Abstract
To report the results of the DECT trial, a phase II study of locally advanced or operable HER2‐positive breast cancer (BC) treated with taxanes and concurrent anthracyclines and trastuzumab. Eligible patients (stage IIA‐IIIB HER2‐positive BC, 18–75 years, normal organ functions, ECOG ≤1, and left ventricular ejection fraction (LVEF) ≥55%) received four cycles of neoadjuvant docetaxel, 100 mg/m2 intravenously, plus trastuzumab 6 mg/kg (loading dose 8 mg/kg) every 3 weeks, followed by four 3‐weekly cycles of epirubicin 120 mg/m2 and cyclophosphamide, 600 mg/m2, plus trastuzumab. Primary objective was pathologic complete response (pCR) rate, defined as ypT0/is ypN0 at definitive surgery. We enrolled 45 consecutive patients. All but six patients (13.3%) completed chemotherapy and all underwent surgery. pCR was observed in 28 patients (62.2%) overall and in 6 (66.7%) from the inflammatory subgroup. The classification and regression tree analysis showed a 100% pCR rate in patients with BMI ≥25 and with hormone negative disease. The median follow up was 46 months (8–78). Four‐year recurrence‐free survival was 74.7% (95%CI, 58.2–91.2). Seven patients (15.6%) recurred and one died. Treatment was well tolerated, with limiting toxicity being neutropenia. No clinical cardiotoxicity was observed. Six patients (13.4%) showed a transient LVEF decrease (<10%). In one patient we observed a ≥10% asymptomatic LVEF decrease persisting after surgery. Notwithstanding their limited applicability due to the current guidelines, our findings support the efficacy of the regimen of interest in the neoadjuvant setting along with a fairly acceptable toxicity profile, including cardiotoxicity. Results on BMI may invite further assessment in future studies. J. Cell. Physiol. 231: 2541–2547, 2016. © 2016 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Neoadjuvant chemotherapy represents the standard treatment for locally advanced breast cancer, and it is currently increasingly used also in patients with operable disease, mainly in unfavourable subsets such as triple negative or human epidermal growth factor‐2 (HER2) positive tumors. HER2 is overexpressed or amplified in 15–20% of breast tumors, and confers a more aggressive clinical behaviour. Trastuzumab, a monoclonal antibody targeting the HER2 receptor, used in combination with chemotherapy, significantly improved the prognosis of this unfavourable subset of breast cancer patients, both in advanced and in adjuvant setting (Slamon et al., 2001; Piccart‐Gebhart et al., 2005; Romond et al., 2005; Joensuu et al., 2006; Perez et al., 2007; Dawood et al., 2010; Slamon et al., 2011).
The role of trastuzumab in combination with chemotherapy has been largely explored in the neoadjuvant setting. Several phase II–III trials have been conducted, both in locally advanced and in operable HER2‐positive breast cancer, with pathological complete response (pCR) rates up to 66% (Buzdar et al., 2005). Yet, the concurrent use of anthracyclines and trastuzumab has been long dismissed due to the high‐rate of cardiotoxicity, that is, 27%, reported in the pivotal phase III trial of metastatic breast cancer from Slamon et al. (2001). Even if data on cardiotoxicity from the concurrent administration were partly downsized by the results of subsequent trials (Buzdar et al., 2005; Gianni et al., 2010; Untch et al., 2010; Guarneri et al., 2012; Untch et al., 2012; Buzdar et al., 2013), warns against administering these drugs in combinations have remained and current guidelines discourage from the concurrent use of these two drugs in early HER2‐positive breast cancer (Denduluri et al., 2016).
In 2008, while the discussion on the concomitant administration of these two drugs was still extremely timely to a research agenda, we designed a phase II prospective trial of neoadjuvant chemotherapy with a sequential regimen of trastuzumab (T) and docetaxel (D) followed by trastuzumab and high‐dose epirubicin in combination with cyclophosphamide (EC), in patients with HER2‐positive operable or locally advanced breast cancer (DECT trial: Docetaxel, Epirubicin, Cyclophosphamide, Trastuzumab). In addition, given the growing interest of our research group towards the role played by anthropometric determinants in affecting treatment outcomes in breast cancer patients across different settings (Vici et al., 2015; D'Aiuto et al., 2016), we relied on data from the DECT trial to further test the association between baseline BMI and rate of pCR in HER2‐positive locally advanced or operable breast cancer and, more in general, to evaluate patient‐ and disease‐related features for their impact on treatment outcomes in the setting and population of interest.
Patients and Methods
The DECT trial was conceived as an open label, phase II prospective trial in women with newly diagnosed locally advanced or operable, HER2‐positive breast cancer. Patients were eligible if diagnosed with a stage IIA to IIIB, histologically proven primary breast cancer. All primary breast cancers had undergone a core biopsy prior to neoadjuvant treatment, and staging work‐up included complete blood count, chemistry, chest radiography, liver ultrasound or computed tomography scan of the liver and bone scan. Cardiac function evaluation included clinical history, a baseline left ventricular ejection fraction (LVEF) evaluation by echocardiogram, and an electrocardiogram, all repeated after four cycles, at the end of neoadjuvant chemotherapy, and during the follow up period, every 6 months or whenever indicated. All the evaluated patients were 18–75 years old, had normal organ functions, an ECOG performance status (PS) ≤1, and a baseline left ventricular ejection fraction (LVEF) of 55% or higher measured by echocardiography. Exclusion criteria included pregnancy, metastatic breast cancer, previous chemotherapy, hormonal therapy, radiotherapy, previous other cancers or contralateral breast cancer, documented history of cardiac disease contraindicating anthracyclines, pre‐existent neuropathy or any other serious illness.
The assessment of proliferative index (percentage of Ki‐67 stained cells, estrogen receptor [ER], progesterone receptor [PgR] and HER2) was performed in pre‐treatment core‐biopsy, and each evaluation was repeated, whenever feasible, at the time of definite surgery. Immunoreactions were revealed by a streptavidin‐biotin enhanced immunoperoxidase technique in an automated autostainer (Bond™ Max, Menarini, Florence, Italy). ER and PgR expression were tested using mAb 6F11 (Menarini) and mAb 1A6 (Menarini), respectively, while Ki‐67 % was assessed using the anti Ki‐67 mAb (MIB1, Dako). ER and PgR were considered positive when >1% of the neoplastic cells showed distinct nuclear immunoreactivity, whereas Ki‐67, based on the median value of our series, was regarded as high if more than 14% of the cell nuclei were immunostained. HER2 overexpression was tested using the polyclonal antibody A0485 (Dako), and was considered positive if grade 3+ staining intensity by immunohistochemistry, or grade 2+ with gene amplification by silver in situ hybridization was detected.
Body mass index (BMI), computed as weight in kilograms divided by height in meters squared, was assessed at baseline. The study was conducted in accordance with Helsinki Declaration and approved by Independent Ethical Committee of Regina Elena National Cancer Institute. All the patients enrolled gave their written informed consent.
The choice of the regimen administered was oriented by previous experience from our team and other groups (Bastholt et al., 1996; Lopez et al., 1998; Vici et al., 2012). Patients received as neoadjuvant treatment four cycles of docetaxel, 100 mg/m2 intravenously, plus trastuzumab (loading dose of 8 mg/kg, followed by 6 mg/kg) every 3 weeks, followed by four cycles of epirubicin, 120 mg/m2 and cyclophosphamide, 600 mg/m2, plus trastuzumab as above, every 3 weeks. Prophylactic administration of G‐CSF was scheduled on days 2–6 of every cycle. Chemotherapy dose‐reductions and delays were scheduled for grade 3–4 haematological and severe non‐haematological toxic effects. In case of signs or symptoms of cardiotoxicity, EC regimen was discontinued. Discontinuation of trastuzumab was scheduled in case of grade 3 acute infusion reaction, severe allergic reaction, cardiotoxicity (≥10% LVEF decrease or a fall to lower than 45% when confirmed by a second evaluation 3 weeks later) or clinical cardiotoxicity (New York Heart Association [NYHA] class II). Adverse events were graded according to NCI Common Toxicity Criteria, version 4. Cardiac function was evaluated by clinical exam, electrocardiogram and echocardiography. Clinical tumor response was assessed by clinical evaluation, mammogram and ultrasound at baseline and before surgery, and pathological response was assessed at definite surgery, which was undertaken within 4 weeks from the last chemotherapy cycle. Pathological complete response (pCR) was defined as no residual invasive cancer both in breast and axilla. Surgery procedures allowed included breast conserving surgery or mastectomy, and complete axillary or sentinel node dissection. Radiotherapy was administered whenever indicated. Adjuvant treatment consisted of 3‐weekly trastuzumab for 6 months in all the patients, and in patients with hormonal receptor positive tumors standard adjuvant hormonal treatment was administered for 5 years.
Power calculation and statistical analysis
The study sample size was established based on ad hoc power calculations. All patients enrolled were included in an intent to treat analysis, and evaluated for efficacy and safety. Pathological complete response was the primary endpoint of the trial. This phase II trial was planned as a single‐stage design as described by A'Hern (2001). A minimum number of 42 patients was considered sufficient to give an 80% probability of rejecting a baseline pCR rate of 40% with an exact 5% one‐sided significance test when the true response is 60%. The regimen would be rejected in case of less than 23 pCR. The response rate was reported with its 95% confidence interval (CI). Relapse free survival (RFS) was calculated from the date of the first cycle to the date of first event (locoregional relapse, distant relapse, progression on neoadjuvant chemotherapy, or death) and overall survival (OS) was calculated from the date of first cycle to the date of death or last follow‐up. The time to event analysis was analyzed according to the Kaplan–Meier method.
Associations between the response rate and clinical or biological characteristics were assessed by Pearson chi‐square test. Variables significantly associated were simultaneously evaluated in regression models and further tested in a Classification And Regression Tree (CART) analysis. This latter classifies patients according to a given dependent variable, that is, pathological response, segregating variables in the way which best differentiate patients in term of the dependent variable chosen. At the end of the analysis each group (node) includes patients with similar behaviour in terms of dependent variable.
BMI was rendered as a categorical variable whose modalities were defined based on a 25 cut off value, in agreement with the directives of the world health organization (http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi, last accessed in April 2016).
Results
Between February 2008 and February 2014, 45 consecutive patients were enrolled in the study.
Pre‐treatment patient characteristics are listed in Table 1. Median age was 45 years, 33 patients (73.3%) were premenopausal, and 16 (35.6%) had baseline BMI ≥25.
Table 1.
Main patient characteristics (N:45)
| Characteristic | N (%) |
|---|---|
| Age | |
| Median | 45 |
| Range | 32–69 |
| Menopausal status | |
| Pre | 33 (73.3) |
| Post | 12 (26.7) |
| BMI | |
| <25 | 29 (64.4) |
| ≥25 | 16 (35.6) |
| Tumor size | |
| T0 | 2 (4.4) |
| T2 | 13 (28.8) |
| T3 | 15 (33.3) |
| T4 | 15 (33.3) |
| Clinical stage | |
| IIA | 3 (6.7) |
| IIB | 13 (28.9) |
| IIIA | 16 (35.5) |
| IIIB | 13 (28.9) |
| Grading | |
| G2 | 3 (6.7) |
| G3 | 25 (55.6) |
| Unknow | 17 (37.8) |
| Ki67 | |
| ≤14% | 4 (8.9) |
| >15% | 31 (68.8) |
| Unknow | 10 (22.2) |
| Estrogen receptor status | |
| Positive | 23 (51.1) |
| Negative | 22 (48.9) |
| Progesterone receptor status | |
| Positive | 17 (37.8) |
| Negative | 28 (62.2) |
| Triple positive tumors | 17 (37.8) |
| HER2 status | |
| Overexpressed | 34 (75.6) |
| Amplified | 11 (24.4) |
The DECT trial. BMI, body mass index.
Sixteen patients (35.6%) had stage II breast cancer and 29 (64.4%) stage III breast cancer, including 9 (20%) patients with inflammatory breast cancer. Twenty four (53.3%) tumors had positive hormonal receptors (ER and/or PgR positive), 17 (37.8%) expressed both hormonal receptors (triple positive). In 34 (75.6%) cases HER2 was overexpressed (HER2 3+), while HER2 gene was amplified in 11 patients (24.4%). All but six patients (13.3%) completed the chemotherapy program and all the patients underwent surgical procedures (Table 1).
Efficacy data
Given the final number of patients enrolled, that is, 45, the primary objective of obtaining ≥23 pCRs in at least 42 consecutive patients was reached. In regard to the clinical response, as expected, two patients judged to have a clinical partial response showed a pCR at definite pathologic evaluation. Overall, a clinical response (cPR + cCR) was observed in all the patients, with 26 (57.7%) of them showing a cCR, and 19 (42.2%) showing a cPR. Radical surgery, consisting in modified or nipple‐sparing mastectomy with sentinel node dissection and/or axillary dissection, was performed in 37 (82.2%) patients, including all the inflammatory carcinomas, while a conservative surgery (quadrantectomy) plus sentinel node dissection or axillary dissection was performed in 8 (17.8%) patients (Table 2).
Table 2.
Types of surgery and pathologic findings (N:45)
| N (%) | |
|---|---|
| Breast surgery | |
| Quadrantectomy | 8 (17.8) |
| Mastectomy | 37 (82.2) |
| Axillary lymph‐node surgery | |
| Sentinel lymph‐node dissection | 2 (4.4) |
| Axillary lymph‐node dissection | 43 (95.6) |
| Residual tumour size (cm) | |
| In situ | 11 (24.4) |
| Microinvasion‐1.0 | 6 (13.4) |
| 1.1‐2 | 3 (6.6) |
| 2.1‐5 | 6 (13.4) |
| No residual tumour | 19 (42.2) |
| Number of positive lymph nodes | |
| 0 | 38 (84.4) |
| 1–3 | 4 (8.9) |
| 4–9 | 3 (6.7) |
The DECT trial.
Pathological CR at definite surgery was observed in 28 (62.2%) patients (95%CI, 48.1–76.4). No residual tumor was found in 19 (42.2%) patients, while 11 (24.4%) patients had “in situ” residual cancer in their breast (Table 3). In the inflammatory subset, namely nine patients, the observed pCR rate was 66.7%. Pathological CRs according to initial stage, hormone receptor status, HER2 overexpression/amplification and other biological parameters are reported in Table 3. As expected, triple positive tumors showed a tendency towards lower pCR compared with tumors not expressing hormonal receptors, which showed a higher response rate (47% vs. 71.4%, respectively, P = 0.09). In univariate models, BMI was predictive of pCR (P = 0.001) and a suggestion was found for a similar role of triple positivity (P = 0.058). In multivariate models, both these latter variables were confirmed (P = 0.005 and P = 0.003, respectively). A CART analysis using these two factors was developed and final nodes identified four risk groups of patients differing by probability of achieving pCR. More specifically, such probability ranged from 100%, in those patients whose BMI was equal to/greater than 25 and HR were not expressed, to 20% in patients with lower BMI and HR positive tumors (Fig. 1).
Table 3.
Pathological responses tumour stage and molecular subtype (N:45)
| Non‐pCR * N (%) | pCR* N (%) | P‐value | |
|---|---|---|---|
| Overall population | 17 (37.8) | 28 (62.2) | |
| Stage at diagnosis | |||
| II | 8 (50.0) | 8 (50.0) | 0.209 |
| III | 9 (31.0) | 20 (69.0) | |
| Inflammatory | |||
| Yes | 3 (33.3) | 6 (66.7) | 0.99 |
| No | 14 (38.9) | 22 (61.1) | |
| HER2 status | |||
| Amplified | 5 (45.5) | 6 (54.5) | 0.55 |
| Overexpressed | 12 (35.3) | 22 (64.7) | |
| Molecular subtypes | |||
| Luminal B | 12 (50.0) | 12 (50.0) | 0.07 |
| HER2‐enriched | 5 (23.8) | 16 (76.2) | |
| Triple positive tumors | |||
| Yes | 9 (52.9) | 8 (47.1) | 0.09 |
| No | 8 (28.6) | 20 (71.4) | |
| BMI | |||
| <25 | 16 (55.2) | 13 (44.8) | 0.001 |
| ≥25 | 1 (6.2) | 15 (93.8) |
The DECT Trial. BMI, body mass index.
Pathological complete response (pCR) was defined as no residual invasive tumor in both breast and axilla.
Figure 1.
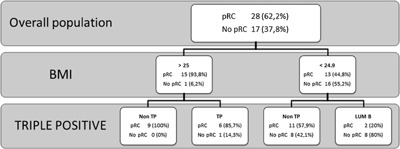
CART analysis by BMI and molecular subgroup (N:45). The DECT Trial. BMI, body mass index; CART, classification and regression tree; pCR, pathological complete response; TP, triple positive (ER/PgR/HER2+).
The median length of follow up was 46 months, within an 8–78 month‐range. Four‐year recurrence‐free survival was 74.7% (95%CI, 58.2–91.2%). Thus far, only seven patients (15.6%) recurred. Two patients developed brain metastases, one patient visceral metastases, two patients bone metastases, and two patients had a local recurrence. Only one patient died, due to multiple brain metastases.
Safety data
Main toxicity data are reported in Table 4. No treatment related deaths occurred. Treatment was generally quite well tolerated, with only six patients (13.4%) not completing the entire neoadjuvant program. The dose‐limiting toxicity was neutropenia, which was of grade 4 in 30 (66.7%) patients, with febrile neutropenia occurring in nine patients (20%). Prophylactic G‐CSF was given to 100% of the patients. A 25% drug dose‐reduction was more frequently performed during the docetaxel/trastuzumab phase of the chemotherapy regimen. Three patients experienced minor allergic reactions to docetaxel not requiring dose‐modifications, but only additional appropriate premedications. None of the patients received reduced doses of drugs due to reason other than myelotoxicity.
Table 4.
Main toxicities (N:45)
| Toxicity | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|
| Hematologic | ||||
| Leukopenia | 6.7 | 24.4 | 46.7 | 11.1 |
| Neutropenia a | 4.4 | 8.9 | 11.1 | 66.7 |
| Thrombocytopenia | 8.9 | 2.2 | — | — |
| Anemia | 24.4 | 24.4 | 2.2 | — |
| Nonhemathologic | ||||
| Arthralgia | 8.9 | 8.9 | — | — |
| Nausea/vomiting | 8.9 | 35.6 | 6.7 | 2.2 |
| Mucositis | 11.1 | 15.6 | 8.9 | — |
| Diarrhea | 11.1 | 6.7 | — | — |
| Myalgia | 4.4 | 6.7 | 2.2 | — |
| Neurotoxicity | 6.7 | 4.4 | 2.2 | — |
| Hypertransaminases | 11.1 | 4.4 | 2.2 | — |
| Hypersensivity | 6.7 | — | — | — |
| Skin toxicity | 4.4 | 4.4 | — | — |
The DECT Trial.
Febrile neutropenia in nine patients (20%).
In this small cohort of patients we did not observe any clinical cardiotoxicity, despite the concurrent use of trastuzumab and an anthracycline. A greater than 10% LVEF decrease was transiently observed in 6 (13.4%) patients at the end of the planned neoadjuvant cycles. LVEF returned to baseline values immediately after surgery, and adjuvant trastuzumab was started as planned. In one patient, there was a ≥10% asymptomatic decrease in LVEF persistently even after surgery, and adjuvant trastuzumab could not be delivered. In this patient LVEF value completely recovered in 3 months, without developing any clinical symptoms.
Discussion
We carried out a phase II prospective neoadjuvant trial of 45 breast cancer patients with operable or locally advanced HER2‐positive breast cancer. We observed a high rate of pCR, that is, 62.2%, which is to our knowledge one of the highest ever reported in the literature among patients treated with trastuzumab‐based neoadjuvant regimens. This is particularly relevant in light of the general consensus on the predictive role of pCR on long‐term outcomes in the HER2‐positive subset (Von Minckwitz et al., 2012; Cortazar et al., 2014). The inclusion of a relatively considerable number of patients with inflammatory breast disease, that is, nine patients out of 45, obtaining 67.6% of pCR, may further reinforce current confidence on the efficacy of the regimen under consideration. When evaluating data on the impact of patient‐ and disease‐related features on the outcome of interest, heavier patients with a HR negative disease were significantly more likely to achieve pCR compared to their counterparts. Toxicity was generally mild and treatment well tolerated, with the combined use of trastuzumab and anthracyclines being not associated with excess in cardiotoxicity.
We bring to the reader attention our results on the efficacy and toxicity of the regimen of interest in a particularly unfavourable historical frame, which will be shortly recalled in the next few lines. The synergistic effect between anthracyclines and trastuzumab has been extensively addressed at a pre‐clinical level (Baselga et al., 1998; Pegram et al., 1999; Pegram et al., 2000; Pegram et al., 2004a,2004b). Yet, this therapeutic approach has been long avoided due to the evidence of cardiac dysfunction which first clearly emerged from the trial led by Slamon et al. (2001). This view has been temporary challenged based on data from subsequent trials showing significantly higher pCR rates and event‐free survival in patients treated simultaneously with trastuzumab and anthracyclines compared to patients treated with chemotherapy alone, along with the lack of evidence of increased cardiac toxicity (Buzdar et al., 2005; Gianni et al., 2010; Untch et al., 2010; Guarneri et al., 2012; Untch et al., 2012; Buzdar et al., 2013). Results from a meta‐analysis including these latter trials for a total of 3292 patients have shown confirmatory evidence of significantly higher likelihood of pCR associated with concurrent anthracyclines and trastuzumab compared with non‐concurrent treatment. However, when addressing toxicity, the authors observed that concurrent administration was indeed associated with significantly increased risk of cardiac‐related adverse events. Subgroup analysis confirmed this finding both in the neo‐adjuvant and metastatic setting (Du et al., 2014). Currently, the use of trastuzumab concomitantly with an anthracycline‐containing regimen is not recommended because of the potential for increased cardiotoxicity (Denduluri et al., 2016). In addition, the 10‐year results from the BIRCG‐006 trial have posed the use of anthracycline‐containing regimens under questioning due to the comparable efficacy and lower rates of clinical congestive heart failure, leukemias and cardiac disfunction showed by the docetaxel, carboplatin and trastuzumab regimen (TCH) compared with AC‐TH for patients with early‐stage HER2‐positive breast cancer (Slamon et al., 2015).
In the current scenario, our reporting is of limited meaning and mainly confined to the particularly high rate of pCR and lack of development of significant cardiotoxicity. In an optimistic view, we may at least partly explain the efficacy rates by the use of epirubicin at a dose of 120 mg/m2 per cycle instead than the canonical dose of 90 mg/m2, since a dose‐response effect has been more clearly demonstrated with epirubin than doxorubicin (Vici et al., 2011). In addition, the choice of a less cardiotoxic anthracycline‐analog such as epirubicin may have conditioned the lack of development of any significant cardiotoxicity, along with the cumulative epirubicin dose not exceeding 480 mg/m2, the strict criteria applied for patient selection, the low median age, and the absence of any previous treatment.
In our study population, we observed better outcomes in patients whose BMI was greater than 25 and whose tumours did not express HR. The finding of lower pCR rates in estrogen‐receptor‐ (ER‐) negative HER2‐positive tumors compared with their ER negative counterpart is not novel and widely consistent across several trials (Collins et al., 2005; Geyer et al., 2006; Loi et al., 2007; Nishimura and Arima, 2007; Cheang et al., 2009; Parker et al., 2009; Sotiriou and Pusztai, 2009; Mackay et al., 2011). Conversely, in the DECT trial, the evidence related to the impact of BMI on pCR seems to diverge from the general consensus on better outcomes in non‐overweigh patients. Indeed, in the pooled analysis of eight neoadjuvant trials from Fontanella and co‐workers, a higher BMI was associated with lower pCR and had a detrimental effect on survival outcomes (Fontanella et al., 2015). Consistently, we have recently observed borderline evidence of higher pCR rates (P = 0.05) and significantly longer overall survival in a clinical series of 86 breast cancer patients aged 45 years or less treated with neoadjuvant chemotherapy (D'Aiuto et al., 2016). Discrepancies in results between our study and those previously reported may be at least partly explained by relevant dissimilarities in disease‐ and patient‐characteristics along with differences in the neoadjuvant regimen of choice. Indeed, although relatively limited from a quantitative stand point, our study population is unique in several respects. First, it is entirely represented by HER2‐positive patients, while in previous studies on BMI and pCR rates both HER2‐positive and ‐negative patients contributed data to test the hypothesis of interest, with obvious consequences in terms of treatment choice and related outcomes. Differences in BMI across the population considered should also be mentioned. Such differences were not particularly remarkable when comparing the DECT population with the clinical series, that is, median BMI 25.3 and 26, respectively, while in the pooled analysis carried out by Fontanella several BMI categories were represented, spanning from underweight to pathologically obese patients (Fontanella et al., 2015; D'Aiuto et al., 2016).
The DECT trial suffers from the particularly limited applicability of its results, given the current recommendations which call for avoidance of concurrent administration of trastuzumab and anthracyclines in HER2+ breast cancer patients. In this view, our communication is an attempt to further inform the scientific community concerning our research experience on the combined use of these drugs in the neo‐adjuvant setting. On the other hand, we produced evidence of acceptable quality which was generated from a phase II clinical trial, which was carefully designed and conducted.
In summary, we carried out a phase II clinical trial testing the efficacy and toxicity of the combined use of epirubicin and trastuzumab in 45 HER2‐positive breast cancer patients with locally advanced operable disease. We observed particularly high pCR rates and no relevant toxicity, including cardiotoxicity. Notwithstanding the fairly acceptable quality of the evidence produced, the applicability of our results is limited by the current guidelines which discourage from the concurrent use of anthracyclines and trastuzumab in the setting and patients considered. In addition, mounting evidence against the use of anthracycline‐containing regimens may further undermine the meaning of our results in the next future. Conversely, our findings on the predictive role of BMI on pCR in this set of HER2 positive breast cancer patients remain unclear, since several aspects related to the study design, patient‐ and disease‐characteristics may have influenced the results observed. In this view, anthropometric and metabolic aspects of the HER2‐positive disease may deserve further consideration in future studies of early HER2‐positive breast cancer.
Authors' Contributions
LP and MB: manuscript drafting, study conduct, data collection and analysis planning. PV: study conception, critical revision of the manuscript for important contents; CN, PM, AG critically revised the manuscript for important intellectual content; DG: statistical analysis; DS, CB, PM, MA, MMS, CN, SDF, TC, FT, AA, SC, LP, MM, LDL, GS, ADB: data collection, database set up and implementation, contribution to data analysis. All authors read and approved the final version of this manuscript and are responsible for all the aspects of the work.
Acknowledgement
This work was supported by Consorzio Interuniversitario Nazionale per la Bio‐Oncologia (CINBO).
L. Pizzuti and M. Barba contributed equally to this work.
Conflicts of interest: The authors declare that they have no conflict of interest.
Literature Cited
- A'Hern RP. 2001. Sample size tables for exact single‐stage phase II designs. Stat Med 20:859–866. [DOI] [PubMed] [Google Scholar]
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. 1998. Recombinant humanized anti‐HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58:2825–2831. [PubMed] [Google Scholar]
- Bastholt L, Dalmark M, Gjedde SB, Pfeiffer P, Pedersen D, Sandberg E, Kjaer M, Mouridsen HT, Rose C, Nielsen OS, Jakobsen P, Bentzen SM. 1996. Dose‐response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: A randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol 14:1146–1155. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. 2005. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2‐positive operable breast cancer. J Clin Oncol 23:3676–3685. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Suman VJ, Meric‐Bernstam F, Leitch AM, Ellis MJ, Boughey JC, Unzeitig G, Royce M, McCall LM, Ewer MS, Hunt KK; American College of Surgeons Oncology Group investigators. 2013. Fluorouracil, epirubicin, and cyclophosphamide (FEC‐75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC‐75 plus trastuzumab as neoadjuvant treatment for patients with HER2‐positive breast cancer (Z1041): A randomised, controlled, phase 3 trial. Lancet Oncol 14:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. 2009. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LC, Botero ML, Schnitt SJ. 2005. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: An analysis of 825 cases. Am J Clin Pathol 123:16–20. [DOI] [PubMed] [Google Scholar]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Pazdur R, Jr. , Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. 2014. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164–172. [DOI] [PubMed] [Google Scholar]
- D'Aiuto M, Chirico A, De Riggi MA, Frasci G, De Laurentiis M, Di Bonito M, Vici P, Pizzuti L, Sergi D, Maugeri‐Saccà M, Barba M, Giordano A. 2016. Body mass index and treatment outcomes following neoadjuvant therapy in women aged 45 y or younger: Evidence from a historic cohort. Cancer Biol Ther 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. 2010. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional‐based review. J Clin Oncol 28:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA, Lyman GH, Partridge AH, Telli ML, Trudeau ME, Wolff AC. 2016. Selection of optimal adjuvant chemotherapy regimens for early breast cancer and adjuvant targeted therapy for human epidermal growth factor receptor 2‐positive breast cancers: An american society of clinical oncology guideline adaptation of the cancer care ontario clinical practice guideline. J Clin Oncol 2016 Apr 18. pii: JCO670182 PMID: 27091714. doi: 10.1200/JCO.2016.67.0182 [DOI] [PubMed] [Google Scholar]
- Du F, Yuan P, Zhu W, Wang J, Ma F, Fan Y, Xu B. 2014. Is it safe to give anthracyclines concurrently with trastuzumab in neo‐adjuvant or metastatic settings for HER2‐positive breast cancer? A meta‐analysis of randomized controlled trials. Med Oncol 31:340. [DOI] [PubMed] [Google Scholar]
- Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Gerber B, Hanusch C, Hilfrich J, Huober J, Schneeweiss A, Paepke S, Jackisch C, Mehta K, Nekljudova V, Untch M, Neven P, von Minckwitz G, Loibl S. 2015. Impact of body mass index on neoadjuvant treatment outcome: A pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat 150:127–139. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello‐Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. 2006. Lapatinib plus capecitabine for HER2‐positive advanced breast cancer. N Engl J Med 355:2733–2743. [DOI] [PubMed] [Google Scholar]
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. 2010. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2negative cohort. Lancet 375:377–384. [DOI] [PubMed] [Google Scholar]
- Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, Untch M, Orlando L, Artioli F, Boni C, Generali DG, Serra P, Bagnalasta M, Marini L, Piacentini F, D'Amico R, Conte P. 2012. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2‐positive operable breast cancer: Results of the randomized phase II CHER‐LOB study. J Clin Oncol 30:1989–1995. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Kellokumpu‐Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi‐Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen AS, Pajunen M, Rauhala M, Kaleva‐Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J; FinHer Study Investigators. 2006. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809–820. [DOI] [PubMed] [Google Scholar]
- Loi S, Haibe‐Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, Ellis P, Harris A, Bergh J, Foekens JA, Klijn JGM, Larsimont D, Buyse M, Bontempi G, Delorenzi M, Piccart MJ, Sotiriou C. 2007. Definition of clinically distinct molecular subtypes in estrogen receptor‐positive breast carcinomas through genomic grade. J Clin Oncol 25:1239–1246. [DOI] [PubMed] [Google Scholar]
- Lopez M, Vici P, Di Lauro K, Conti F, Paoletti G, Ferraironi A, Sciuto R, Giannarelli D, Maini CL. 1998. Randomized prospective clinical trial of high‐dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol 16:86–92. [DOI] [PubMed] [Google Scholar]
- Mackay A, Weigelt B, Grigoriadis A, Kreike B, Natrajan R, A'hern R, Tan DSP, Dowsett M, Ashworth A, Reis‐Filho JS. 2011. Microarray‐based class discovery for molecular classification of breast cancer: Analysis of interobserver agreement. J Natl Cancer Inst 103:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Arima N. 2007. Clinical significance of proliferative activity evaluated by MIB‐1 in the treatment and postoperative follow‐up of early breast cancer. J Clin Oncol 18S:20154. [Google Scholar]
- Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AN, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. 2009. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D. 1999. Inhibitory effects of combinations of HER‐2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18:2241–2251. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. 2004a. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:739–749. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Pienkowski T, Northfelt DW, Eiermann W, Patel R, Fumoleau P, Quan E, Crown J, Toppmeyer D, Smylie M, Riva A, Blitz S, Press MF, Reese D, Lindsay MA, Slamon DJ. 2004b. Results of two open‐label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2‐positive advanced breast cancer. J Natl Cancer Inst 96:759–769. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Lopez A, Konecny G, Slamon DJ. 2000. Trastuzumab and chemotherapeutics: Drug interactions and synergies. Semin Oncol 27:21–25; discussion 92–100. [PubMed] [Google Scholar]
- Perez EA, Lerzo G, Pivot X, Thomas E, Vahdat L, Bosserman L, Viens P, Cai C, Mullaney B, Peck R, Hortobagyi GN. 2007. Efficacy and safety of ixabepilone (BMS‐247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 25:3407–3414. [DOI] [PubMed] [Google Scholar]
- Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; for the Herceptin Adjuvant (HERA) Trial Study Team. 2005. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 353:1659–1672. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr. , Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. 2005. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 353:1673–1684. [DOI] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah‐Fisch I, Lindsay MA, Riva A, Crown J; Breast Cancer International Research Group. 2011. Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med 365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Eiermann W, Robert NJ, Giermek J, Martin M, Jasiowka M, Mackey JR, Chan A, Liu M‐C, Pinter T, Valero V, Falkson C, Fornander T, Shiftan TA, Bensfia S, Hitier S, Xu N, Bée‐Munteanu V, Drevot P, Press MF, Crown J. On Behalf of the BCIRG‐006 Investigators. Ten‐year follow‐up of BCIRG‐006 comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2‐positive early breast cancer. San Antonio Breast Cancer Symposium. Abstract S5‐04. Presented December 11, 2015.
- Slamon DJ, Leyland‐Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Pusztai L. 2009. Gene‐expression signatures in breast cancer. N Engl J Med 360:790–800. [DOI] [PubMed] [Google Scholar]
- Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, Gerber B, Rezai M, Jackisch C, Huober J, Kühn T, Nekljudova V, von Minckwitz G; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie‐Breast (AGO‐B) Study Group. 2012. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline‐taxane‐based chemotherapy (GeparQuinto, GBG 44): A randomised phase 3 trial. Lancet Oncol 13:135–144. [DOI] [PubMed] [Google Scholar]
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kühn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G. 2010. Neoadjuvant treatment with trastuzumab in HER2‐positive breast cancer: Results from the GeparQuattro study. J Clin Oncol 28:2024–2031. [DOI] [PubMed] [Google Scholar]
- Vici P, Brandi M, Giotta F, Foggi P, Schittulli F, Di Lauro L, Gebbia N, Massidda B, Filippelli G, Giannarelli D, Di Benedetto A, Mottolese M, Colucci G, Lopez M. 2012. A multicenter phase III prospective randomized trial of high‐dose epirubicin in combination with cyclophosphamide (EC) versus docetaxel followed by EC in node‐positive breast cancer. GOIM (Gruppo Oncologico Italia Meridionale) 9902 study. Ann Oncol 23:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Colucci G, Giotta F, Sergi D, Filippelli G, Perri P, Botti C, Vizza E, Carpino A, Pizzuti L, Latorre A, Giannarelli D, Lopez M, Di Lauro L. 2011. A multicenter prospective phase II randomized trial of epirubicin/vinorelbine versus pegylated liposomal doxorubicin/vinorelbine as first‐line treatment in advanced breast cancer. A GOIM study. J Exp Clin Cancer Res 30:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Crispo A, Giordano A, Di Lauro L, Sperati F, Terrenato I, Pizzuti L, Sergi D, Mottolese M, Botti C, Grimaldi M, Capasso I, D'Aiuto G, Di Bonito M, Di Paola F, Maugeri‐Saccà M, Montella M, Barba M. 2015. Anthropometric, metabolic and molecular determinants of human epidermal growth factor receptor 2 expression in luminal B breast cancer. J Cell Physiol 230:1708–1712. [DOI] [PubMed] [Google Scholar]
- Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. 2012. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. [DOI] [PubMed] [Google Scholar]


