Abstract
This descriptive, cross‐sectional analysis evaluated the impact of baseline characteristics on health‐related quality of life (HR‐QoL) at different stages of multiple myeloma (MM). The bortezomib clinical‐trial programme evaluated HR‐QoL early and consistently, producing a large multi‐study dataset. Baseline data, captured using the European Organization for Research and Treatment of Cancer (EORTC) quality‐of‐life questionnaire (QLQ‐C30), were pooled from six bortezomib randomized trials conducted in different disease‐stage categories: ‘New’ (previously untreated; n = 753), ‘Early’ (1–3 prior therapies; n = 1569) and ‘Late’ (≥4 prior therapies; n = 239) disease. Mean EORTC global health scores were similar across the three stages. Unexpectedly, emotional, physical and role functioning were higher in the later stages, indicating better perceived health. Symptom scores, including pain, were largely similar or lower in the later versus earlier stages, signifying a lower symptom burden/better symptom control with more advanced disease. Notable variation in HR‐QoL was observed by age and clinical parameters within and across stages. Multivariate modelling indicated that opioid use and performance status were key factors driving overall HR‐QoL across stages. Using an age‐restricted analysis, transplant eligibility had little impact on HR‐QoL in New disease patients. Thus, changes in HR‐QoL over the treatment course of MM are complex and impacted by baseline factors. A prospective observational international inception cohort study that captures key clinical, HR‐QoL and demographic characteristics, along with safety and supportive care information, is needed.
Keywords: multiple myeloma, quality of life, EORTC QLQ‐C30, disease stages, integrated analysis
Substantial advances in treatment options for multiple myeloma (MM) over recent years have led to improvements in patient survival (Engelhardt et al, 2014; Kumar et al, 2014; Liwing et al, 2014; Ludwig et al, 2014). Nevertheless, MM remains a generally incurable disease and patients often live with pronounced symptoms due to bone involvement and fractures, recurrent bacterial infections, impaired renal function and anaemia (Gulbrandsen et al, 2004). With the prospect of premature mortality and on‐going complications, optimizing health‐related quality of life (HR‐QoL) over the disease course becomes an important treatment goal (Sonneveld et al, 2013; Kvam & Waage, 2015).
At diagnosis and during treatment, many patients with MM report pain and fatigue, reduced functional capabilities and impaired overall HR‐QoL, compared with age‐ and gender‐matched controls (Gulbrandsen et al, 2004; Wagner et al, 2012; Baz et al, 2015). The extent of HR‐QoL impairment may vary depending on disease‐related factors, including stage and extent of bone involvement and organ impairment, as well as patient‐related factors, such as age and comorbidities. MM treatments, while potentially improving patients’ symptoms, can also result in side effects/toxicities that may negatively impact patients’ HR‐QoL.
Increasingly, HR‐QoL analyses are being included in clinical trials to assess how HR‐QoL is affected by a course of treatment (Osborne et al, 2012; Sonneveld et al, 2013; Maes & Delforge, 2015). In MM, a number of recent randomized phase II (Ludwig et al, 2013) and phase III/IIIb (Delforge et al, 2012; Hjorth et al, 2012; Dimopoulos et al, 2013, 2014; Stewart et al, 2013; Niesvizky et al, 2015; Song et al, 2015) trials of bortezomib‐ and immunomodulatory drug‐based therapies have measured the impact of treatment on patient‐reported HR‐QoL. Validated instruments commonly used in this setting include the European Organization for the Research and Treatment of Cancer (EORTC) Quality‐of‐Life Questionnaire (QLQ)‐C30 (Aaronson et al, 1993; Osborne et al, 2012), the Functional Assessment of Cancer Therapy (FACT) series of questionnaires (Cella, 1997; Calhoun et al, 2003) and the EuroQoL five dimensions (EQ‐5D) questionnaire (formerly the EuroQoL questionnaire) [EuroQoL Group, 1990; Brooks, 1996; http://www.euroqol.org/eq-5d-products/eq-5d-3l.html (Accessed March 2016)]. Although HR‐QoL analyses are becoming increasingly important in clinical trial evaluations in MM (Sonneveld et al, 2013), much remains to be understood about the extent and type of HR‐QoL impairment across the disease and treatment course: from diagnosis and initial therapy to advanced disease following receipt of multiple therapies (Osborne et al, 2012). Indeed, to our knowledge, there have been no longitudinal, observational cohort studies in MM that have investigated changes in patient‐reported HR‐QoL over the disease course, spanning multiple therapeutic interventions.
The efficacy and safety of the proteasome inhibitor bortezomib has been studied in numerous prospective clinical trials in MM involving both previously untreated patients and those with relapsed and/or refractory disease. From early phase II trials onward, the bortezomib clinical trial programme has consistently incorporated a standard set of HR‐QoL assessments (Richardson et al, 2003, 2005; Orlowski et al, 2007, 2015; San Miguel et al, 2008; Ludwig et al, 2013). Integration and analysis of these large datasets therefore provides a unique opportunity to study changes in baseline HR‐QoL patterns throughout the MM treatment course in the era of novel agents. As autologous stem cell transplantation (ASCT) remains a standard frontline treatment for MM in eligible patients, (Engelhardt et al, 2014) integrating these datasets also allows for a comparison of baseline EORTC QLQ‐C30 scores among newly diagnosed patients based on their transplant eligibility status, a parameter closely related to age and co‐morbidity.
Here, we report results from an integrated, descriptive, cross‐sectional analysis of baseline HR‐QoL data from a large number of patients at distinct stages of the MM disease and treatment pathway. The goals of this research were to: (i) explore the association between baseline demographic/clinical factors and HR‐QoL scores in patients at different points in the MM treatment pathway, (ii) identify fixed and modifiable factors that could affect HR‐QoL at these stages, (iii) place future HR‐QoL data into a broader context, and (iv) provide parameter estimates for cost‐utility analyses.
Methods
Study dataset
This was an integrated analysis of baseline patient‐level data from six clinical studies of bortezomib in patients with previously untreated or relapsed and/or refractory MM. The six studies selected were all company‐sponsored, registration‐oriented trials, published between 2003 and 2015, which had consistent HR‐QoL evaluations as an endpoint. All studies were supported by Millennium Pharmaceuticals, Inc. and Janssen Research & Development. A summary of key inclusion/exclusion criteria for the six studies is shown in Table SI. For the purpose of this analysis, patients were grouped by disease stage as follows: ‘New disease’, defined as previously untreated patients; ‘Early disease’, defined as patients who had received 1–3 prior therapies; and ‘Late disease’, defined as patients who had received ≥4 prior therapies.
The New disease group included both transplant‐eligible and transplant‐ineligible patients from the VISTA and MMY‐2043 studies. VISTA (NCT00111319) was a randomized phase III study of melphalan and prednisone versus bortezomib, melphalan and prednisone in 682 previously untreated, elderly MM patients ineligible for ASCT (median age 71 years [range, 48–91]) (San Miguel et al, 2008). MMY‐2043 (NCT00531453) was a randomized phase II study of bortezomib, thalidomide and dexamethasone ± cyclophosphamide in 98 previously untreated, transplant‐eligible MM patients (median age 57·5 years [range, 18–70]) (Ludwig et al, 2013).
The Early disease group included patients from the APEX, DOXIL‐MMY‐3001 and T06 studies. APEX (NCT00048230) was a randomized phase III study of bortezomib versus high‐dose dexamethasone in 669 patients with relapsed MM (median age 61 years [range, 47–74]) who had received 1–3 prior therapies (Richardson et al, 2005). DOXIL‐MMY‐3001 (NCT00103506) was a randomized phase III study of pegylated liposomal doxorubicin and bortezomib versus bortezomib alone in 646 patients with relapsed/refractory MM (Orlowski et al, 2007); in this study, patients had a median age of 61 years (range, 28–88) and 66% had received ≥2 prior therapies. T06 (NCT00401843) was a randomized phase II study of bortezomib plus siltuximab (CNTO 328; an anti‐interleukin‐6 monoclonal antibody) versus bortezomib plus placebo in 307 patients with relapsed/refractory MM who had received 1–3 prior therapies [median age 64/61 years (range, 36–82)] (Orlowski et al, 2015).
The Late disease group included patients from the SUMMIT and APEX trials. SUMMIT was a non‐randomized, phase II study of bortezomib in 202 patients with relapsed/refractory MM (Richardson et al, 2003), who had a mean age of 60 years (range, 34–84) and had received a median of 6 (range, 2–15) prior therapies. While the APEX study criteria specified patients with relapsed MM who had received 1–3 prior therapies (Richardson et al, 2005), 37 patients had received >3 prior therapies; these 37 patients were included in the Late disease group in the present analysis.
All six trials were conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines, and all patients provided written informed consent for participation.
Assessment of patient‐reported HR‐QoL
Patients with available baseline HR‐QoL data were selected for analysis. The EORTC QLQ‐C30 core instrument version 3.0 was incorporated in all of the above clinical trials. This instrument is a 30‐item questionnaire divided into 14 domains and a global health status/QoL scale (Aaronson et al, 1993). The domains include five functional domains (physical, role, emotional, social and cognitive), eight symptom‐related domains (appetite loss, constipation, diarrhoea, dyspnoea, fatigue, insomnia, nausea and vomiting, and pain) and one domain related to financial difficulties. Scores for each domain range from 0 to 100. For the global health and functional domains, higher scores represent better HR‐QoL, signifying better overall health and functioning, whereas for the symptom domains, lower scores represent better HR‐QoL, or less severe symptoms. Data from the financial difficulties domain are not presented in this analysis.
In the present analysis, baseline HR‐QoL values were defined as EORTC QLQ‐C30 scores obtained on the first day of the first treatment cycle, i.e. the first dosing day. If baseline data were not available, data from the screening visit were used. Physician‐assessed performance status was standardized on the Eastern Cooperative Oncology Group (ECOG) scale with Karnofsky Performance Status (KPS) mapped to ECOG performance status values as follows: ECOG 0 = KPS 100; ECOG 1 = KPS 80–90; ECOG 2 = KPS 60–70; and ECOG 3 = KPS <60 (Ma et al, 2010).
Statistical analyses
Data were summarized descriptively. Mean EORTC QLQ‐C30 global health status scores across age‐ and disease‐stage groups in the present study were compared with the EORTC normal value for the general adult population (n = 7802) (Scott et al, 2008). Previous studies have demonstrated that a 6‐point difference in EORTC QLQ‐C30 score was estimated to be a clinically meaningful improvement in HR‐QoL for patients with MM (Fayers & Bottomley, 2002; Kvam et al, 2010a,b; Jordan et al, 2013); by extension, this value was applied in the present study as a basis for the comparison of differences in mean EORTC QLQ‐30 scores between groups.
The two studies of previously untreated MM patients included in this analysis (VISTA and MMY‐2043) enrolled patient populations with differing eligibility for ASCT: transplant‐ineligible (VISTA) and transplant‐eligible (MMY‐2043). As ASCT is a standard frontline treatment for MM in eligible patients (Ludwig et al, 2014), HR‐QoL was assessed according to transplant eligibility in New disease patients using an age‐restrictive method to ensure comparability. Those transplantation‐ineligible patients from VISTA whose age was outside the range of the transplant‐eligible patients enrolled in MMY‐2043 (48–69 years; age‐restricted analysis approach) were removed from the analysis. Clinical characteristics were then compared across the two patient populations to test for selection effects.
Data on patient demographics, disease characteristics and treatments that were hypothesized to be associated with patients’ HR‐QoL were obtained: age, albumin levels, analgesia use, anaemia treatment, bone lesions, calcium levels, creatinine clearance, ECOG performance status, gender, geographic region, haemoglobin levels, International Staging System (ISS) disease stage, myeloma isotype and opioid use. To evaluate the association between these characteristics or clinical measures and HR‐QoL, data for all EORTC QLQ‐C30 domains were analysed in patient subgroups defined by baseline characteristics as well as within and across all disease stages. Parametric P‐values for univariate subgroup comparisons were determined using a t‐test when comparing scores between two groups or an anova when comparing scores between three groups or more. Multivariate linear regressions were also performed to determine the independent contribution of the different baseline characteristics in order to identify the key drivers of overall HR‐QoL within each disease stage. For the creation of the multivariate models, variables with a P‐value of <0·2 in univariate models were included in a backward selection process retaining only variables with P‐values of <0·05.
Given the large number of comparisons in this analysis, type I error risk was inflated and a false discovery rate (FDR) approach was applied (Benjamini & Hochberg, 1995). This approach aims to control for the FDR: i.e. the expected proportion of type I errors among the rejected hypotheses, instead of the family‐wise error rate (FWER). The FDR approach is less conservative than FWER approaches and is particularly appropriate for exploratory analyses, such as those presented in this analysis. In practice, q‐values – the equivalent of P‐values in the FDR approach – were computed for each comparison and are reported here. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients
Baseline HR‐QoL data were available for 2561 patients enrolled in all study arms, whether they contained bortezomib or not, across the six clinical trials (Tables SI and SII). The Late disease group included an additional non‐baseline time point for one patient in the SUMMIT trial, which could not be excluded from the analysis. Table SII shows the number of patients by disease stage derived from each of the studies.
Patient demographics and baseline characteristics by disease stage are summarized in Table 1. Patients were predominantly White and most had an ECOG performance status of 1 in all three disease stages. As a result of the locations of trial execution, the majority of patients in the New and Early disease stage groups were from Europe, whereas most patients in the Late disease stage group were from North America. The median time from diagnosis was 0·1, 3·0 and 3·9 years, respectively. Gender and disease characteristics, including ISS stage, myeloma isotype and baseline haemoglobin levels, were comparable across the disease stages.
Table 1.
Patient demographics and baseline disease characteristics by disease stage
| New disease (n = 753) | Early disease (n = 1569) | Late disease (n = 239)e | |
|---|---|---|---|
| Median age, years (range) | 70 (33–91) | 62 (27–88) | 59 (32–84) |
| <65 years, n (%) | 112 (15) | 958 (61) | 153 (64) |
| 65–75 years, n (%) | 485 (64) | 498 (32) | 77 (32) |
| >75 years, n (%) | 156 (21) | 113 (7) | 10 (4) |
| Male, n (%) | 374 (50) | 884 (56) | 143 (60) |
| Ethnicity, n (%) | |||
| White | 674 (90) | 1413 (90) | 200 (83) |
| Black | 10 (1) | 87 (6) | 22 (9) |
| Latino/Hispanic | 1 (<1) | 12 (<1) | 2 (<1) |
| Asian/Pacific | 67 (9) | 28 (2) | 6 (3) |
| Other/Missing | 1 (<1) | 29 (2) | 10 (4) |
| Geographical location, n (%)a | |||
| North America | 56 (7) | 389 (25) | 216 (90) |
| Latin America | 14 (2) | 25 (2) | 0 |
| Europe | 607 (81) | 1061 (68) | 24 (10) |
| Asia | 66 (9) | 5 (<1) | 0 |
| Other | 10 (1) | 89 (6) | 0 |
| ISS disease stage at baseline, n (%)b | |||
| I | 147 (20) | 556 (35) | 66 (28) |
| II | 355 (47) | 531 (34) | 93 (39) |
| III | 251 (33) | 442 (28) | 64 (27) |
| Missing | 0 | 40 (3) | 17 (7) |
| Performance status (ECOG) at baseline, n (%)b | |||
| 0 | 100 (13) | 514 (33) | 20 (8) |
| 1 | 392 (52) | 884 (56) | 167 (70) |
| 2 | 258 (34) | 143 (9) | 46 (19) |
| ≥3 | 2 (<1) | 3 (<1) | 0 |
| Missing | 1 (<1) | 25 (2) | 7 (3) |
| Median time since diagnosis, years (range) | 0·1 (0–11·2) | 3·0 (0·2–24·9) | 3·9 (0·6–18·5) |
| Myeloma isotype, n (%) | |||
| IgA | 189 (25) | 375 (24) | 58 (24) |
| IgG | 473 (63) | 968 (62) | 140 (58) |
| IgM | 4 (1) | 6 (<1) | 1 (<1) |
| IgD or IgE | 9 (1) | 19 (1) | 3 (1) |
| Missing | 78 (10) | 201 (13) | 38 (16) |
| Prior ASCT, n (%)c | 0 | 246 (16) | 96 (40) |
| Creatinine clearance rate, ml/sb | |||
| Median (range) | 1·0 (0·2–3·0) | 1·2 (0·2–9·5) | 1·2 (0·2–3·7) |
| <0·5, n (%) | 34 (5) | 42 (3) | 9 (4) |
| 0·5–1·0, n (%) | 331 (44) | 420 (27) | 63 (26) |
| >1·0, n (%) | 388 (52) | 1087 (69) | 166 (69) |
| Missing, n (%) | 0 | 20 (1) | 2 (1) |
| Haemoglobin levels at baseline, g/l | |||
| Median (range) | 105 (64–165) | 110 (59–174) | 103 (54–146) |
| Comorbidities at baseline, n (%)d | |||
| Cardiovascular | 518 (69) | 888 (57) | 114 (48) |
| Endocrine | 284 (38) | 572 (37) | 69 (29) |
| Gastrointestinal | 379 (50) | 765 (49) | 133 (55) |
| Genitourinary | 294 (39) | 614 (39) | 118 (49) |
| Haematological | 286 (38) | 633 (40) | 61 (25) |
| Analgesic use at baseline, n (%) | |||
| Patients receiving analgesics at baseline | 378 (50) | 548 (35) | 120 (50) |
| Non‐opioidc,d | 235 (62) | 219 (40) | 37 (31) |
| Weak opioidc,d | 145 (38) | 192 (35) | 48 (40) |
| Strong opioidc,d | 143 (38) | 260 (47) | 53 (44) |
| GABA analoguesc,d | 1 (<1) | 0 | 0 |
| Anaemia treatments at baseline, n (%) | 135 (18) | 219 (14) | 98 (41) |
| ESA use | 85 (11) | 186 (12) | 93 (39) |
ASCT, autologous stem cell transplant; ECOG, Eastern Cooperative Oncology Group; ESA, erythropoiesis‐stimulating agents; GABA, gamma‐aminobutyric analogues; ISS, International Staging System.
Differences in geographical distribution between groups were associated with the different areas in which the respective studies were conducted. For example, patients in the New disease group were mostly derived from the VISTA study, which was conducted predominantly in Europe, whereas patients in the Late disease group were predominantly derived from the SUMMIT study, which was conducted solely in North America.
Patients with missing data are included all percentage calculations.
Percentages calculated using the number of patients who received the relevant treatment type as the denominator.
Categories are not mutually exclusive.
The Late disease group included an additional non‐baseline time point for n = 1 patient in the SUMMIT trial, which could not be excluded from the analysis. Thus, the Late disease group includes 239 patients but data values are based on a denominator of 240.
The most notable disparity between the disease stage groups was the pronounced difference in age, due to variations in patient characteristics between the contributing studies. In the New, Early and Late disease stages, respectively, median age was 70, 62 and 59 years, with 85%, 39% and 36% of patients aged ≥65 years. A higher proportion of patients in the New disease stage had baseline creatinine clearance <1·0 ml/s compared with patients in the Early or Late disease stages. In the Late disease group, the proportion of patients with baseline cardiovascular comorbidities was lower versus the New and Early disease groups; similarly, the proportion of patients with endocrine or haematological comorbidities was lower in the Late disease group. Overall analgesic use at baseline was similar between the New and Late disease groups, with lowest usage seen in patients with Early disease. Of these, use of non‐opioids at baseline was highest in patients with New disease and lowest in those with Late stage disease, while weak opioid use was similar among the disease stages. Strong opioid use was recorded more frequently in Early and Late disease versus New disease.
Comparison of baseline HR‐QoL scores by age and disease stage
As patient age is one of the factors that may influence the extent of HR‐QoL impairment in patients with MM (Dimopoulos et al, 2014), a comparative analysis of HR‐QoL across different MM disease stages in this study would have been biased due to the aforementioned marked difference in median patient age across the disease stages. In addition, age‐matched reference values for the general adult population were not available. We therefore compared baseline EORTC QLQ‐C30 scores (global health status and individual functioning and symptom domain scores) in patients grouped by age (<65 vs. 65–75 vs. >75 years) within each disease stage (Table 2 and Fig S1). As only 10 patients in the Late disease group were aged >75 years, this subset was excluded from analysis.
Table 2.
Mean (SD) EORTC QLQ‐C30 scores at baseline by age and disease stage
| Mean score (SD) | New disease | Early disease | Late diseasea, b | |||||
|---|---|---|---|---|---|---|---|---|
| <65 years (n = 112) | 65–75 years (n = 485) | >75 years (n = 156) | <65 years (n = 958) | 65–75 years (n = 498) | >75 years (n = 113) | <65 years (n = 153) | 65–75 years (n = 77) | |
| EORTC QLQ‐C30 scores | ||||||||
| Global health status | 55·2 (23·0) | 50·6 (21·7) | 48·3 (21·2) | 58·8 (22·2) | 56·7 (22·3) | 55·4 (24·3) | 56·1 (24·1) | 55·9 (21·3) |
| Functional domain scores | ||||||||
| Cognitive functioning | 81·7 (24·4) | 76·6 (23·2) | 75·5 (22·9) | 82·1 (21·8) | 81·7 (21·8) | 72·3 (26·9) | 82·4 (18·2) | 78·0 (22·3) |
| Emotional functioning | 67·6 (25·6) | 70·3 (24·1) | 71·1 (24·9) | 74·5 (22·4) | 74·5 (22·2) | 73·4 (23·3) | 76·8 (19·1) | 78·8 (21·5) |
| Physical functioning | 65·9 (26·5) | 61·0 (26·8) | 57·7 (26·6) | 70·8 (22·8) | 67·4 (23·1) | 65·0 (23·2) | 70·3 (23·4) | 69·0 (21·8) |
| Role functioning | 54·1 (33·9) | 54·7 (35·1) | 53·2 (32·7) | 64·7 (30·9) | 64·5 (30·7) | 66·7 (30·3) | 61·9 (32·6) | 68·7 (31·6) |
| Social functioning | 69·0 (30·9) | 66·2 (32·4) | 70·2 (28·8) | 71·3 (28·7) | 73·8 (28·5) | 75·1 (26·7) | 62·8 (30·8) | 64·6 (28·9) |
| Symptom domain scores | ||||||||
| Appetite loss | 20·8 (28·3) | 25·0 (30·6) | 28·0 (32·9) | 16·5 (25·0) | 20·3 (30·2) | 25·9 (30·9) | 21·1 (27·0) | 21·8 (30·7) |
| Constipation | 20·2 (26·5) | 25·0 (32·3) | 27·1 (30·6) | 15·6 (25·8) | 19·6 (28·5) | 25·1 (31·8) | 17·0 (24·4) | 18·8 (27·4) |
| Diarrhoea | 6·7 (16·2) | 6·3 (16·8) | 6·3 (16·1) | 7·8 (17·0) | 8·5 (18·3) | 7·3 (18·1) | 9·3 (18·8) | 11·4 (19·6) |
| Dyspnoea | 21·6 (27·5) | 23·9 (29·5) | 25·7 (30·3) | 19·7 (25·3) | 23·9 (29·2) | 21·4 (29·8) | 21·7 (28·8) | 21·8 (28·1) |
| Fatigue | 43·1 (27·9) | 45·0 (26·4) | 48·7 (26·6) | 38·2 (25·4) | 39·6 (25·9) | 43·1 (27·6) | 45·0 (25·9) | 40·6 (23·9) |
| Insomnia | 25·4 (27·9) | 30·4 (29·8) | 35·3 (34·9) | 30·7 (31·4) | 24·4 (28·8) | 29·1 (33·6) | 35·3 (31·9) | 22·2 (29·6) |
| Nausea and vomiting | 7·0 (18·3) | 9·3 (19·2) | 8·7 (19·5) | 7·0 (14·7) | 7·6 (17·4) | 5·7 (11·8) | 7·3 (17·0) | 5·8 (11·9) |
| Pain | 43·7 (33·0) | 45·6 (32·3) | 42·4 (30·7) | 38·6 (31·0) | 35·3 (29·4) | 36·4 (31·3) | 37·9 (31·1) | 31·3 (29·6) |
Higher scores for EORTC QLQ‐C30 global health status and EORTC QLQ‐C30 functional domains indicate better overall HR‐QoL. Lower EORTC QLQ‐C30 symptom domain scores indicate better HR‐QoL. Scores highlighted in bold indicate a ≥6 point difference between age subgroups within a specific disease stage group.
SD, standard deviation.
As there were only 10 patients aged >75 years in the Late disease group, this subgroup of patients was omitted from the analysis.
The Late disease group included an additional non‐baseline time point for n = 1 patient in the SUMMIT trial, which could not be excluded from the analysis.
In patients aged <65 years, mean EORTC QLQ‐C30 global health status scores appeared similar between patients at different stages of disease (Table 2). In general, mean functional domain scores tended to be higher (i.e. better) in the later disease stages; notably, emotional functioning in Late disease was ≥6 points higher than in New disease. One exception was social functioning, which was ≥6 points higher in New versus Late disease. Higher (i.e. worse) insomnia and borderline lower (i.e. better) pain scores at the ≥6‐point level were observed in Late versus New disease.
In patients aged 65–75 years, mean EORTC QLQ‐C30 global health status scores were 6·1 points higher (i.e. better) in Early versus New disease. As in patients aged <65 years, most function domain scores tended to be higher at the later disease stages: mean emotional, physical and role function scores were all ≥6 points higher (i.e. better) in Late versus New disease. In contrast, mean social functioning scores were ≥6 points higher (i.e. better) in Early versus New and Late disease; the latter two subgroups having virtually the same scores. Of interest, mean symptom scores for constipation, insomnia and pain were all ≥6 points lower (i.e. better) in Early or Late disease compared with New disease.
In the oldest subgroup of patients (aged >75 years), mean EORTC QLQ‐C30 global health status scores, and physical and role functioning scores were ≥6 points higher (i.e. better) in Early versus New disease. Likewise, mean pain and insomnia symptom scores were ≥6 points lower (i.e. better) in Early versus New disease.
In the New and Early disease groups, there was a trend for higher (i.e. better) mean EORTC QLQ‐C30 global health status scores in younger patients (<65 vs. 65–75 vs. >75 years). This difference reached the ≥6‐point threshold in the New disease group when comparing patients aged <65 years with those aged >75 years. The more limited comparison of global health status scores in the Late disease group revealed little difference between patients aged <65 and >75 years (Table 2). As with the global health status scale, mean scores for cognitive and physical functioning met the ≥6‐point threshold for a meaningful difference, as did the individual mean scores for appetite, constipation and insomnia, when younger patients were compared with older patients within the disease stages, although the age subset comparisons meeting the threshold varied by scale: sometimes <65 versus 65–75 years; other times <65 versus >75 years. Paradoxically, in the Late disease group, mean scores for insomnia and pain were ≥6 points lower (i.e. better) in patients aged 65–75 years compared with those aged <65 years.
Although EORTC QLQ‐C30 global health status scores were no worse in the later stages of disease versus New disease, the mean normative global health status score for the general adult population was ≥6 points higher (i.e. better) than the mean scores seen across all MM age and disease stage categories (Fig 1).
Figure 1.
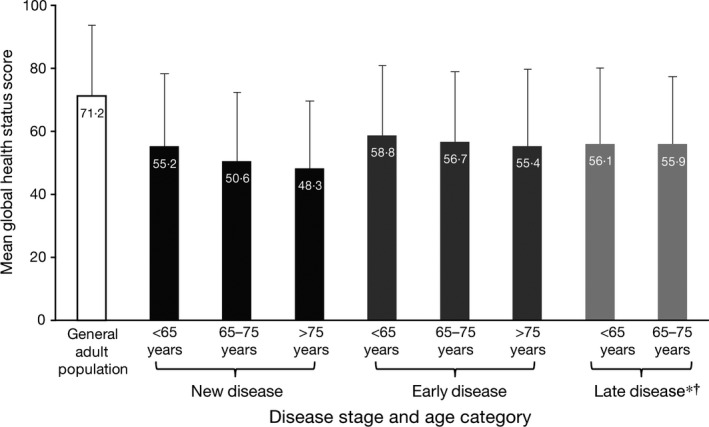
Mean EORTC QLQ‐C30 global health status scores for the EORTC general adult population and for the present MM study population by disease stage* and age category†. *The Late disease group included an additional non‐baseline time point for n = 1 patient in the SUMMIT trial, which could not be excluded from the analysis. †Due to there being only 10 patients aged >75 years in the Late disease group, this subgroup of patients was omitted. Error bars represent the standard deviation from the mean.
Univariate and multivariate analysis of baseline HR‐QoL scores by clinical measures in the overall study population
Statistically significant differences in mean scores for EORTC QLQ‐C30 global health status, plus the majority of individual EORTC QLQ‐C30 functioning and symptom domains, were observed between subgroups for all baseline characteristics and clinical measures tested, with the exception of myeloma isotype (Table 3). Most of these differences were estimated to be clinically relevant (except for myeloma isotype) when comparisons were made between the larger sample sizes and gender. Scores for diarrhoea and insomnia, in particular, were generally not significantly different between subgroups.
Table 3.
Univariate comparison of (A) EORTC QLQ‐C30 global health status and functioning scores and (B) EORTC QLQ‐C30 symptom scores for patients grouped by baseline characteristics and clinical measures
| (A) | ||||||
|---|---|---|---|---|---|---|
| Clinical measure | EORTC QLQ‐C30 scores, mean (SD) | |||||
| Global health status | Functional domain scores | |||||
| Cognitive functioning | Emotional functioning | Physical functioning | Role functioning | Social functioning | ||
| Age, years | ||||||
| <65 (n = 1223) | 58·1 (22·6) | 82·1 (21·7) | 74·1 (22·4) | 70·3 (23·3) | 63·4 (31·5) | 70·0 (29·3) |
| 65–75 (n = 1060) | 53·8 (22·1) | 79·0 (22·6) | 72·8 (23·2) | 64·5 (25·0) | 60·1 (33·3) | 69·5 (30·7) |
| >75 (n = 279) | 51·7 (22·6) | 74·5 (24·5) | 72·2 (24·2) | 60·8 (25·3) | 59·1 (32·4) | 72·4 (27·9) |
| P‐value | <0·0001 | <0·0001 | 0·3277 | <0·0001 | 0·0408 | 0·4216 |
| Creatinine clearance rate, ml/s | ||||||
| <1·0 (n = 898) | 51·9 (22·2) | 77·3 (23·8) | 71·1 (24·1) | 63·2 (24·8) | 57·9 (32·6) | 67·6 (30·8) |
| ≥1·0 (n = 1642) | 57·5 (22·4) | 81·5 (21·7) | 74·6 (22·2) | 68·8 (24·1) | 63·5 (32·2) | 71·3 (29·0) |
| P‐value | <0·0001 | <0·0001 | 0·0007 | <0·0001 | <0·0001 | 0·0057 |
| Gender | ||||||
| Male (n = 1401) | 56·7 (22·5) | 81·6 (22·1) | 75·9 (21·9) | 69·5 (24·6) | 62·5 (32·8) | 71·1 (29·3) |
| Female (n = 1161) | 54·3 (22·4) | 78·1 (22·8) | 70·3 (23·8) | 63·6 (24·0) | 60·5 (31·9) | 68·8 (30·2) |
| P‐value | 0·0160 | 0·0002 | <0·0001 | <0·0001 | 0·1579 | 0·0760 |
| Geographical location | ||||||
| North America (n = 661) | 59·9 (23·4) | 80·6 (21·7) | 76·9 (21·2) | 70·5 (23·2) | 65·2 (31·8) | 68·7 (29·5) |
| Latin America (n = 39) | 62·0 (19·4) | 90·6 (13·7) | 73·7 (18·6) | 75·9 (20·9) | 73·5 (26·1) | 82·9 (26·9) |
| Europe (n = 1692) | 53·5 (21·8) | 79·5 (23·1) | 71·4 (23·6) | 65·3 (24·8) | 59·9 (32·7) | 70·0 (30·2) |
| Asia (n = 71) | 48·5 (21·8) | 71·1 (24·4) | 75·4 (24·3) | 51·1 (26·8) | 48·3 (32·5) | 64·3 (28·0) |
| Other (n = 99) | 66·6 (21·9) | 86·6 (15·8) | 81·5 (17·2) | 77·6 (18·0) | 71·1 (27·1) | 78·3 (22·7) |
| P‐value | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | 0·0016 |
| Haemoglobin levels, g/l | ||||||
| <80 (n = 62) | 49·2 (23·3) | 76·1 (30·0) | 64·7 (27·7) | 62·4 (23·7) | 53·6 (33·3) | 62·6 (33·3) |
| 80–99 (n = 756) | 51·1 (22·6) | 77·8 (22·9) | 72·3 (24·2) | 60·9 (24·8) | 54·6 (33·0) | 65·2 (31·2) |
| 100–120 (n = 1107) | 56·6 (22·5) | 80·0 (22·6) | 73·2 (22·5) | 67·6 (24·4) | 63·3 (32·1) | 70·9 (29·5) |
| >120 (n = 624) | 59·6 (21·5) | 82·9 (20·9) | 75·5 (21·5) | 73·0 (22·7) | 67·3 (30·7) | 74·9 (27·1) |
| P‐value | <0·0001 | 0·0008 | 0·0036 | <0·0001 | <0·0001 | <0·0001 |
| ISS disease stage | ||||||
| I (n = 769) | 61·5 (21·6) | 83·9 (19·6) | 76·7 (20·5) | 74·6 (21·8) | 69·9 (30·3) | 76·4 (27·2) |
| II (n = 979) | 54·8 (22·2) | 79·1 (23·3) | 72·7 (22·8) | 65·5 (24·9) | 60·7 (32·6) | 69·2 (29·8) |
| III (n = 757) | 50·1 (22·4) | 77·1 (23·8) | 70·5 (25·1) | 60·4 (24·5) | 53·5 (32·2) | 64·6 (31·1) |
| P‐value | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 |
| Myeloma isotype | ||||||
| IgA (n = 622) | 56·9 (23·4) | 78·6 (23·1) | 73·4 (23·2) | 67·5 (24·6) | 62·8 (32·9) | 71·8 (29·5) |
| IgG (n = 1581) | 55·7 (22·3) | 80·7 (22·3) | 73·8 (23·0) | 66·9 (24·3) | 62·0 (32·1) | 70·7 (29·3) |
| IgM (n = 11) | 69·1 (15·0) | 86·4 (18·0) | 75·8 (12·0) | 80·5 (13·7) | 74·2 (20·2) | 83·3 (14·9) |
| IgD/IgE (n = 31) | 50·0 (21·5) | 75·9 (27·3) | 66·1 (26·3) | 67·8 (25·6) | 60·3 (30·3) | 67·8 (32·4) |
| P‐value | 0·1010 | 0·1758 | 0·3887 | 0·3501 | 0·6375 | 0·4649 |
| Performance status (ECOG) | ||||||
| 0 (n = 634) | 68·2 (19·6) | 87·3 (17·4) | 81·1 (18·0) | 82·5 (17·1) | 79·7 (24·2) | 84·5 (21·1) |
| 1 (n = 1443) | 55·4 (20·7) | 80·7 (21·4) | 73·6 (22·1) | 67·7 (21·4) | 62·5 (30·2) | 70·5 (28·2) |
| 2 (n = 447) | 38·3 (20·2) | 67·9 (26·7) | 62·5 (26·3) | 42·5 (23·1) | 33·7 (29·8) | 48·9 (32·2) |
| ≥3 (n = 5) | 18·3 (14·9) | 36·7 (38·0) | 21·7 (17·3) | 18·7 (19·7) | 3·3 (7·5) | 23·3 (25·3) |
| P‐value | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 |
| (B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical measure | EORTC QLQ‐C30 symptom domain scores, mean (SD) | |||||||
| Appetite loss | Constipation | Diarrhoea | Dyspnoea | Fatigue | Insomnia | Nausea and vomiting | Pain | |
| Age, years | ||||||||
| <65 (n = 1223) | 17·5 (25·6) | 16·2 (25·7) | 7·9 (17·2) | 20·1 (25·9) | 39·5 (25·8) | 30·7 (31·2) | 7·0 (15·3) | 39·0 (31·2) |
| 65–75 (n = 1060) | 22·6 (30·5) | 22·1 (30·4) | 7·7 (17·7) | 23·7 (29·3) | 42·2 (26·1) | 27·1 (29·5) | 8·3 (18·0) | 39·9 (31·3) |
| >75 (n = 279) | 26·7 (31·9) | 26·1 (30·6) | 7·0 (17·7) | 23·8 (29·9) | 46·1 (27·0) | 32·2 (34·1) | 7·5 (16·8) | 39·3 (30·9) |
| P‐value | <0·0001 | <0·0001 | 0·7775 | 0·0093 | 0·0007 | 0·0109 | 0·2735 | 0·8183 |
| Creatinine clearance rate, ml/s | ||||||||
| <1·0 (n = 898) | 26·8 (31·9) | 23·3 (30·4) | 8·5 (18·4) | 24·0 (30·0) | 45·1 (26·0) | 31·1 (31·6) | 9·6 (19·2) | 41·9 (31·8) |
| ≥1·0 (n = 1642) | 17·4 (26·1) | 17·9 (27·3) | 7·3 (16·9) | 20·9 (26·5) | 39·3 (25·9) | 28·4 (30·5) | 6·6 (15·0) | 38·1 (30·8) |
| P‐value | <0·0001 | <0·0001 | 0·1258 | 0·0131 | <0·0001 | 0·0629 | <0·0001 | 0·0068 |
| Gender | ||||||||
| Male (n = 1401) | 18·4 (27·2) | 18·4 (27·7) | 7·4 (17·7) | 20·9 (27·1) | 38·9 (25·9) | 27·4 (30·1) | 6·2 (14·4) | 36·9 (30·9) |
| Female (n = 1161) | 23·3 (30·0) | 21·4 (29·3) | 8·1 (17·2) | 23·4 (28·6) | 44·3 (26·0) | 31·8 (31·7) | 9·3 (18·8) | 42·4 (31·3) |
| P‐value | <0·0001 | 0·0145 | 0·3634 | 0·0349 | <0·0001 | 0·0009 | <0·0001 | <0·0001 |
| Geographical location | ||||||||
| North America (n = 661) | 19·7 (27·5) | 18·9 (26·9) | 9·0 (18·7) | 22·9 (28·3) | 39·7 (26·1) | 29·4 (31·5) | 6·9 (14·4) | 35·5 (30·8) |
| Latin America (n = 39) | 17·1 (24·0) | 17·1 (25·2) | 4·3 (11·3) | 5·1 (12·2) | 25·4 (21·6) | 26·5 (26·7) | 3·8 (11·1) | 34·2 (30·8) |
| Europe (n = 1692) | 20·9 (29·0) | 20·0 (29·1) | 7·3 (17·1) | 22·1 (27·7) | 42·5 (26·2) | 29·6 (30·9) | 7·8 (17·4) | 41·3 (31·4) |
| Asia (n = 71) | 33·3 (34·3) | 25·4 (32·1) | 10·3 (19·2) | 19·7 (31·2) | 48·7 (27·8) | 30·0 (31·4) | 13·8 (23·1) | 46·9 (30·1) |
| Other (n = 99) | 13·3 (22·1) | 17·4 (25·9) | 5·8 (16·1) | 23·0 (27·2) | 34·0 (21·5) | 25·9 (29·0) | 5·0 (10·9) | 28·5 (25·7) |
| P‐value | 0·0005 | 0·3902 | 0·1068 | 0·0055 | <0·0001 | 0·8185 | 0·0051 | <0·0001 |
| Haemoglobin level, g/l | ||||||||
| <80 (n = 62) | 33·3 (32·1) | 34·6 (37·7) | 9·3 (19·9) | 32·7 (27·8) | 54·1 (26·9) | 38·8 (35·6) | 14·5 (20·3) | 51·5 (34·0) |
| 80–99 (n = 756) | 25·7 (31·1) | 22·1 (29·5) | 8·3 (18·2) | 26·5 (30·3) | 47·4 (26·5) | 30·2 (31·8) | 9·1 (18·7) | 42·8 (32·4) |
| 100–120 (n = 1107) | 20·5 (28·3) | 20·7 (29·1) | 7·5 (17·1) | 21·0 (27·3) | 40·6 (26·0) | 29·2 (30·2) | 7·7 (17·0) | 38·8 (30·7) |
| >120 (n = 624) | 13·8 (23·8) | 13·9 (23·8) | 7·1 (17·0) | 17·5 (24·7) | 34·3 (23·7) | 27·7 (30·4) | 4·9 (12·0) | 35·4 (29·7) |
| P‐value | <0·0001 | <0·0001 | 0·5999 | <0·0001 | <0·0001 | 0·0771 | <0·0001 | <0·0001 |
| ISS disease stage | ||||||||
| I (n = 769) | 13·0 (23·6) | 14·8 (25·0) | 7·1 (16·2) | 17·3 (24·3) | 33·6 (24·1) | 27·8 (30·0) | 5·6 (14·3) | 34·0 (29·3) |
| II (n = 979) | 20·7 (28·8) | 20·9 (29·3) | 7·3 (17·2) | 22·6 (28·0) | 42·3 (26·2) | 29·2 (31·1) | 7·5 (16·3) | 41·2 (31·2) |
| III (n = 757) | 28·8 (30·9) | 23·4 (30·3) | 9·0 (19·2) | 26·3 (30·3) | 48·4 (26·1) | 31·3 (31·4) | 10·1 (19·1) | 43·0 (32·6) |
| P‐value | <0·0001 | <0·0001 | 0·0811 | <0·0001 | <0·0001 | 0·1125 | <0·0001 | <0·0001 |
| Myeloma isotype | ||||||||
| IgA (n = 622) | 21·8 (29·2) | 20·8 (30·2) | 7·4 (17·2) | 19·7 (26·2) | 40·9 (27·2) | 30·6 (31·1) | 7·8 (16·7) | 39·8 (32·2) |
| IgG (n = 1581) | 19·5 (27·9) | 19·0 (27·9) | 7·5 (17·0) | 22·5 (28·2) | 40·9 (26·0) | 28·9 (30·8) | 6·9 (15·6) | 38·3 (30·8) |
| IgM (n = 11) | 18·2 (17·4) | 9·1 (15·6) | 9·1 (15·6) | 18·2 (27·3) | 34·3 (15·3) | 18·2 (27·3) | 3·0 (6·7) | 27·3 (20·1) |
| IgD/IgE (n = 31) | 31·0 (34·4) | 27·6 (36·8) | 5·7 (15·6) | 20·7 (28·7) | 43·7 (28·2) | 39·1 (33·4) | 18·4 (26·9) | 40·2 (29·7) |
| P‐value | 0·0859 | 0·1841 | 0·9401 | 0·2616 | 0·8203 | 0·1613 | 0·0018 | 0·5185 |
| Performance status (ECOG) | ||||||||
| 0 (n = 634) | 9·3 (19·0) | 11·3 (21·7) | 7·1 (15·9) | 15·1 (22·1) | 26·6 (21·2) | 22·1 (28·1) | 3·8 (9·9) | 21·9 (24·1) |
| 1 (n = 1443) | 19·0 (26·8) | 18·9 (27·6) | 7·5 (17·4) | 22·9 (28·0) | 41·6 (24·6) | 29·9 (30·5) | 7·3 (15·9) | 40·0 (29·4) |
| 2 (n = 447) | 41·3 (34·3) | 33·8 (33·8) | 9·0 (19·3) | 28·0 (31·6) | 60·7 (24·0) | 37·0 (33·1) | 13·3 (23·3) | 61·5 (30·9) |
| ≥3 (n = 5) | 53·3 (38·0) | 80·0 (29·8) | 0 | 60·0 (43·5) | 86·7 (12·2) | 53·3 (50·6) | 30·0 (18·3) | 86·7 (29·8) |
| P‐value | <0·0001 | <0·0001 | 0·3074 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 |
Scores for baseline albumin level; analgesia use; bone lesions; non‐opioid, weak opioid or strong opioid use; and baseline calcium level are not shown in the table.
P‐values are corrected for false discovery rate (FDR); values of <0·05 are shown in bold text.
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System; SD, standard deviation.
A general trend for higher (i.e. better) EORTC QLQ‐C30 global health status and functioning scores, and lower (i.e. better) EORTC QLQ‐C30 symptom scores at baseline was observed for patients with the following: younger age; male gender; no anaemia or analgesia treatment; fewer bone lesions; higher creatinine clearance; higher haemoglobin levels; less advanced ISS disease stage; lower ECOG performance status score; higher albumin levels; and lower calcium levels (Table 3 and data not shown).
In multivariate analysis, analgesia use and ECOG performance status were common factors driving overall HR‐QoL within each disease stage group (Table 4). In the New disease group, albumin ≥35 g/l, creatinine clearance ≥1·0 ml/s, ECOG performance status 0, geographic region and no strong opioid use were all significantly (P ≤0·05) associated with better EORTC QLQ‐C30 global health status. In the Early disease group, ISS stage I and ECOG performance status 0/1 were significantly associated with better EORTC QLQ‐C30 global health status, while European region and either weak or strong opioid use were significantly associated with poorer overall health status. Finally, in the Late disease group, the presence of bone lesions, ECOG performance status 0/1 and no non‐opioid use were significantly associated with better EORTC QLQ‐C30 global health status.
Table 4.
Multivariate regression models of EORTC QLQ‐C30 global health status scores by disease stage
| Variable | Level | New disease (n = 731) | Early disease (n = 1409) | Late disease (n = 180) | |||
|---|---|---|---|---|---|---|---|
| Parameter estimate | P‐value | Parameter estimate | P‐value | Parameter estimate | P‐value | ||
| Intercept | 37·66 | 35·70 | 37·59 | ||||
| Geographical location | North America | 10·73 | <0·0001 | ‐2·23 | <0·0001 | ||
| Latin America | 5·59 | ‐4·48 | |||||
| Europe | ‐5·32 | ‐9·96 | |||||
| Asia | ‐0·18 | 8·47 | |||||
| Other | Reference | Reference | |||||
| Albumin | <35 g/l | ‐3·57 | 0·01 | ||||
| Creatinine clearance rate | <1·0 ml/s | ‐4·33 | <0·01 | ||||
| Bone lesions | 6·44 | 0·04 | |||||
| ISS disease stage | I | 6·65 | <0·0001 | ||||
| II | 0·79 | ||||||
| III | Reference | ||||||
| Performance status (ECOG) | 0 | 37·00 | <0·0001 | 37·57 | <0·0001 | 31·98 | <0·0001 |
| 1 | 25·39 | 26·22 | 21·48 | ||||
| 2 | 12·96 | 11·90 | Reference | ||||
| ≥3 | Reference | Reference | |||||
| Strong opioid | ‐12·11 | <0·0001 | ‐8·87 | <0·0001 | |||
| Weak opioid | ‐5·07 | <0·01 | |||||
| Non‐opioid analgesics | ‐15·63 | <0·001 | |||||
| R 2 | 0·29 | 0·23 | 0·22 | ||||
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System. P‐values of <0.05 are shown in bold text.
Baseline HR‐QoL scores by transplant eligibility status in the New disease stage
Overall, there was a good balance of baseline characteristics between transplant‐eligible and transplant‐ineligible patients in the New disease stage, although there was still a difference of 8 years in the median age between the two groups [59 years (range, 48–68)] vs. [67 years (range, 48–69)] (Table SIII).
Despite this age difference, mean EORTC QLQ‐C30 global health status scores appeared similar between transplant‐eligible and ‐ineligible patients in the New disease stage (Table 5). The same held true for all other QLQ‐C30 scales, none of which exceeded the ≥6‐point threshold for a clinically meaningful difference.
Table 5.
Mean (SD) EORTC QLQ‐C30 scores by transplant eligibility in patients aged 48–69 years in the New disease group
| Mean (SD) score | Transplant eligible | |
|---|---|---|
| Yes (n = 84) | No (n = 253) | |
| EORTC QLQ‐C30 scores | ||
| Global health status | 53·0 (21·2) | 50·7 (22·3) |
| Functional domain scores | ||
| Cognitive functioning | 82·1 (23·8) | 77·8 (24·9) |
| Emotional functioning | 67·3 (24·0) | 69·9 (24·3) |
| Physical functioning | 65·7 (25·7) | 62·6 (26·5) |
| Role functioning | 53·9 (33·9) | 56·6 (33·9) |
| Social functioning | 66·9 (31·8) | 68·5 (31·2) |
| Symptom domain scores | ||
| Appetite loss | 19·1 (26·7) | 22·4 (30·0) |
| Constipation | 22·8 (27·2) | 21·3 (30·9) |
| Diarrhoea | 5·7 (13·7) | 6·5 (16·8) |
| Dyspnoea | 21·8 (27·5) | 24·2 (30·1) |
| Fatigue | 43·1 (25·5) | 44·1 (27·5) |
| Insomnia | 26·4 (28·1) | 27·6 (29·5) |
| Nausea and vomiting | 8·3 (20·7) | 7·9 (18·3) |
| Pain | 45·7 (33·2) | 45·4 (32·0) |
Higher scores for EORTC QLQ‐C30 global health status and EORTC QLQ‐C30 functional domain scores indicate better overall HR‐QoL. Lower EORTC QLQ‐C30 symptom domain scores indicate better HR‐QoL.
SD, standard deviation.
Discussion
To the best of our knowledge, this report is one of the largest examinations of HR‐QoL in patients with MM across different disease stages to date. These results indicate that the observed differences in HR‐QoL over the MM disease course are complex – with some appearing counter‐intuitive – and are influenced by demographic factors, particularly age, as well as clinical parameters. Our findings also show that eligibility for ASCT had little association with HR‐QoL among New disease patients after adjusting for age.
This analysis showed that, when comparing HR‐QoL scores for common age groups across the three disease stages, overall health status was not worse, with a trend towards better global health status in relapsed disease (both the Early and Late disease groups) when compared with the New disease group. Emotional, physical and role functioning also appeared to be better in the later versus earlier disease stages, while cognitive function was mainly similar. In general, symptom scores were similar or better in relapsed disease.
These findings support the hypothesis that, for relapsed patients, symptoms are better controlled than for newly diagnosed patients. Of interest, a trend for higher pain scores in New versus relapsed disease (Early and Late disease groups) was observed, suggesting that pain is either less well controlled in New disease or that patients have not developed strategies to cope with that pain. Also, patients with New stage disease tended to have more fatigue and greater loss of appetite compared with Early stage disease. A possible explanation for these observations is ‘response shift’ or ‘adaptation’, a phenomenon whereby patients adjust to their HR‐QoL limitations or shift their expectations as their experience with a health condition deepens over time (Schwartz et al, 1999; Kvam et al, 2010b; Ubel et al, 2010).
Contrary to our expectations at the onset of this research, these results suggest that HR‐QoL does not necessarily worsen with every relapse or as a result of substantial prior treatment, at least in patients who remain eligible for participation in clinical trials. A number of factors may have influenced this observation: differing selection criteria for the trials included in this analysis according to when the studies were conducted (see Table SI); the different availability of prior/subsequent therapies over time; and improvements in supportive care. In addition, trials of treatments for New disease included all types of patients, whereas in progressively later disease stages, those patients who died or who did poorly with treatments would not be included, thereby ‘hardier’ patients who have derived benefit from, and were able to tolerate, multiple lines of therapy are selected. This suggestion is reinforced by the lower proportion of baseline cardiovascular, endocrine and haematological comorbidities in the Late versus Early disease stage groups.
Despite these limitations, these findings may reflect a true observation, in that patients in the later stages of MM may be better able to cope with their disease versus those who have been newly diagnosed, are more adapted to their disease resulting in better emotional functioning, and may have a subjectively lower symptom and pain burden. In contrast, younger, fitter patients who proceed to transplantation may have considerable distress since they are presented with both a cancer diagnosis and a complex transplant option. Thus, while there may be a selection bias for patients in clinical trials, the findings reported here may reflect a real‐world situation.
In the present analysis, age appeared to be a key factor associated with patient‐reported HR‐QoL in MM, thus confirming previous reports (Slovacek et al, 2008; Dimopoulos et al, 2014). Our findings demonstrate that global health status in MM tends to decrease with increasing age in New and Early disease, although this did not hold true for Late disease, possibly due to the more ‘hardy’ nature of these patients who had survived multiple lines of prior MM therapy, as well as the much smaller size of this group. As might be expected, physical, role and cognitive functioning all tended to decline with advancing age. Of interest, emotional and social functioning did not follow this pattern, with emotional function remaining stable and social function improving as patient age increased. These findings may be suggestive of a generally better well‐being in younger patients with earlier stage MM. However, a new MM diagnosis may have a greater psychological impact on this type of patient, who may have inherently higher expectations for these aspects of functioning and perhaps are still adapting psychologically to the new reality of being diagnosed with MM.
Patient subgroup analyses showed that, in addition to age, various other demographic and baseline clinical variables impacted significantly on HR‐QoL. Notably, ECOG performance status and opioid use were identified in multivariate analysis as factors independently associated with EORTC global health status across all three disease stages. While poorer ECOG performance status was associated with lower global health scores, as would be expected, the impact of opioid use was less apparent. Baseline use of non‐opioids tended to decrease as disease stage progressed, whereas use of strong opioids was higher in relapsed disease. Multivariate analysis showed that pain scores were generally worse in those patients receiving analgesics regardless of disease stage – significantly worse pain scores in patients receiving non‐opioids (Early and Late disease), weak opioids (New and Early disease) or strong opioids (all disease stages). Taken together, our data suggest that analgesic pain management may not have been the key driver behind the differences in pain scores across the disease stages observed in the main HR‐QoL analysis. The data also suggest that pain management with analgesia may have been inadequate from the patient perspective.
To our knowledge, this is the first analysis contrasting the HR‐QoL of newly diagnosed MM patients by transplant eligibility. Despite efforts to age match the populations, transplant‐ineligible patients were a median of 8 years older than transplant‐eligible patients. However, despite the age gap, clinical characteristics and HR‐QoL scores were generally similar between the two groups. None of the observed HR‐QoL differences approached the threshold for a clinically meaningful difference. It appears, therefore, that the overall clinical characteristics of the two populations matter more with respect to HR‐QoL than age per se. Nonetheless, these findings were surprising given that transplant‐eligible patients tend to be of younger age, have fewer comorbidities and may be generally in better health compared with transplant‐ineligible patients (Moreau et al, 2011; Ludwig et al, 2012). It is possible that emotional functioning, particularly the impact of the new diagnosis and/or fear of therapy, as well as social functioning might counterbalance the otherwise better health status that these younger patients would be expected to have. Additional prospective studies are required to further elucidate the relationship between transplant eligibility and HR‐QoL in MM.
Despite the preferential influence of age on overall HR‐QoL in the earlier stages of MM, global health status scores across all age groups and disease stages were lower than the mean EORTC adult normal, demonstrating that HR‐QoL is already impaired in patients with MM from the time of initial diagnosis (Gulbrandsen et al, 2004). While the EORTC values are currently the best retrospective reference data available for the general adult population (Scott et al, 2008), age‐matched data would provide a more robust comparison, especially given that the median age of the reference population was lower than those in any of the MM groups in the present study. Consistently, other prospective studies using the EORTC QLQ‐C30 questionnaire have reported similar reductions in HR‐QoL in MM patients compared with age‐ and gender‐matched populations (Gulbrandsen et al, 2004; Mols et al, 2012).
As mentioned previously, there are several limitations to this study. Firstly, baseline patient HR‐QoL data were derived from six clinical studies of bortezomib‐based therapies which used somewhat different enrolment criteria, and whose data were published over a 12‐year period (from 2003 to 2015). Although many high‐risk patients were included in these trials, some adverse clinical characteristics, such as hypercalcaemia or neurotoxicity, were typically excluded [Richardson et al, 2003, 2005; Orlowski et al, 2007; San Miguel et al, 2008; http://www.euroqol.org/eq-5d-products/eq-5d-3l.html (Accessed March 2016); Ludwig et al, 2013]. Therefore, there may have been an over‐representation of ‘healthier’ patients in this analysis. Secondly, over the past decade, advances in therapies for MM, such as ASCT and the novel agents, bortezomib, lenalidomide and thalidomide, have improved patient survival (Gentile et al, 2012; Ludwig et al, 2014). Consequently, our results may be confounded by a ‘history effect’ whereby patient HR‐QoL data collected from clinical trials conducted over the course of more than 12 years may be influenced by the changing availability of certain treatment options. Similarly, supportive care practices for pain and anaemia may have changed over this protracted data collection period (Snowden et al, 2011). In addition, some studies were geographically localized, while others were conducted on a global scale. This could increase cultural diversity in the population, thus influencing patient‐reported HR‐QoL. It should be emphasized that our results suggest that clinical and demographic parameters, and supportive care practices have a much greater influence on patient‐reported HR‐QoL than the categorical schema used in this analysis, which was based on the number of prior therapies.
Our analysis is also limited by the fact that some important clinical characteristics, in particular the type of cytogenetic abnormality in the malignant plasma cells, were not consistently available across the studies. In addition, baseline HR‐QoL scores were captured using the same standard as the clinical variables, whereby HR‐QoL data available before the first dosing day were carried forward if it was missing at the first dosing day. While this may introduce some temporal variability in patient‐reported HR‐QoL, it was deemed worthwhile in order to boost the sample sizes for subgroup analyses. Finally, the dataset used in the present analysis incorporated an erroneous non‐baseline time point from one patient from the SUMMIT study; however, given the large sample size (n = 2561), inclusion of this time point was expected to have a negligible impact on the results.
Given the non‐curative nature of MM treatment, HR‐QoL is an important consideration for patient care, as outlined in a recent systematic review (Sonneveld et al, 2013). Our results suggest that differences in HR‐QoL across the MM disease course appear to be complex and mitigated by clinical and demographic factors, especially age. There were some surprising results, such as the observed increase in global health status over the course of disease progression. However, these unexpected findings may reflect the realities of clinical practice more than originally anticipated. Other researchers have also reported conflicting results when evaluating HR‐QoL in MM. For example, age did not predict EORTC QLQ‐C30 global health status in a smaller European cohort study in which a cross‐sectional analysis of all presenting MM patients, regardless of disease or treatment stage, was conducted (Jordan et al, 2013). Instead, disease duration, symptom burden, bone symptoms (as also observed in our analysis) and being currently on therapy were found to be strong predictors of global health status on multiple regression analysis. In a separate, small Dutch longitudinal patient registry study of MM patients up to 10 years post‐diagnosis, length of survivorship was found to have no impact on EORTC QLQ‐C30 global health status or QLQ‐C30 subscales (Mols et al, 2012). However, a steady decline in global health status, as well as worsening in symptoms, such as pain and fatigue, was observed over the 1‐year follow‐up period. A subsequent larger study of the same Dutch registry population, which was designed to explore the impact of age on HR‐QoL, found no association between age and EORTC QLQ‐C30 global health status, while comorbidity was found to be an influential factor (van der Poel et al, 2015). As in this report, both of the two latter studies reported inferior HR‐QoL among MM patients across all scales of the EORTC QLQ‐C30 when compared to a normative reference population.
These results should be interpreted with some caution as the selection of patients who are candidates for clinical trials may have led to some bias. However, they may also provide important observations for treating physicians on: (i) the impact of a new diagnosis on the emotional and role functioning of patients, suggesting a potential role for psychological counselling, and (ii) the importance of early and effective pain control which may result in improved physical functioning. Further validation would be warranted, either in other database studies, prospective evaluations or by analysing trials that occurred concurrently. A large naturalistic, prospective, international, multi‐region, inception cohort study, following patients from diagnosis to advanced disease, would be particularly valuable to help elucidate the complex relationships explored in this paper. Such a study should use a standard longitudinal set of clinical, demographic and HR‐QoL assessments criteria across all therapies (Osborne et al, 2012; Jordan et al, 2013), with rigorous data capture over time, including safety monitoring and the use of supportive care.
Funding sources
This research was supported by Janssen Global Services, LLC.
Author contribution
DRJ: Conceived the project, developed the analytical plan, interpreted the data and co‐wrote the manuscript. HvdV: Developed the analytical plan, interpreted the data and co‐wrote the manuscript. DLE: Developed the analytical plan, interpreted the data and co‐wrote the manuscript. AR: Developed the analytical plan, interpreted the data and co‐wrote the manuscript. JM: Developed the analytical plan, performed statistical analysis, interpreted the data and co‐wrote the manuscript. KL: Interpreted data and co‐wrote the manuscript.
Conflicts of interest
DRJ: Employment (Janssen Global Services); Stock (Johnson & Johnson). HvdV: Employment (Takeda, formerly Janssen Research & Development); Stock (Johnson & Johnson). DLE: Employment (Takeda); Stock (Johnson & Johnson). AR: Employment (MAPI). JM: Employment (MAPI). KL: Employment (Janssen R&D, LLC).
Supporting information
Table SI. Key inclusion and exclusion criteria of the six studies included in the analysis.
Table SII. Patients with baseline HR‐QoL data included in this study by trial and disease stage.
Table SIII. Baseline characteristics by transplant eligibility status of patients in the New disease group.
Fig S1. Mean EORTC QLQ‐C30 scores across disease stages* in patients aged (A) <65 years, (B) 65–75 years and (C) >75 years†.
Acknowledgements
The authors would like to thank Stephanie J. Lee, MD, MPH (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) who assisted in the development of the analytical plan and in the interpretation of the data, while also providing valuable feedback on the manuscript, and Suzanne Viselli (Janssen Research & Development, Raritan, NJ, USA) for her contributions to the creation of the analysis datasets utilized for the analysis and for assisting with any data‐related questions.
They would also like to acknowledge Emma Landers and Catherine Crookes, medical writers with FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc. and Janssen Global Services, LLC.
References
- Aaronson, N.K. , Ahmedzai, S. , Bergman, B. , Bullinger, M. , Cull, A. , Duez, N.J. , Filiberti, A. , Flechtner, H. , Fleishman, S.B. & de Haes, J.C. (1993) The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376. [DOI] [PubMed] [Google Scholar]
- Baz, R. , Lin, H.M. , Hui, A.M. , Harvey, R.D. , Colson, K. , Gallop, K. , Swinburn, P. , Laubach, J. , Berg, D. & Richardson, P. (2015) Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health‐related quality of life. Supportive Care in Cancer, 23, 2789–2797. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. & Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B, 57, 289–300. [Google Scholar]
- Brooks, R. (1996) EuroQol: the current state of play. Health Policy, 37, 53–72. [DOI] [PubMed] [Google Scholar]
- Calhoun, E.A. , Welshman, E.E. , Chang, C.H. , Lurain, J.R. , Fishman, D.A. , Hunt, T.L. & Cella, D. (2003) Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (Fact/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. International Journal of Gynecological Cancer, 13, 741–748. [DOI] [PubMed] [Google Scholar]
- Cella, D. (1997) The Functional Assessment of Cancer Therapy‐Anemia (FACT‐An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Seminars in Hematology, 34, 13–19. [PubMed] [Google Scholar]
- Delforge, M. , Dhawan, R. , Robinson, D. Jr , Meunier, J. , Regnault, A. , Esseltine, D.L. , Cakana, A. , van de Velde, H. , Richardson, P.G. & San Miguel, J.F. (2012) Health‐related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. European Journal of Haematology, 89, 16–27. [DOI] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Delforge, M. , Hajek, R. , Kropff, M. , Petrucci, M.T. , Lewis, P. , Nixon, A. , Zhang, J. , Mei, J. & Palumbo, A. (2013) Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health‐related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica, 98, 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Palumbo, A. , Hajek, R. , Kropff, M. , Petrucci, M.T. , Lewis, P. , Millar, S. , Zhang, J. , Mei, J. & Delforge, M. (2014) Factors that influence health‐related quality of life in newly diagnosed patients with multiple myeloma aged >/= 65 years treated with melphalan, prednisone and lenalidomide followed by lenalidomide maintenance: results of a randomized trial. Leukaemia & Lymphoma, 55, 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt, M. , Terpos, E. , Kleber, M. , Gay, F. , Wasch, R. , Morgan, G. , Cavo, M. , van de Donk, N. , Beilhack, A. , Bruno, B. , Johnsen, H.E. , Hajek, R. , Driessen, C. , Ludwig, H. , Beksac, M. , Boccadoro, M. , Straka, C. , Brighen, S. , Gramatzki, M. , Larocca, A. , Lokhorst, H. , Magarotto, V. , Morabito, F. , Dimopoulos, M.A. , Einsele, H. , Sonneveld, P. & Palumbo, A. (2014) European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica, 99, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQoL Group (1990) A new facility for the measurement of health‐related quality of life. Health Policy, 16, 199–208. [DOI] [PubMed] [Google Scholar]
- Fayers, P. & Bottomley, A. (2002) Quality of life research within the EORTC‐the EORTC QLQ‐C30. European Organisation for Research and Treatment of Cancer. European Journal of Cancer, 38(Suppl 4), S125–S133. [DOI] [PubMed] [Google Scholar]
- Gentile, M. , Recchia, A.G. , Mazzone, C. & Morabito, F. (2012) Emerging biological insights and novel treatment strategies in multiple myeloma. Expert Opinion on Emerging Drugs, 17, 407–438. [DOI] [PubMed] [Google Scholar]
- Gulbrandsen, N. , Hjermstad, M.J. & Wisloff, F. (2004) Interpretation of quality of life scores in multiple myeloma by comparison with a reference population and assessment of the clinical importance of score differences. European Journal of Haematology, 72, 172–180. [DOI] [PubMed] [Google Scholar]
- Hjorth, M. , Hjertner, O. , Knudsen, L.M. , Gulbrandsen, N. , Holmberg, E. , Pedersen, P.T. , Andersen, N.F. , Andreasson, B. , Billstrom, R. , Carlson, K. , Carlsson, M.S. , Flogegard, M. , Forsberg, K. , Gimsing, P. , Karlsson, T. , Linder, O. , Nahi, H. , Othzen, A. & Swedin, A. (2012) Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: a randomized study. European Journal of Haematology, 88, 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, K. , Proskorovsky, I. , Lewis, P. , Ishak, J. , Payne, K. , Lordan, N. , Kyriakou, C. , Williams, C.D. , Peters, S. & Davies, F.E. (2013) Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health‐related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Supportive Care in Cancer, 22, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S.K. , Dispenzieri, A. , Lacy, M.Q. , Gertz, M.A. , Buadi, F.K. , Pandey, S. , Kapoor, P. , Dingli, D. , Hayman, S.R. , Leung, N. , Lust, J. , McCurdy, A. , Russell, S.J. , Zeldenrust, S.R. , Kyle, R.A. & Rajkumar, S.V. (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia, 28, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam, A.K. , Fayers, P. & Wisloff, F. (2010a) What changes in health‐related quality of life matter to multiple myeloma patients? A prospective study. European Journal of Haematology, 84, 345–353. [DOI] [PubMed] [Google Scholar]
- Kvam, A.K. , Wisloff, F. & Fayers, P.M. (2010b) Minimal important differences and response shift in health‐related quality of life; a longitudinal study in patients with multiple myeloma. Health and Quality of Life Outcomes, 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam, A.K. & Waage, A. (2015) Health‐related quality of life in patients with multiple myeloma–does it matter? Haematologica, 100, 704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liwing, J. , Uttervall, K. , Lund, J. , Aldrin, A. , Blimark, C. , Carlson, K. , Enestig, J. , Flogegard, M. , Forsberg, K. , Gruber, A. , Haglof, K.H. , Johansson, P. , Lauri, B. , Mellqvist, U.H. , Swedin, A. , Svensson, M. , Nasman, P. , Alici, E. , Gahrton, G. , Aschan, J. & Nahi, H. (2014) Improved survival in myeloma patients: starting to close in on the gap between elderly patients and a matched normal population. British Journal of Haematology, 164, 684–693. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Avet‐Loiseau, H. , Blade, J. , Boccadoro, M. , Cavenagh, J. , Cavo, M. , Davies, F. , de la Rubia, J. , Delimpasi, S. , Dimopoulos, M. , Drach, J. , Einsele, H. , Facon, T. , Goldschmidt, H. , Hess, U. , Mellqvist, U.H. , Moreau, P. , San‐Miguel, J. , Sondergeld, P. , Sonneveld, P. , Udvardy, M. & Palumbo, A. (2012) European perspective on multiple myeloma treatment strategies: update following recent congresses. The Oncologist, 17, 592–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, H. , Viterbo, L. , Greil, R. , Masszi, T. , Spicka, I. , Shpilberg, O. , Hajek, R. , Dmoszynska, A. , Paiva, B. , Vidriales, M.B. , Esteves, G. , Stoppa, A.M. , Robinson, D. Jr , Ricci, D. , Cakana, A. , Enny, C. , Feng, H. , van de Velde, H. & Harousseau, J.L. (2013) Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. Journal of Clinical Oncology, 31, 247–255. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Sonneveld, P. , Davies, F. , Blade, J. , Boccadoro, M. , Cavo, M. , Morgan, G. , de la Rubia, J. , Delforge, M. , Dimopoulos, M. , Einsele, H. , Facon, T. , Goldschmidt, H. , Moreau, P. , Nahi, H. , Plesner, T. , San‐Miguel, J. , Hajek, R. , Sondergeld, P. & Palumbo, A. (2014) European perspective on multiple myeloma treatment strategies in 2014. The Oncologist, 19, 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Bandukwala, S. , Burman, D. , Bryson, J. , Seccareccia, D. , Banerjee, S. , Myers, J. , Rodin, G. , Dudgeon, D. & Zimmermann, C. (2010) Interconversion of three measures of performance status: an empirical analysis. European Journal of Cancer, 46, 3175–3183. [DOI] [PubMed] [Google Scholar]
- Maes, H. & Delforge, M. (2015) Optimizing quality of life in multiple myeloma patients: current options, challenges and recommendations. Expert Review of Hematology, 8, 355–366. [DOI] [PubMed] [Google Scholar]
- Mols, F. , Oerlemans, S. , Vos, A.H. , Koster, A. , Verelst, S. , Sonneveld, P. & van de Poll‐Franse, L.V. (2012) Health‐related quality of life and disease‐specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population‐based study using the PROFILES registry. European Journal of Haematology, 89, 311–319. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Avet‐Loiseau, H. , Harousseau, J.L. & Attal, M. (2011) Current trends in autologous stem‐cell transplantation for myeloma in the era of novel therapies. Journal of Clinical Oncology, 29, 1898–1906. [DOI] [PubMed] [Google Scholar]
- Niesvizky, R. , Flinn, I.W. , Rifkin, R. , Gabrail, N. , Charu, V. , Clowney, B. , Essell, J. , Gaffar, Y. , Warr, T.A. , Neuwirth, R.A. , Zhu, Y. , Elliott, J. , Esseltine, D.L. , Niculescu, L. & Reeves, J. ; For the UPFRONT study investigators (2015) Community‐Based Phase IIIb Trial of Three Upfront Bortezomib‐Based Myeloma Regimens. Journal of Clinical Oncology, 33, 3921–9. [DOI] [PubMed] [Google Scholar]
- Orlowski, R.Z. , Nagler, A. , Sonneveld, P. , Blade, J. , Hajek, R. , Spencer, A. , San, M.J. , Robak, T. , Dmoszynska, A. , Horvath, N. , Spicka, I. , Sutherland, H.J. , Suvorov, A.N. , Zhuang, S.H. , Parekh, T. , Xiu, L. , Yuan, Z. , Rackoff, W. & Harousseau, J.L. (2007) Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. Journal of Clinical Oncology, 25, 3892–3901. [DOI] [PubMed] [Google Scholar]
- Orlowski, R.Z. , Gercheva, L. , Williams, C. , Sutherland, H. , Robak, T. , Masszi, T. , Goranova‐Marinova, V. , Dimopoulos, M.A. , Cavenagh, J.D. , Spicka, I. , Maiolino, A. , Suvorov, A. , Blade, J. , Samoylova, O. , Puchalski, T.A. , Reddy, M. , Bandekar, R. , van de Velde, H. , Xie, H. & Rossi, J.F. (2015) A phase 2, randomized, double‐blind, placebo‐controlled study of siltuximab (anti‐IL‐6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. American Journal of Hematology, 90, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, T.R. , Ramsenthaler, C. , Siegert, R.J. , Edmonds, P.M. , Schey, S.A. & Higginson, I.J. (2012) What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. European Journal of Haematology, 89, 437–457. [DOI] [PubMed] [Google Scholar]
- van der Poel, M.W. , Oerlemans, S. , Schouten, H.C. & van de Poll‐Franse, L.V. (2015) Elderly multiple myeloma patients experience less deterioration in health‐related quality of life than younger patients compared to a normative population: a study from the population‐based PROFILES registry. Annals of Hematology, 94, 651–661. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Barlogie, B. , Berenson, J. , Singhal, S. , Jagannath, S. , Irwin, D. , Rajkumar, S.V. , Srkalovic, G. , Alsina, M. , Alexanian, R. , Siegel, D. , Orlowski, R.Z. , Kuter, D. , Limentani, S.A. , Lee, S. , Hideshima, T. , Esseltine, D.L. , Kauffman, M. , Adams, J. , Schenkein, D.P. & Anderson, K.C. (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. The New England Journal of Medicine, 348, 2609–2617. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Sonneveld, P. , Schuster, M.W. , Irwin, D. , Stadtmauer, E.A. , Facon, T. , Harousseau, J.L. , Ben‐Yehuda, D. , Lonial, S. , Goldschmidt, H. , Reece, D. , San‐Miguel, J.F. , Blade, J. , Boccadoro, M. , Cavenagh, J. , Dalton, W.S. , Boral, A.L. , Esseltine, D.L. , Porter, J.B. , Schenkein, D. & Anderson, K.C. (2005) Bortezomib or high‐dose dexamethasone for relapsed multiple myeloma. N.Engl . Journal of Medicine, 352, 2487–2498. [DOI] [PubMed] [Google Scholar]
- San Miguel, J.F. , Schlag, R. , Khuageva, N.K. , Dimopoulos, M.A. , Shpilberg, O. , Kropff, M. , Spicka, I. , Petrucci, M.T. , Palumbo, A. , Samoilova, O.S. , Dmoszynska, A. , Abdulkadyrov, K.M. , Schots, R. , Jiang, B. , Mateos, M.V. , Anderson, K.C. , Esseltine, D.L. , Liu, K. , Cakana, A. , van de Velde, H. & Richardson, P.G. (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. The New England Journal of Medicine, 359, 906–917. [DOI] [PubMed] [Google Scholar]
- Schwartz, C.E. , Feinberg, R.G. , Jilinskaia, E. & Applegate, J.C. (1999) An evaluation of a psychosocial intervention for survivors of childhood cancer: paradoxical effects of response shift over time. Psychooncology, 8, 344–354. [DOI] [PubMed] [Google Scholar]
- Scott, N.W. , Fayers, P.M. , Aaronson, N.K. , Bottomley, A. , de Graeff, A. , Groenvold, M. , Gundy, C. , Koller, M. , Petersen, M.A. & Sprangers, M.A.G. ; on behalf of the EORTC Quality of Life Group . (2008) EORTC‐QLQ‐C30 Reference Values (2008). European Organization for Research and Treatment of Cancer, Brussels, Belgium. http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf.
- Slovacek, L. , Slovackova, B. , Pavlik, V. , Hrstka, Z. , Macingova, Z. , Jebavy, L. & Horacek, J.M. (2008) Health‐related quality of life in multiple myeloma survivors treated with high dose chemotherapy followed by autologous peripheral blood progenitor cell transplantation: a retrospective analysis. Neoplasma, 55, 350–355. [PubMed] [Google Scholar]
- Snowden, J.A. , Ahmedzai, S.H. , Ashcroft, J. , D'Sa, S. , Littlewood, T. , Low, E. , Lucraft, H. , Maclean, R. , Feyler, S. , Pratt, G. & Bird, J.M. (2011) Guidelines for supportive care in multiple myeloma 2011. British Journal of Haematology, 154, 76–103. [DOI] [PubMed] [Google Scholar]
- Song, K.W. , Dimopoulos, M.A. , Weisel, K.C. , Moreau, P. , Palumbo, A. , Belch, A. , Schey, S. , Sonneveld, P. , Sternas, L. , Yu, X. , Amatya, R. , Monzini, M.S. , Zaki, M. , Jacques, C. & San, M.J. (2015) Health‐related quality of life from the MM‐003 trial of pomalidomide plus low‐dose dexamethasone versus high‐dose dexamethasone in relapsed and/or refractory multiple myeloma. Haematologica, 100, e63–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld, P. , Verelst, S.G. , Lewis, P. , Gray‐Schopfer, V. , Hutchings, A. , Nixon, A. & Petrucci, M.T. (2013) Review of health‐related quality of life data in multiple myeloma patients treated with novel agents. Leukemia, 27, 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, A.K. , Trudel, S. , Bahlis, N.J. , White, D. , Sabry, W. , Belch, A. , Reiman, T. , Roy, J. , Shustik, C. , Kovacs, M.J. , Rubinger, M. , Cantin, G. , Song, K. , Tompkins, K.A. , Marcellus, D.C. , Lacy, M.Q. , Sussman, J. , Reece, D. , Brundage, M. , Harnett, E.L. , Shepherd, L. , Chapman, J.A. & Meyer, R.M. (2013) A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality‐of‐life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood, 121, 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubel, P.A. , Peeters, Y. & Smith, D. (2010) Abandoning the language of “response shift”: a plea for conceptual clarity in distinguishing scale recalibration from true changes in quality of life. Quality of Life Research, 19, 465–471. [DOI] [PubMed] [Google Scholar]
- Wagner, L.I. , Robinson, D. Jr , Weiss, M. , Katz, M. , Greipp, P. , Fonseca, R. & Cella, D. (2012) Content development for the Functional Assessment of Cancer Therapy‐Multiple Myeloma (FACT‐MM): use of qualitative and quantitative methods for scale construction. Journal of Pain and Symptom Management, 43, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Key inclusion and exclusion criteria of the six studies included in the analysis.
Table SII. Patients with baseline HR‐QoL data included in this study by trial and disease stage.
Table SIII. Baseline characteristics by transplant eligibility status of patients in the New disease group.
Fig S1. Mean EORTC QLQ‐C30 scores across disease stages* in patients aged (A) <65 years, (B) 65–75 years and (C) >75 years†.


