension of the cervical vertebral column in patients with DA‐CSM. We hypothesized that kMRI would identify areas of spinal cord compression that were not noted with neutral MRI alone, and that the dimensions of both the spinal cord and the vertebral column would vary depending upon the position of the cervical vertebral column.
Materials and Methods
Animals
This prospective study was approved by and conducted in accordance with the Institutional Animal Care and Use Committee and the Clinical Research Advisory Committee at The Ohio State University. Nine client‐owned Doberman Pinschers that were presented to The Ohio State University Veterinary Medical Center for evaluation of presumed DA‐CSM were enrolled between January 2014 and October 2015. Complete physical and neurologic examinations, CBC, serum biochemical profile and cervical spinal radiography were performed in all cases. A patient was excluded from study enrollment if examination findings were not consistent with cervical myelopathy, if cervical spinal radiographs identified infectious or neoplastic lesions, or if general anesthesia was considered unsafe in the patient based upon physical examination or laboratory findings. Owner informed consent was required at the time of enrollment. Patients were monitored constantly during recovery from general anesthesia. Once the patient was ambulatory, it was determined if the patient was neurologically worse compared with the pre‐MRI status. Owners also were contacted a few days after discharge to see if they noticed any late neurologic worsening.
MRI Protocol
All dogs underwent general anesthesia and MRI using a 3.0 Tesla machine1 and surface coil. Standard MRI of the cervical vertebral column was performed with the patient in dorsal recumbency. T1‐weighted (TR = 450–700 milliseconds; TE = 8 milliseconds) and T2‐weighted (TR = 3500–5000 milliseconds; TE = 110 milliseconds) images were obtained in the sagittal plane from C2‐T3. T1‐weighted (TR = 500–650 milliseconds; TE = 8 milliseconds) and T2‐weighted (TR = 3000–4000 milliseconds; TE = 120 milliseconds) transverse images were obtained at each disc space from C2‐C3 through T2‐T3. Three transverse slices were obtained at each disc space, positioned perpendicular to the vertebral canal and centered on the intervertebral disc. Slice thickness was 3 millimeters (contiguous).
After standard MRI was performed, linear traction was applied to the cervical vertebral column using a cervical harness and a weight. The total weight applied to the patient's cervical vertebral column was approximately 20% of the patient's total body weight in kilograms. T2‐weighted sagittal images then were obtained from C2‐T3 (TR = 3500–5000 milliseconds; TE = 110 milliseconds).
For kMRI, the patient was placed in right lateral recumbency using the MRI‐compatible positioning device (Fig 1). The cervical vertebral column was flexed and held in place with the positioning device. T2‐weighted sagittal (TR = 3500–5000 milliseconds; TE = 110 milliseconds) and transverse (TR = 3000–4000 milliseconds; TE = 120 milliseconds) images were obtained from C2‐T1. Transverse images were acquired as described above. After MRI in flexion, the patient's cervical vertebral column was extended and held in place with the positioning device. Magnetic resonance imaging was performed as was described for flexion.
Figure 1.
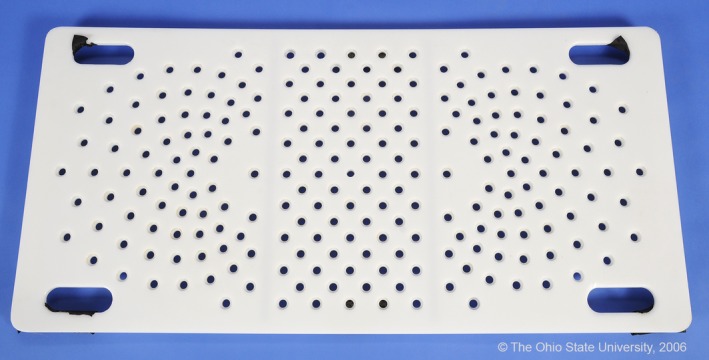
Photograph of novel, nonferromagnetic positioning device.
Morphologic Analysis
Three evaluators (MP, RDC, AH) used the mid‐sagittal and transverse T2‐weighted images to independently evaluate the subjective degree of spinal cord compression at each disc space from C2‐C3 through C7‐T1. A compression score was assigned at each disc space based upon a previously described modified spinal cord compression scale: 0, no spinal cord compression; 1, mild (<25%) spinal cord compression; 2, moderate (25–50%) spinal cord compression; and, 3, severe (>50%) spinal cord compression.23 The worst site of spinal cord compression was recorded for each position. The direction of spinal cord compression was noted at each disc space as follows: no compression; dorsal compression; dorsolateral compression; lateral compression; ventral compression; or multiple directions of compression.
Both T1‐weighted and T2‐weighted images were used to evaluate the signal intensity within the spinal cord, the methodology of which has been described previously.24 The following signal intensity changes were noted: isointense to normal spinal cord parenchyma, hyperintense to normal spinal cord parenchyma, or hyperintense on T2‐weighted imaging and hypointense on T1‐weighted imaging.
Morphometric Analysis
A computer‐based software program2 was used to calculate morphometric parameters and the degree of flexion and extension for each patient. In our study, the degree of flexion was defined as the degree of spinal cord deviation from the lamina of C5, and the degree of extension was defined as the degree of spinal cord deviation from the dorsal aspect of the C6‐C7 disc space (Fig 2).
Figure 2.
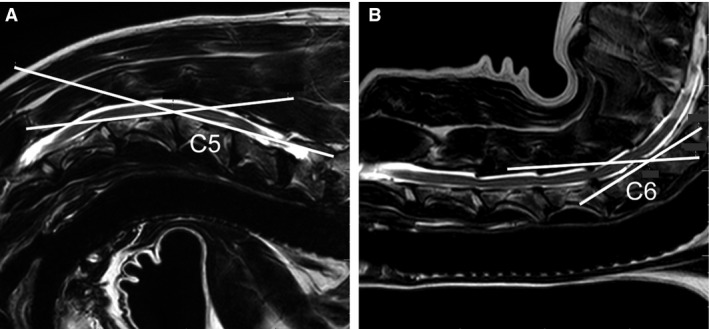
Sagittal T2‐weighted MRI images demonstrating measurement of the degree of flexion (A) and extension (B). The C5 (A) and C6 (B) vertebral bodies are labeled. The degree of flexion is 22° and the degree of extension is 28°.
The following morphometric parameters were acquired in all patients based on previously published definitions: spinal cord height (T2‐weighted, midsagittal images), intervertebral disc width (T2‐weighted, midsagittal images), spinal cord height (T2‐weighted, transverse images), spinal cord width (T2‐weighted, transverse images), spinal cord area (T2‐weighted, transverse images), and vertebral canal height (T2‐weighted, transverse images; Fig 3).23 Measurements were performed at each disc space (at the center of the intervertebral disc) from C2‐C3 through C7‐T1 in all patients. A single evaluator (MP) performed measurements on all patients. The same evaluator then repeated measurements in 3 patients to assess intraobserver agreement. A second evaluator (AH) performed measurements in the same 3 patients to assess interobserver agreement.
Figure 3.
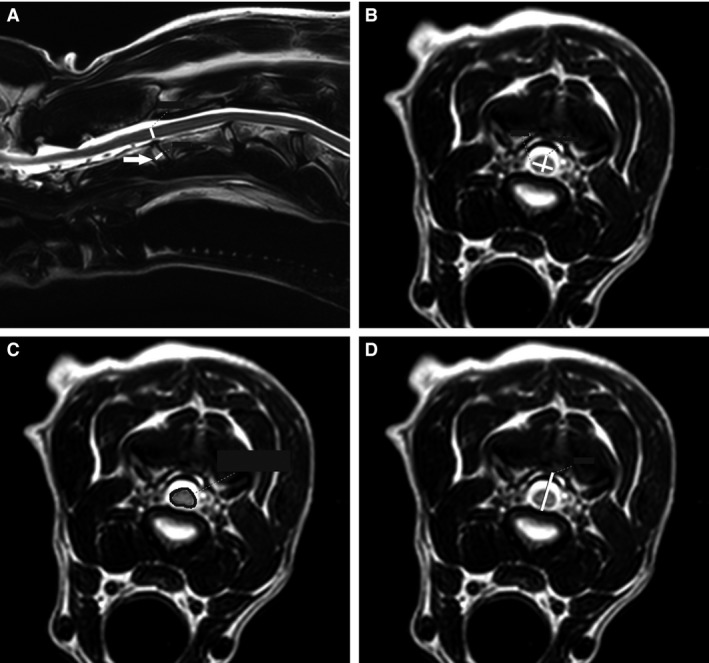
Morphometric measurements demonstrating the following at C2‐C3, neutral position: spinal cord height and intervertebral disc width, mid‐sagittal plane (A), spinal cord height and width, transverse plane (B), vertebral canal height, transverse plane (C), spinal cord area, transverse plane (D). White lines represent all measurements with the exception of spinal cord area, which is outlined in black. The arrow is pointing to the intervertebral disc at C2‐C3.
Statistical Analysis
Mixed‐effects linear regression followed by linear contrast statements were used to analyze the initial morphometric data of the primary evaluator (MP). Mixed‐effects linear regression also was used to calculate the interobserver and intraobserver agreement for the morphometric data (ρ). A Fisher's exact test was used to determine if an association existed between the position of the cervical vertebral column and the modified spinal cord compression score at each disc space. Sidak's method was used to adjust P‐values and the 95% confidence intervals to conserve the overall type I error at 0.05 because of multiple testing. An adjusted P‐value was considered significant if <.05.
A kappa analysis was performed to assess interobserver agreement for the following: modified spinal cord compression score, direction of spinal cord compression, signal changes within the spinal cord, worst site of spinal cord compression, and number of spinal cord compressions compared to a neutral position (flexion and extension only). Kappa (κ) values were interpreted as follows: less than chance agreement (κ < 0), slight agreement (0.01–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80), almost perfect agreement (0.81–0.99), and perfect agreement (κ = 1.00).25 All analyses were run using a computer‐based software program.3
Results
Clinical Data
The group contained 3 female and 6 male patients; all patients were neutered and their mean age was 7.1 years (range, 6–8 years). Median patient weight was 36.8 kg (range, 30.7–45.6 kg). Eight of 9 patients had gait abnormalities characterized by ambulatory tetraparesis and general proprioceptive ataxia. In 1 patient, only mild paraparesis was observed. Four of 9 patients had hyperesthesia on palpation of the caudal cervical region.
None of the patients worsened neurologically after kMRI was performed. The median degrees of flexion and extension obtained were 23° (range, 15–33°) and 28° (range, 19–35°), respectively. The median anesthesia time required for positioning and kinematic imaging (flexion and extension) was 50 minutes (range, 40–90 minutes). Approximately half of this time was used to properly position patients parallel to the table in lateral recumbency and the other half adjusting MRI settings.
Morphologic Data
With neutral positioning, 14 total compressions were noted; 5/9 patients had 1 compression, 3/9 patients had 2 compressions, and 1/9 patients had 3 compressions. The distribution of compressive lesions was as follows: 1/14 lesions, C3‐C4; 1/14 lesions, C4‐C5; 5/14 lesions, C5‐C6; and, 7/14 lesions, C6‐C7. Eleven of 14 lesions were considered mild and 3/14 lesions were considered moderate. The direction of compression was ventral (from an intervertebral disc protrusion) at 10/14 sites, dorsal (from bulging of the ligamentum flavum) at 2/14 sites, ventral and dorsal (from an intervertebral disc protrusion and bulging of the ligamentum flavum) at 1/14 sites and lateral (from articular facet proliferation) at 1/14 sites. Five of 13 lesions resolved with traction. The worst site of compression was at C4‐C5 in 1/9 patients, C5‐C6 in 2/9 patients, and C6‐C7 in 6/9 patients. T2‐weighted hyperintensity was noted within the spinal cord at C6‐C7 in 4/9 patients and at both C5‐C6 and C6‐C7 in 1/9 patients.
With flexion, 12 total compressions were noted; 1/9 patients had no compressions, 5/9 patients had 1 compression, 2/9 patients had 2 compressions, and 1/9 patients had 3 compressions. The distribution of lesions was as follows: 1/12 lesions, C2‐C3; 1/12 lesions, C4‐C5; 3/12 lesions, C5‐C6; and 7/12 lesions, C6‐C7. Eleven of 12 lesions were considered mild and 1/12 lesions was considered moderate. The direction of compression was ventral at 11/12 sites and lateral at 1/12 sites. The worst site of compression was at C4‐C5 in 1/9 patients, C5‐C6 in 1/9 patients, and C6‐C7 in 6/9 patients; a compressive lesion was not identified in 1/9 patients. T2‐weighted hyperintensity was noted within the spinal cord at C6‐C7 in 4/9 patients and at both C5‐C6 and C6‐C7 in 1/9 patients. In 2/9 patients, the T2‐hyperintense region was shifted cranially, so that it was positioned over the vertebral body instead of at the disc space (Figs 4 and 5).
Figure 4.
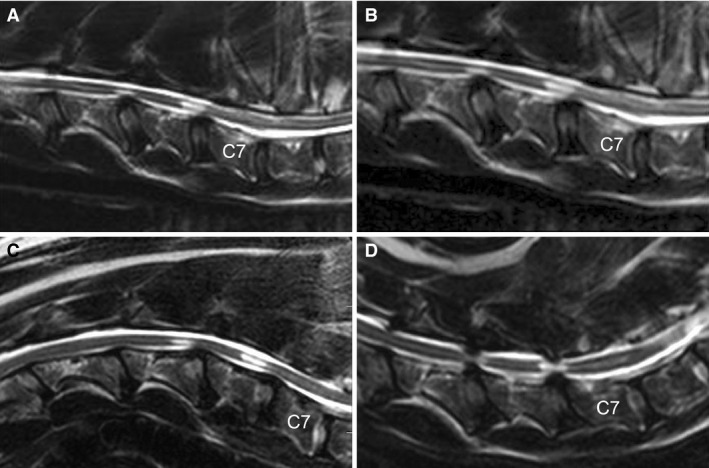
Sagittal T2‐weighted MRI images of a dog with DA‐CSM. Neutral position (A), traction (B), flexion (C) and extension (D) are represented. Flexion of the cervical vertebral column results in cranial migration of the T2‐weighted hyperintensities within the spinal cord at C5‐C6 and C6‐C7. Extension is associated with both ventral and dorsal compression (pincer effect) at C5‐C6 and C6‐C7.
Figure 5.
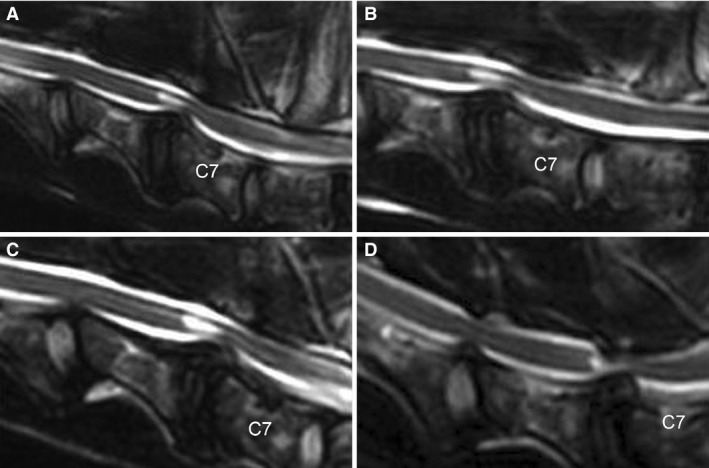
Sagittal T2‐weighted MRI images of a dog with DA‐CSM. Neutral position (A), traction (B), flexion (C) and extension (D) are represented. With extension of the cervical vertebral column, there is significant worsening of the C6‐C7 compression primarily dorsally, as well as a new compressive lesion identified at C5‐C6.
Flexion was associated with improvement or resolution of spinal cord compression in 4/9 patients (Fig 4). Four of 12 compressive lesions present in a neutral position resolved with flexion and 1/12 lesions improved by 1 score. A new area of spinal cord compression was identified in flexion for 2/9 patients. The new areas of compression were located at C2‐C3 (mild) and C6‐C7 (mild); the direction of compression was ventral for both lesions.
With extension, 20 total compressions were noted; 1/9 patients had 1 compression, 6/9 patients had 2 compressions, 1/9 patients had 3 compressions, and 1/9 patients had 4 compressions. The distribution of lesions was as follows: 2/20 lesions, C3‐C4; 4/20 lesions, C4‐C5; 5/20 lesions, C5‐C6; and, 9/20 lesions, C6‐C7. There were 13/20 mild lesions, 3/20 moderate lesions, and 4/20 severe lesions. The direction of compression was dorsal at 4/20 sites, ventral at 3/20 sites, dorsal and ventral at 12/20 sites, and dorsal and lateral at 1/20 sites. There was a significant association between extension of the cervical vertebral column and the presence of multiple directions of compression at both C5‐C6 (P = .021) and C6‐C7 (P = .031). The worst site of compression was located at C5‐C6 in 2/9 patients and at C6‐C7 in 7/9 patients. T2‐weighted hyperintensity was noted within the spinal cord at C6‐C7 in 3/9 patients and at both C5‐C6 and C6‐C7 in 1/9 patients (Fig 4).
There was worsening of compressive lesions at 7/14 neutral sites with extension; 2/7 lesions were located at C5‐C6 and 5/7 lesions were located at C6‐C7 (Fig 5). Four of 7 compressive lesions worsened by 1 score and 3/7 lesions worsened by 2 scores. Six new sites of compression were identified with extension; 1 new compressive lesion was identified in 4/9 patients and 2 new compressive lesions were identified in 1/9 patients. One of 6 new compressive lesion was at C3‐C4, 3/6 were at C4‐C5 and 2/6 were at C6‐C7; the association between extension and the presence of a compressive lesion at C4‐C5 was significant (P = .041). The directions of compression were as follows: dorsal (2/6 sites), ventral (1/6 sites), and dorsal and ventral (3/6 sites).
Extension was associated with improvement of spinal cord compression in 1/9 patients. In total, there were 2/14 compressive lesions in neutral position that improved with extension. Improvement occurred at C3‐C4 and C4‐C5; both lesions improved by 1 score.
Interobserver agreement was perfect (κ = 1.00) for the worst site of spinal cord compression in a neutral position and almost perfect for both traction (κ = 0.83) and extension (κ = 0.82). Substantial agreement existed with regard to the signal intensity within the spinal cord (κ = 0.79). There was moderate agreement for both the modified spinal cord compression scoring (κ = 0.42) and the direction of compression (κ = 0.56). Fair agreement was noted with regard to the worst site of spinal cord compression in flexion (κ = 0.40). There was slight agreement in the number of compressive lesions (compared to neutral position) in flexion and extension (κ = 0.02 and 0.07, respectively).
Morphometric Data
A total of 1,043 measurements were performed; data were summarized as the mean and standard deviation of each variable at each spinal cord level in neutral position, traction, flexion, and extension (Table 1). Traction images were not obtained in the transverse plane and variables measured included only the spinal cord height (mid‐sagittal) and intervertebral disc width. One patient lacked traction imaging, 1 patient did not have transverse images in flexion at C2‐C3 and 1 patient lacked transverse images in flexion and extension at C7‐T1; the absence of images in these patients was a result of technical difficulty. The cranial and caudal margins of 14 intervertebral discs could not be identified, and measurement was not performed.
Table 1.
Morphometric data (mean ± SD) comparing neutral, traction, flexion, and extension in 9 Doberman Pinschers with DA‐CSM
| SC level | Position | SCH, sag (mm) | SCH, trans (mm) | DW (mm) | SCA (mm) | SCW (mm) | VCH (mm) |
|---|---|---|---|---|---|---|---|
| C2‐C3 | Neutral | 4.40 ± 0.69 | 4.51 ± 0.65 | 4.88 ± 0.77 | 3.14 ± 0.41 | 7.99 ± 0.83 | 12.22 ± 1.61a |
| Traction | 4.36 ± 0.65 | 5.00 ± 0.77 | |||||
| Flexion | 4.50 ± 0.48 | 4.78 ± 0.44 | 5.37 ± 1.00 | 3.23 ± 0.56 | 7.85 ± 0.78 | 10.16 ± 1.18a | |
| Extension | 4.48 ± 0.44 | 4.62 ± 0.64 | 4.91 ± 0.64 | 3.38 ± 0.59 | 8.32 ± 0.62 | 11.22 ± 1.16 | |
| C3‐C4 | Neutral | 3.86 ± 0.74 | 4.28 ± 0.52 | 4.47 ± 0.53 | 3.08 ± 0.45 | 8.14 ± 0.91 | 9.99 ± 2.14 |
| Traction | 4.09 ± 0.79 | 4.90 ± 0.51 | |||||
| Flexion | 4.17 ± 0.73 | 4.56 ± 0.22 | 4.82 ± 0.73 | 3.19 ± 0.58 | 8.06 ± 1.08 | 8.49 ± 1.53 | |
| Extension | 4.09 ± 0.87 | 4.70 ± 0.49 | 4.74 ± 0.39 | 3.32 ± 0.53 | 8.14 ± 1.25 | 9.57 ± 1.31 | |
| C4‐C5 | Neutral | 4.03 ± 0.92 | 4.29 ± 0.55 | 4.77 ± 0.80 | 3.18 ± 0.60 | 8.14 ± 0.79 | 10.30 ± 0.94a |
| Traction | 4.06 ± 0.76 | 5.25 ± 0.79 | |||||
| Flexion | 4.27 ± 0.86 | 4.34 ± 0.43 | 5.38 ± 0.88 | 3.19 ± 0.48 | 7.99 ± 0.88 | 8.29 ± 1.35a | |
| Extension | 3.91 ± 1.04 | 4.36 ± 0.62 | 5.16 ± 0.63 | 3.33 ± 0.62 | 7.98 ± 1.10 | 8.48 ± 1.19 | |
| C5‐C6 | Neutral | 4.24 ± 1.10 | 4.51 ± 1.01 | 5.13 ± 0.71 | 3.59 ± 0.77 | 8.42 ± 0.95 | 9.78 ± 1.77b |
| Traction | 4.26 ± 1.12 | 5.74 ± 0.60 | |||||
| Flexion | 4.21 ± 0.98 | 4.43 ± 0.91 | 5.54 ± 0.62 | 3.57 ± 0.85 | 8.36 ± 1.40 | 8.20 ± 1.85 | |
| Extension | 3.66 ± 1.30 | 4.51 ± 1.36 | 5.37 ± 0.64 | 3.52 ± 0.85 | 7.94 ± 1.00 | 7.57 ± 2.53b | |
| C6‐C7 | Neutral | 4.01 ± 0.82 | 4.53 ± 0.72 | 4.87 ± 0.96 | 3.58 ± 0.88 | 8.37 ± 1.18 | 9.77 ± 1.89b |
| Traction | 4.01 ± 0.94 | 5.63 ± 0.88 | |||||
| Flexion | 4.08 ± 1.00c | 4.46 ± 0.90 | 5.46 ± 1.23 | 3.52 ± 1.00 | 8.29 ± 1.25 | 8.61 ± 1.38 | |
| Extension | 3.16 ± 1.26b , c | 4.59 ± 1.69 | 5.08 ± 0.94 | 3.62 ± 1.16 | 8.86 ± 1.36 | 7.63 ± 2.34b | |
| C7‐T1 | Neutral | 5.33 ± 0.39b | 5.58 ± 0.49 | 4.90 ± 0.39 | 3.28 ± 0.45 | 7.50 ± 0.47 | 14.42 ± 1.79a |
| Traction | 5.12 ± 0.67 | 5.30 ± 0.39 | |||||
| Flexion | 4.77 ± 0.56c | 4.99 ± 0.84 | 5.42 ± 0.46 | 3.16 ± 0.52 | 7.24 ± 0.87 | 12.34 ± 2.01a | |
| Extension | 5.47 ± 0.49c | 5.69 ± 0.71 | 5.28 ± 0.53 | 3.40 ± 0.46 | 7.29 ± 0.79 | 13.05 ± 2.37 |
SC, spinal cord; SCH, spinal cord height; sag, sagittal; trans, transverse; DW, disc width; SCA, spinal cord area; SCW, spinal cord width; VCH, vertebral canal height; mm, millimeters.
Statistically significant difference between neutral and flexion (P < .05).
Statistically significant difference between neutral and extension (P < .05).
Statistically significant difference between flexion and extension (P < .05).
The spinal cord height (SCH) on mid‐sagittal imaging was significantly shorter in extension than in neutral position (P = .003) or flexion (P = .001) at C6‐C7. A significant decrease in SCH was observed from flexion to extension at C7‐T1 (P = .029). The mid‐sagittal SCH at C5‐C6 was shorter in extension (mean, 3.7 mm; SD, 1.3) than in neutral position (mean, 4.4 mm; SD, 1.1) or flexion (mean, 4.2 mm; SD, 1.0), but these differences were not significant (P = .12 and .17, respectively). No significant differences were observed in mid‐sagittal SCH with change in position of the cervical vertebral column at the remaining spinal cord levels. Similarly, change in position of the cervical vertebral column did not result in a significant change in transverse SCH, intervertebral disc width, spinal cord width, or spinal cord area at any level.
A significant decrease in vertebral canal height (VCH) was observed from neutral position to extension at C5‐C6 (P = .011) and C6‐C7 (P = .017). The VCH at C4‐C5 in neutral (mean, 10.3 mm; SD, 0.9) was taller than the VCH in extension (mean, 8.5 mm; SD, 1.2); this difference was not significant (P = .073). The VCH in flexion was significantly shorter than in neutral position at C2‐C3 (P = .012), C4‐C5 (P = .031) and C7‐T1 (P = .038). No other significant differences were noted in VCH with change in position of the cervical vertebral column at the remaining spinal cord levels.
Intraobserver agreement was best for vertebral canal height (ρ = 0.91) and worst for spinal cord height in the transverse plane (ρ = 0.75), whereas interobserver agreement was best for spinal cord width (ρ = 0.80) and worst for spinal cord area (ρ = 0.35).
Discussion
Ours is the first study to prospectively assess the feasibility and effect of kinematic MRI in dogs with DA‐CSM. In general, flexion of the cervical vertebral column was associated with improvement in spinal cord compression, whereas extension was associated with worsening spinal cord compression and development of compressive lesions not identified with neutral positioning. Cranial migration of T2‐weighted hyperintense regions also was noted with flexion of the cervical vertebral column. This finding may be evidence of spinal cord lengthening, which has been reported in humans.5, 26 Spinal cord height (mid‐sagittal plane) and VCH were considered the most useful morphometric measurements in our study.
In our study, extension of the cervical vertebral column was associated with areas of mild spinal cord compression that were not present in neutral position, especially at C4‐C5. In more than half of these compressions, a disc protrusion was identified, which suggests that extension of the cervical vertebral column identifies disc protrusions that may not be apparent in a neutral position. Our findings are consistent with what has been observed in humans. In a study of humans that evaluated kMRI in patients with cervical myelopathy, extension identified new spinal cord compressions most commonly at C5‐C6, followed by C4‐C5.17 In a study that evaluated kMRI in human patients with cervical pain, extension caused the number of cervical disc protrusions to increase by 16.4%.19, which resulted in the conclusion that kMRI added value to imaging protocols, especially when standard imaging is unremarkable.19
We observed that extension of the cervical vertebral column is associated with worsening of spinal cord compression at C5‐C6 and C6‐C7. In some patients, compressions that were mild in a neutral position became severe in extension. In these instances, extension may aid in the diagnostic assessment of dogs with DA‐CSM by enhancing visualization of compressions. Our results are in partial agreement with studies performed in humans. In 1 study that evaluated kMRI in humans with cervical pain, extension caused a significant worsening of cervical disc bulges; but this change occurred from C2‐C3 through C7‐T1.19 In another study of human patients, a significant decrease in the spinal cord cross‐sectional area was observed with extension of the cervical vertebral column; and this change occurred at multiple levels, with the largest decrease occurring at C5‐C6.27 In Doberman Pinschers with DA‐CSM, relative stenosis of the vertebral canal is more pronounced in the caudal cervical vertebral column,23, 28 which may be why we noted worsening only at C5‐C6 and C6‐C7 with extension. The caudal cervical vertebral column of dogs has higher torsional forces, which have been associated with intervertebral disc degeneration and protrusion.29 The combination of relative vertebral canal stenosis and disc protrusions in the caudal cervical vertebral region of dogs with DA‐CSM accounts for the differences as compared to CSM in humans.
In our patients, extension often was associated with spinal cord compression secondary to ventral disc protrusion and buckling of the ligamentum flavum dorsally. In humans, this phenomenon is known as the pincer effect.30 In 1 study of human patients, this effect was noted with extension in 14 of 23 compressions in patients with CSM.15 In another study of humans, bulging of the ligamentum flavum in extension was more apparent in patients with cervical pain, especially at C4‐C5 and C5‐C6.31
Our study showed improvement or resolution of ventral spinal cord compression with flexion, which has been noted previously in dogs in a myelographic study14 and suggests that disc protrusions may improve with flexion of the cervical vertebral column. This observation is in contrast to a study in humans, in which flexion caused worsening of disc protrusions in 3% of patients.19 An alternate explanation for the improvement that we found with flexion is that it is secondary to an increase in the VCH, which was noted in a recent biomechanical study in dogs.7 An unexpected finding regarding our morphometric results was that with flexion, shortening of the vertebral canal height at C2‐C3, C4‐C5, and C7‐T1 occurred. A retrospective study of humans did find a decrease in cranial‐caudal diameter of the vertebral canal with flexion at C7‐T1, but the cranial‐caudal diameter was increased in flexion at all remaining levels (C2‐C3 through C6‐C7).6 We suspect that the decrease in VCH that we found was secondary to imprecise measurements caused by image distortion, which was 1 limitation of this study.
We found that flexion of the cervical vertebral column identified new areas of spinal cord compression in 2/9 patients. In humans, flexion has been shown to identify new compressive lesions that were not apparent in neutral position. In 1 study of human patients, the number of compressive lesions identified at C5‐C6 increased by 3.7% with flexion.17
Our results suggest that kMRI is a safe procedure when performed in a controlled manner. None of the patients experienced neurologic worsening after kMRI. This finding is in contrast to previous reports involving flexion/extension imaging using myelography, in which 20% of dogs worsened after imaging.14 The lack of clinical worsening in our study suggests that previously reported neurologic deterioration may have been secondary to myelography rather than flexion or extension of the cervical vertebral column. Possible causes of neurologic worsening after flexion/extension myelography include filling of the subarachnoid space with contrast agent (which may cause further compression of the spinal cord), lack of controlled flexion and extension of the cervical vertebral column, possibly more flexion or extension of the cervical vertebral column without the constraints of magnet bore, neurotoxicity of the contrast agent, which has been suggested as a cause for neurologic deterioration postmyelogram or some combination of these factors.32
Some limitations were associated with positioning of patients and analysis of kMRI images in our study. The technique added a considerable amount of time to the standard MRI protocol and positioning large patients on the device was labor intensive. These factors improved as more patients were imaged. The cases that took longest did so because of technical difficulties with image settings (eg, adjusting the field of view, changing the plane from sagittal to dorsal to image patients in lateral recumbency). Imaging in right lateral recumbency was associated with image distortion and repositioning of the chemical shift artifact, which caused the spinal cord and vertebral canal boundaries to be difficult to distinguish. This factor resulted in imprecision of measurements and poor interobserver agreement with regard to morphometric analysis. In addition, to avoid crosstalk artifact, it was challenging to obtain all transverse images perpendicular to the vertebral canal, which contributed to image distortion.
Conclusion
Our results support the use of kMRI in patients with DA‐CSM, recognizing that this technique adds time to a standard imaging protocol. From a clinical perspective, imaging in extension is most useful, and should be considered when neutral positioning does not provide a clear reason for the patient's clinical signs, or when compressive lesions noted in neutral positioning do not explain the severity of a patient's clinical signs. Surgical correction of additional compressive lesions found with kMRI could yield better outcomes and longer survival times in dogs with DA‐CSM than those currently available.
Acknowledgments
The authors thank Denise Bailey, Julie Morris, and the staff at The OSU Wright Center of Innovation in Biomedical Imaging for their assistance with MR imaging. The authors also thank Marc Hardman for his assistance with illustrations. The authors thank Dr. Jean Schelhorn, Erica Braun and Paul Reeder for their assistance with the design and prototype creation of the kinematic board.
Conflict of Interest Declaration:
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration:
Authors declare no off‐label use of antimicrobials.
Grant Support:
This project was funded by the Gray Lady Foundation.
This project was completed at The Ohio State University Veterinary Medical Center in Columbus, Ohio.
Footnotes
Achieva 3.0‐T, Philips Healthcare, Best, The Netherlands
ClearCanvas Workstation, Synaptive Medical, Toronto, Canada
Stata 14.0, StataCorp, College Station, TX
References
- 1. da Costa RC. Cervical spondylomyelopathy (wobbler syndrome) in dogs. Vet Clin North Am Small Anim Pract 2010;40:881–913. [DOI] [PubMed] [Google Scholar]
- 2. De Decker S, Gielen MV, Duchateau L, et al. Evolution of clinical signs and predictors of outcome after conservative medical treatment for disk‐associated cervical spondylomyelopathy in dogs. J Am Vet Med Assoc 2012;240:848–857. [DOI] [PubMed] [Google Scholar]
- 3. da Costa RC. Pathogenesis of cervical spondylomyelopathy: lessons from recent years. Seattle WA: Proc of 25th ACVIM; 2007 Jun 6–9;318–320. [Google Scholar]
- 4. Ichihara K, Taguchi T, Sakuramoto I, et al. Mechanism of the spinal cord injury and cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg (Spine 3) 2003;99:278–285. [DOI] [PubMed] [Google Scholar]
- 5. Henderson F, Geddes J, Vaccaro A, et al. Stretch‐associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery 2005;56:1101–1113. [PubMed] [Google Scholar]
- 6. Sayit E, Aghdasi B, Daubs M, et al. The occupancy of the components in the cervical vertebral column and their changes with extension and flexion. Global Spine J 2015;5:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramos RM, da CR, Oliveira ALA, et al. Effects of flexion and extension on the diameter of the caudal cervical vertebral canal in dogs. Vet Surg 2015;44:459–466. [DOI] [PubMed] [Google Scholar]
- 8. Muhle C, Resnick D, Ahn J, et al. In vivo changes in the neuroforaminal size at flexion‐extension and axial rotation of the cervical spine in healthy persons examined using kinematic magnetic resonance imaging. Spine 2001;13:E287–E293. [DOI] [PubMed] [Google Scholar]
- 9. Ramos RM, da Costa RC, Oliveira ALA, et al. Morphological changes of the cervical intervertebral foramen due to flexion‐extension and compression‐tension movements in the canine cervical vertebral column. BMC Vet Res 2015;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid JD. Effects of flexion‐extension movements of the head and spine upon the spinal cord and nerve roots. J Neurol Neurosurg Psychiatry 1960;23:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muhle C, Weinert D, Falliner A, et al. Dynamic changes of the spinal canal in patients with cervical spondylosis at flexion and extension using magnetic resonance imaging. Invest Radiol 1998;33:444–449. [DOI] [PubMed] [Google Scholar]
- 12. Penderis J, Dennis R. Use of traction during magnetic resonance imaging of caudal cervical spondylomyelopathy (“wobbler syndrome”) in the dog. Vet Radiol Ultrasound 2004;45:216–219. [DOI] [PubMed] [Google Scholar]
- 13. da Costa RC, Parent J, Dobson H, et al. Comparison of magnetic resonance imaging and myelography in the diagnosis of cervical spondylomyelopathy in Doberman pinscher dogs ‐ 18 cases. Vet Radiol Ultrasound 2006;47:23–531. [DOI] [PubMed] [Google Scholar]
- 14. Seim HB, Withrow SJ. Pathophysiology and diagnosis of caudal cervical spondylo‐myelopathy with emphasis on the Doberman pinscher. J Am Anim Hosp Assoc 1982;18:241–251. [Google Scholar]
- 15. Muhle C, Metzner J, Weinert D, et al. Kinematic MR imaging in surgical management of cervical disc disease, spondylosis and spondylotic myelopathy. Acta Radiol 1999;40:146–153. [DOI] [PubMed] [Google Scholar]
- 16. Muhle C, Wiskirchen J, Weiner D, et al. Biomechanical aspects of the subarachnoid space and cervical cord in healthy individuals examined with kinematic magnetic resonance imaging. Spine 1998;23:556–567. [DOI] [PubMed] [Google Scholar]
- 17. Hayashi T, Wang J, Suzuki A, et al. Risk factors for missed dynamic canal stenosis in the cervical spine. Spine 2014;39:812–819. [DOI] [PubMed] [Google Scholar]
- 18. Harada T, Tsuji Y, Mikami Y, et al. The clinical usefulness of preoperative dynamic MRI to select decompression levels for cervical spondylotic myelopathy. Mag Res Imaging 2010;28:820–825. [DOI] [PubMed] [Google Scholar]
- 19. Lao L, Daubs MD, Scott TP, et al. Missed cervical disc bulges diagnosed with kinematic magnetic resonance imaging. Eur Spine J 2014;23:1725–1729. [DOI] [PubMed] [Google Scholar]
- 20. Gilbert JW, Wheeler GR, Lingreen RA, et al. Imaging in the position that causes pain. Surg Neurol 2008;69:463–465. [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Zeitoun D, Rangel A, et al. Preoperative evaluation of the cervical spondylotic myelopathy with flexion‐extension magnetic resonance imaging. Spine 2011;36:E1134–E1139. [DOI] [PubMed] [Google Scholar]
- 22. Miura J, Doita M, Miyata K, et al. Dynamic evaluation of the spinal cord in patients with cervical spondylotic myelopathy using a kinematic magnetic resonance imaging technique. J Spinal Disord Tech 2009;22:8–13. [DOI] [PubMed] [Google Scholar]
- 23. da Costa RC, Parent J, Partlow G, et al. Morphologic and morphometric magnetic resonance imaging features of doberman pinschers with and without clinical signs of cervical spondylomyelopathy. Am J Vet Res 2006;67:1601–1612. [DOI] [PubMed] [Google Scholar]
- 24. Martin‐Vaquero P, da Costa RC. Magnetic resonance imaging features of Great Danes with and without clinical signs of cervical spondylomyelopathy. J Am Vet Med Assoc 2014;245:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360–363. [PubMed] [Google Scholar]
- 26. Endo K, Suzuki H, Nishimura H, et al. Kinematic analysis of the cervical cord and cervical canal by dynamic neck motion. Asian Spine J 2014;8:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Machino M, Yukawa Y, Ito K, et al. Dynamic changes in dural sac and spinal cord cross‐sectional area in patients with cervical spondylotic myelopathy. Spine 2011;36:399–403. [DOI] [PubMed] [Google Scholar]
- 28. De Decker S, Gielen I, Duchateau L, et al. Magnetic resonance imaging vertebral canal and body ratios in doberman pinschers with and without disk‐associated cervical spondylomyelopathy and clinically normal English foxhounds. Am J Vet Res 2011;72:1496–1504. [DOI] [PubMed] [Google Scholar]
- 29. Johnson JA, da Costa RC, Bhattacharya S, et al. Kinematic motion patterns of the cranial and caudal canine cervical spine. Vet Surg 2011;40:720–727. [DOI] [PubMed] [Google Scholar]
- 30. Penning L, van der Zwaag P. Biomechanical aspects of spondylotic myelopathy. Acta Radiol 1966;5:1090–1103. [DOI] [PubMed] [Google Scholar]
- 31. Zhong G, Buser Z, Lao L, et al. Kinematic relationship between missed ligamentum flavum bulge and degenerative factors in the cervical spine. Spine J 2015;15:2216–2221. [DOI] [PubMed] [Google Scholar]
- 32. Lewis DD, Hosgood G. Complications associated with the use of iohexol for myelography of the cervical vertebral column in dogs: 66 cases (1988–1990). J Am Vet Med Assoc 1992;9:1381–1384. [PubMed] [Google Scholar]


