Abstract
Statins exhibit neuroprotective effects after spinal cord injury (SCI). However, the molecular mechanism underlying these effects remains unknown. This study demonstrates that the hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin (Simv) exhibits neuroprotective effects on neuronal apoptosis and supports functional recovery in a rat SCI model by activating the Wnt/β‐catenin signaling pathway. In specific, Simv administration after SCI significantly up‐regulated the expression of low density lipoprotein receptor‐related protein 6 phosphorylation and β‐catenin protein, increased the mRNA expression of lymphoid enhancer factor‐1 and T‐cell factor‐1, and suppressed the expression of β‐catenin phosphorylation in the spinal cord neurons. Simv enhanced motor neuronal survival in the spinal cord anterior horn and decreased the lesion of spinal cord tissues after SCI. Simv administration after SCI also evidently reduced the expression levels of Bax, active caspase‐3, and active caspase‐9 in the spinal cord neurons and the proportion of transferase UTP nick end labeling (TUNEL)‐positive neuron cells, but increased the expression level of Bcl‐2 in the spinal cord neurons. However, the anti‐apoptotic effects of Simv were reduced in cultured spinal cord nerve cells when the Wnt/β‐catenin signaling pathway was suppressed in the lipopolysaccharide‐induced model. Furthermore, the Basso, Beattie, and Bresnahan scores indicated that Simv treatment significantly improved the locomotor functions of rats after SCI. This study is the first to report that Simv exerts neuroprotective effects by reducing neuronal apoptosis, and promoting functional and pathological recovery after SCI by activating the Wnt/β‐catenin signaling pathway.
We verified the neuroprotective properties associated with simvastatin following spinal cord injury (SCI). Simvastatin reduced neuronal apoptosis, improved the functional and pathological recovery via activating Wnt/β‐catenin signal pathway, however, the anti‐apoptosis effects of simvastatin were reversed following suppressing Wnt/β‐catenin signaling pathway in primary spinal cord neurons. The significant findings may provide clinical therapeutic value of simvastatin for treating SCI.
Keywords: apoptosis, neuroprotection, simvastatin, spinal cord injury, Wnt/β‐catenin
Abbreviations used
- APC
adenomatous polyposis coli
- BBB
Basso, Beattie, and Bresnahan
- CNS
central nervous system
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DMSO
dimethyl sulfoxide
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- HMG‐COA
3‐hydroxy‐3‐methylglutaryl co‐enzyme A
- LEF‐1
lymphoid enhancer factor‐1
- LPS
lipopolysaccharide
- LRP‐6
low‐density lipoprotein receptor‐related protein‐6
- MTT
reduction of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide to a purple formazan product
- PCP
planar cell polarity
- PBS
phosphate‐buffered saline
- PFA
paraformaldehyde
- RT‐PCR
reverse‐transcription polymerase chain reaction analysis
- SCI
spinal cord injury
- Simv
simvastatin
- siRNA
small interference RNA
- TUNEL
transferase UTP nick end labeling
- TCF‐1
T‐cell factor‐1
Neural cell apoptosis influences the development of diseases in the central nervous system (CNS) and plays a significant part in the secondary injury of spinal cord injury (SCI), which influences the microglia and oligodendrocytes, as well as inhibits the recovery of white matter and neurological functions (Yuan and Yankner 2000; Beattie et al. 2002). Effective treatments remain lacking despite intensive research on SCI (Courtine et al. 2011). Simvastatin (Simv) is a 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor widely used to reduce cholesterol, and treat coronary heart disease and atherosclerosis (Mangili et al. 2014). Simv treatment also improves the conditions of patients with cancer, human rhabdomyosarcoma, Alzheimer's disease, rheumatoid arthritis, and traumatic CNS injury. However, these beneficial effects are not related to the cholesterol‐lowering activity of Simv (Crisby 2003; Hadi et al. 2013). A recent study has reported that Simv promotes hippocampal neurogenesis and osteogenic differentiation, but inhibits mesangial cell apoptosis by regulating the Wnt/β‐catenin signaling pathway (Lin et al. 2008; Qiao et al. 2011; Robin et al. 2014). Simv improves functional recovery in various CNS diseases by enhancing neurotrophic factors, mobilizing bone marrow stromal cells, and restricting inflammation (Han et al. 2011, 2012; Esposito et al. 2012). Nevertheless, the specific mechanism by which Simv promotes functional recovery after SCI remains to be elucidated.
Wnts are a family of glycoproteins that participate in neural development, axonal guidance, cell proliferation, and neural cell survival (Gonzalez‐Fernandez et al. 2014; Zhang et al. 2014). Several studies suggested that the Wnt/β‐catenin signaling pathway is activated after SCI and promotes functional recovery (Fernandez‐Martos et al. 2011; Suh et al. 2011; Sun et al. 2013). In addition, the Wnt/β‐catenin signaling pathway reportedly regulates apoptotic activity in several diseases, including acute respiratory distress syndrome and colorectal cancer (Hsu et al. 2014; Li et al. 2015).
In this study, we hypothesized that Simv exerts neuroprotective effects on SCI by activating the Wnt/β‐catenin signaling pathway. This study aimed to explore the expected neuroprotective mechanism of action of Simv in SCI treatment. The findings of this study may provide a novel mechanism by which Simv exerts its therapeutic effects and may be of potential therapeutic value in future treatments of SCI.
Materials and methods
Animals and Simv treatment
All experimental procedures were approved by the Animal Ethics Committee of the Liaoning Medical University, Jinzhou, China. Adult male Sprague–Dawley rats (250–300 g) were purchased from the Laboratory Animal Center of Liaoning Medical University (Jinzhou city, Liaoning province, China). Animals were housed in a specific pathogen‐free laboratory at 23 ± 0.5°C with a 12 h light–dark cycle, and ad libitum food and water access. Simv (TOCRIS, Bristol, UK) was dissolved in 100% ethanol and 1 N NaOH and allowed to stand for 30 min, after which the pH was adjusted to 7.2. Stock solutions were stored at −20°C until further use (Saito et al. 2011; Ching et al. 2013). The animals were randomly divided into three groups: sham, vehicle, and Simv. Only laminectomy was performed in the sham group. The vehicle and Simv groups were intraperitoneally injected with the vehicle with or without Simv (10 mg/kg), respectively. All injections were immediately administered after SCI and then once daily for 2 days as previously described (Esposito et al. 2012; Ching et al. 2013).
SCI model
An SCI model was established using the modified weight‐drop method as previously described (Yacoub et al. 2014). In brief, the rats were intraperitoneally injected with chloral hydrate (0.33 mL/kg) for anesthesia and placed in a stereotactic frame. After a standard T9‐10 laminectomy, the spinal cords of the rats were aseptically exposed, and a 2‐mm diameter impactor weight (10 g) device was dropped from a height of 25 mm to compress the T9‐10 spinal cord and induce hind limb paralysis and neurogenic bladder. After SCI, the spinal cords were irrigated with saline, the incisions were sutured shut, and antibiotics were administered for 3 consecutive days. Manual bladder expression was performed three times daily until bladder function was re‐established.
Behavioral assessment
The Basso, Beattie, Bresnahan (BBB) locomotor rating scale was used to determine the motor function prior to SCI at 24 and 72 h after SCI and then weekly for 7 weeks (Basso et al. 1995). In brief, the open‐field locomotor activity score was determined by observing and calculating the behaviors involving all hind limb joint movements, plantar placement, forelimb and hind limb coordination, and trunk stability. The BBB scores ranged from 0 (complete paralysis) to 21 (unimpaired locomotion). All of the rats were evaluated by three trained examiners through a double blind method.
Primary cell culture
The E18 Sprague–Dawley rats were killed, and the spinal cords were dissociated. Then, spinal cord tissues were digested in papain (Invitrogen, Carlsbad, CA, USA) after being minced. Cells were seeded at 1 × 104 cells/well in 96‐well plates for cell viability assays and at 3 × 104 cells/well in 100‐mm culture dishes for western blot analysis. The cells were then coated with poly‐d‐lysine (Sigma‐Aldrich, St Louis, MO, USA) in a mixture of minimum essential medium, 10% fetal bovine serum, and 0.6% glucose supplemented by antibiotics (penicillin/streptomycin). The medium was discarded a day after neuroplating, and the cells were cultured in neurobasal media including B27, GlutaMAX, and penicillin/streptomycin (Invitrogen, Carlsbad) in a humidified 5 % CO2 incubator at 37°C. The cells were used in the subsequent experiment after being cultured for 8 days to 10 days.
RNA transfection and cell treatment
Small interference RNA (siRNA; Qiagen, Cambridge, MA, USA) was utilized to silence the β‐catenin gene. In brief, primary neuron cells were transfected with siRNA (30 nM concentration) targeting β‐catenin and non‐silencing siRNA using Lipofectamine 2000™ transfection reagent (Invitrogen, Carlsbad). After 6 h, the medium including transfection reagents was decanted. Then, the primary neuron cells were further incubated in the fresh media for 48 h. The β‐catenin low‐expressing cells were incubated for subsequent experiments (Figure S1).
Cells were randomly divided into five groups: (i) control group; (ii) lipopolysaccharide (LPS) group, where cells were treated with LPS (100 ng/mL, Sigma‐Aldrich); (iii) LPS + siRNA group, where cells were treated with LPS (100 ng/mL) after being transfected with the siRNA; (iv) LPS + Simv group, where cells were treated with LPS (100 ng/mL) and Simv (10 μM); and (v) LPS + siRNA + Simv group, where cells were treated with LPS (100 ng/mL) and Simv (10 μM) after being transfected with siRNA. Furthermore, the Wnt antagonist XAV939 (1 μM; Selleckchem, Houston, TX, USA) (Huang et al. 2009) was used to replace β‐catenin siRNA in the five groups to observe the effect of Simv on neuronal apoptosis after inhibiting the Wnt/β‐catenin signaling pathway in primary spinal cord neurons.
Cell viability assay
The reduction in 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide to a purple formazan product (MTT) assay was utilized to detect the cell viability for subsequent Simv treatment. In brief, the cells were incubated with Simv (0, 2, 4, 6, 8, 10, 12, 15, and 20 μM) for 24 h at 37°C. MTT (20 μL, Sigma‐Aldrich) was added to each well, and the cells were incubated for 4 h at 37°C after which the media were decanted and 150 μL of dimethyl sulfoxide (Sigma‐Aldrich) was added to each well. Finally, the absorbency at 490 nm was determined on a Thermo Scientific™ Varioskan™ Flash Multimode Reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Western blot
Western blot analysis was performed as previously described (Su et al. 2012). In brief, rats were anesthetized with 10% chloral hydrate (0.33 mL/kg) at 3, 7, and 14 days after SCI. Spinal cord tissues (2 mm cephalad and caudally around the epicenter) were obtained from the rats. Spinal cord tissue and cultured cells were homogenized in radioimmunoprecipitation assay lysis buffer (50 mM Tris‐HCl, pH 7.4, 150 mM NaCl, 1% NP‐40, 0.25% Na‐deoxycholate, and 1 mM EDTA). Total protein (30 μg) was loaded in each well, separated through 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred to polyvinylidene membranes. The membranes were blocked for 2 h at 23 ± 2°C and then incubated overnight at 4°C with the following primary antibodies: anti‐p‐LRP‐6 (1 : 500; Cell Signaling Technology, Inc., Boston, USA), anti‐β‐catenin (1 : 500; Cell Signaling Technology, Inc.), anti‐p‐β‐catenin (Ser33/37/Thr41) (1 : 500; Cell Signaling Technology, Inc.), anti‐active caspase‐3 (1 : 1000; Novus Biologicals, Littleton, CO, USA), anti‐active caspase‐9 (1 : 1000; Novus Biologicals), anti‐Bcl‐2 (1 : 1000; Novus Biologicals), anti‐Bax (1 : 1000; Novus Biologicals), anti‐NeuN antibody (1 : 300; Abcam, Cambridge, UK), and anti‐β‐actin (1 : 1000; Abcam). The membranes were incubated with secondary antibodies (1 : 2000; Abcam) for 2 h at 23 ± 2°C and visualized using ChemiDoc‐It™TS2 Imager (UVP, LLC, Upland, CA, USA).
Reverse transcription polymerase chain reaction analysis
The rats were intraperitoneally injected with 10% chloral hydrate (0.3 mL/kg) for anesthesia at 3, 7, and 14 days after SCI, and spinal cord tissues (2‐mm cephalad and caudal from the epicenter) were removed. Total RNA was extracted from spinal cord tissue using TRIZOL (Invitrogen, Grand Island), and cDNA was synthesized from 1 μg of total RNA using the TaKaRa RNA PCR™ Kit (AMV) Ver. 3.0 (Takara Bio Inc., Shiga, Japan). cDNA was used as a template for reverse transcription polymerase chain reaction analysis (RT‐PCR) amplification (Mastercycler Gradient, Eppendorf, Germany) to observe the mRNA expression of lymphoid enhancer factor‐1 (LEF‐1) and T‐cell factor‐1 (TCF‐1) by using the following primers: LEF‐1 (forward primer 5′‐ TACGCTAAAGGAGAGCGCAG‐3′ and reverse primer 5′‐GCTGTCATTCTGGGACCTGT‐3′; GenBank accession no: NM 130429.1); TCF‐1 (forward primer 5′‐GAGCTGCCAACCAAAAAGGG‐3′ and reverse primer 5′‐CCAGTTGTAGACACGCACCT‐3′; GenBank accession no: NM 012669.1); and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (forward primer 5′‐CATGAGAAGTATGACAACAGCCT‐3′ and reverse primer 5′‐AGTCCTTCCACGATACCAAAGT‐3′). The cycling conditions were as follows: 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 60°C, 30 s at 72°C, and a final extension at 72°C for 10 min.
Tissue preparation
At 14 days after SCI, rats were intraperitoneally injected with 10% chloral hydrate (0.3 mL/kg) for anesthesia and perfused with 0.9% saline and 4% paraformaldehyde in phosphate‐buffered saline (PBS) (pH 7.4). The T8–L2 segments of the spinal cord (including the epicenter) were removed from the rats and immersed in 4% paraformaldehyde for 2 days. Using the cryostat Microtome (Leica CM3050S; Heidelberg, Germany), we prepared 5 μm crosswise sections (3 mm caudally to the epicenter) for TUNEL staining and 20 μm crosswise sections (3 mm caudally to the epicenter) for Nissl staining. Subsequently, all sections were mounted on slides.
Nissl staining
The 20 μm crosswise sections were immersed overnight in an ethanol/chloroform solution (1 : 1) and then dehydrated in a series of decreasing alcohol concentrations (100% alcohol, 95% alcohol, and distilled water). The sections were stained in 0.05% cresyl violet solution (pH 3.0) for 10 min at 40°C, quickly rinsed in distilled water, differentiated in 95% alcohol for 6 min, dehydrated in 100% alcohol, and then cleared with xylene. Five Nissl stained sections were randomly selected to calculate the average quantities of spinal cord anterior horn motor neurons and the proportion of lesion sizes in the three groups.
TUNEL staining
The 5 μm crosswise sections were immersed in 0.01 M citric acid (pH 6.0) for antigen retrieval. The sections were blocked with blocking buffer (5% normal goat serum and 0.1% Triton X‐100 in PBS) at 4°C for 2 h and then incubated overnight with the primary antibody anti‐NeuN (1 : 300; Abcam) for neuron marking. The sections were rinsed with PBS, incubated with goat anti‐rabbit IgG conjugated to Alexa Fluor® 488 (1:400, Invitrogen, Carlsbad, CA, USA) for 2 h at 23 ± 2°C, and then incubated with TUNEL reaction mixture (In Situ Cell Death Detection Kit, TMR red; Roche, Mannheim, Germany) at 37°C for 1 h. The sections were rinsed with PBS, and the nuclei were counter‐stained with 4′,6‐diamidino‐2‐phenylindole (1 : 1000). All sections were analyzed under a fluorescence microscope (Leica), and the proportion of TUNEL‐positive neuron cells in four random sections was counted for each animal in the three groups.
Statistical analysis
All data are presented as mean ± SD. Comparisons between two groups and among multiple groups were performed using unpaired Student's t‐test and one‐way anova, respectively. The BBB scores were analyzed using the Mann–Whitney U‐test. Statistical analyses were performed using the Graph Prism Program, Version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was considered at p < 0.05.
Results
Simv treatment improves locomotor functional recovery after SCI
BBB scores were measured to evaluate the effect of Simv on locomotor functional recovery after SCI (Fig. 1). Following SCI, the hindquarters of the rats were paralyzed in the Simv‐treated and vehicle‐treated groups, and the locomotor functional recovery in both groups gradually improved between 1 day and 2 weeks after SCI. The BBB scores were consistently and significantly higher in the Simv‐treated group than in the vehicle‐treated group between 2 and 7 weeks after SCI (p < 0.05). The scores in both groups reached a plateau between 6 and 7 weeks after SCI.
Figure 1.
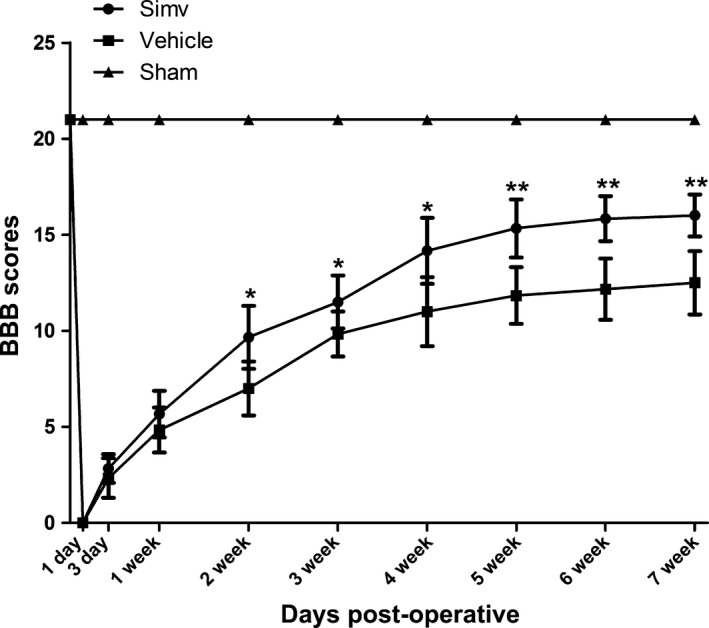
Simvastatin improved locomotor functional recovery after spinal cord injury (SCI). The Basso, Beattie, and Bresnahan (BBB) scores were evaluated to observe locomotor functional recovery in the three groups. Compared with the vehicle‐treated group, the Simv‐treated group was distinctly higher in the period between 2 and 7 weeks after SCI. Simv, simvastatin; *p < 0.05, **p < 0.01 compared with the vehicle‐treated group. n = 6 per group
Simv increases motor neuronal survival and reduces lesion size after SCI
Nissl staining was conducted to determine the quantities of motor neurons in the spinal cord anterior horn and to evaluate the tissue lesion in the spinal cord 14 days after SCI (Fig. 2). Nissl staining revealed greater numbers of motor neurons in the anterior horn of the Simv‐treated rats than in that of the vehicle‐treated rats (Fig. 2a and b). In addition, the Simv‐treated rats had a lower proportion of lesion size within the spinal cords compared with the vehicle‐treated rats (Fig. 2a and c).
Figure 2.
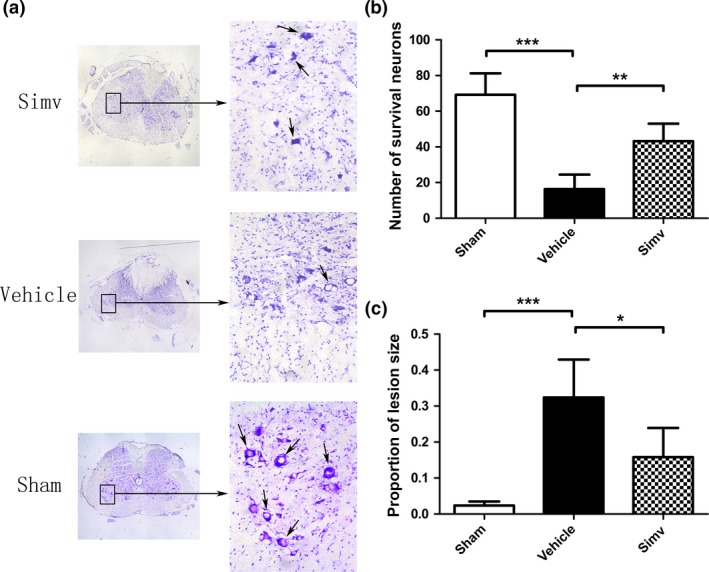
Simvastatin increased motor neuronal survival and decreased tissue lesion after spinal cord injury. (a) Nissl staining indicated (b) increased motor neuronal number and (c) reduced lesion size in the Simv‐treated rats compared with the vehicle‐treated rats. Simv, simvastatin; scale bar = 500 μm and 100 μm, respectively; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle‐treated group. n = 5 per group
Simv activates the Wnt/β‐catenin signaling pathway in the spinal cord neurons after SCI
Western blot (Fig. 3a) and RT‐PCR (Fig. 4a) analyses were utilized to observe the effects of Simv treatment on the Wnt/β‐catenin signaling pathway after SCI. Western blot results showed that the Simv‐treated rats had significantly higher protein levels of p‐LRP6 and β‐catenin, but lower p‐β‐catenin levels in the spinal cord neurons at 3, 7, and 14 days after SCI compared with the vehicle‐treated and sham rats (Fig. 3b–d). Furthermore, Simv significantly enhanced the mRNA expression patterns of LEF‐1 and TCF‐1 at 3, 7, and 14 days after SCI (Fig. 4b and c).
Figure 3.

Simvastatin treatment activated the Wnt/β‐catenin signaling pathway in the spinal cord neurons after spinal cord injury (SCI). (a) The expression levels of p‐low density lipoprotein receptor‐related protein 6 (LRP‐6), β‐catenin, and p‐β‐catenin in the spinal cord neurons in the three groups at 3, 7, and 14 days after SCI, respectively, were detected using western blot analysis. (b) Simv treatment significantly increased p‐LRP‐6 and (c) β‐catenin expression and (d) decreased p‐β‐catenin expression in the spinal cord neurons compared with the vehicle‐treated group. NeuN was used as the loading control. Simv, simvastatin; *p < 0.05, **p < 0.01 compared with the vehicle‐treated group. n = 4 per group.
Figure 4.
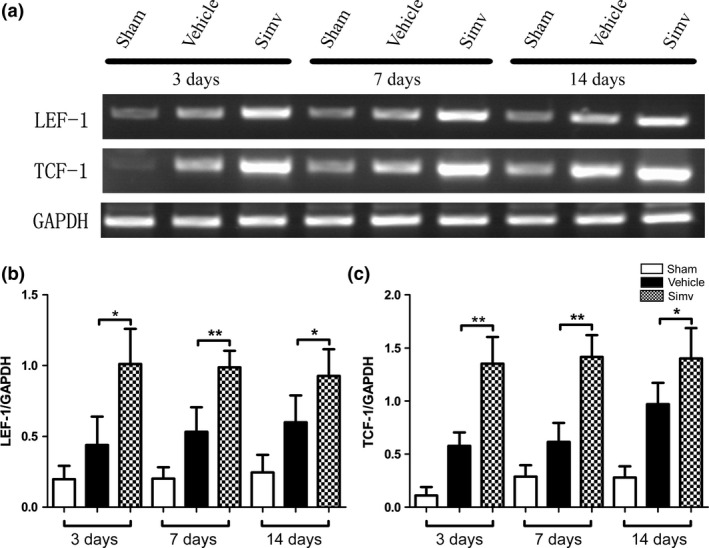
Simvastatin treatment increased lymphoid enhancer factor‐1 (LEF‐1) and T‐cell factor‐1 (TCF‐1) mRNA expression after spinal cord injury (SCI). (a) Expression levels of LEF‐1 and TCF‐1 mRNA at 3, 7, and 14 days after SCI in the three experimental groups. (b) LEF‐1 and (c) TCF‐1 mRNA expression significantly increased in the Simv‐treated animals compared with the sham and vehicle‐treated groups. GAPDH was used as a loading control. Simv, simvastatin; *p < 0.05, **p < 0.01 compared with the vehicle‐treated group. n = 4 per group.
Simv inhibits neuronal apoptosis in SCI rats
Western blot analysis revealed that Bax, active caspase‐3, and active caspase‐9 are markedly up‐regulated and Bcl‐2 protein is down‐regulated in the spinal cord neurons of rats after SCI (Fig. 5a). However, Simv administration after SCI significantly reduced the expression levels of Bax, active caspase‐3, and active caspase‐9 and up‐regulated Bcl‐2 expression (Fig. 5b–e). Furthermore, TUNEL/NeuN/4′,6‐diamidino‐2‐phenylindole double staining was utilized by jointly marking the NeuN (neuronal marker) and DNA fragmentation in the three groups to detect the variation in neuronal apoptosis. Results showed a greater number of TUNEL‐positive neuron cells in the vehicle‐treated group than in the sham group. However, the proportion of TUNEL‐positive neuron cells was significantly lower in the Simv‐treated group than in the vehicle‐treated group (Fig. 6a and b). The above results indicate that Simv can evidently suppress neuronal apoptosis in SCI rats.
Figure 5.
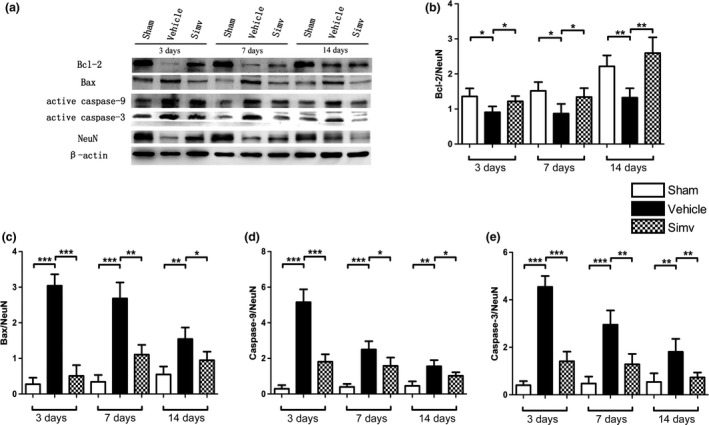
Neuronal apoptosis is inhibited by simvastatin after spinal cord injury (SCI). (a) The expression levels of Bcl‐2, Bax, active caspase‐3, and caspase‐9 in the spinal cord neurons were detected using western blot analysis at 3, 7, and 14 days after SCI. (b) Bcl‐2 expression decreased, whereas (c) Bax, (d) active caspase‐9, and (e) active caspase‐3 levels increased in the vehicle‐treated rats compared with the sham‐treated rats after SCI. However, Simv treatment significantly reduced the expression of Bax, active caspase‐9, and active caspase‐3, and increased the expression of Bcl‐2, compared with vehicle treatment. NeuN was used as the loading control. Simv, simvastatin; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle‐treated group. n = 4 per group.
Figure 6.
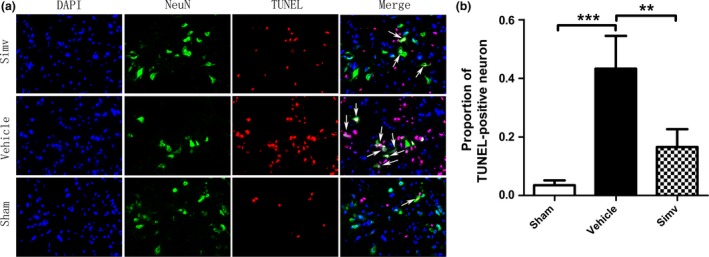
Simvastatin reduced TUNEL‐positive neurons after spinal cord injury. (a) TUNEL/NeuN/4′,6‐diamidino‐2‐phenylindole (DAPI) double staining was utilized to detect the variation in neuronal apoptosis in the three groups. (a) The proportion of TUNEL‐positive neurons was significantly higher in the vehicle‐treated rats compared with the sham group. Conversely, Simv treatment decreased the number of TUNEL‐positive neurons compared with vehicle treatment. (b) The proportion of TUNEL‐positive neurons was lower in the Simv‐treated group than in the vehicle‐treated group. Simv, simvastatin; scale bar = 50 μm, **p < 0.01, ***p < 0.001 compared with the vehicle‐treated group. n = 4 per group.
Selection of concentrations for Simv treatment
Primary spinal cord neuron cells were incubated with Simv (0, 2, 4, 6, 8, 10, 12, 15, and 20 μM) for 24 h, and cell viability was detected using the MTT assay. As assessed by IC50 concentration, no detrimental effects to cells were found after treatment with Simv at concentrations between 8 and 10 μM (Fig. 7). Therefore, a concentration of 10 μM was used in all subsequent experiments.
Figure 7.
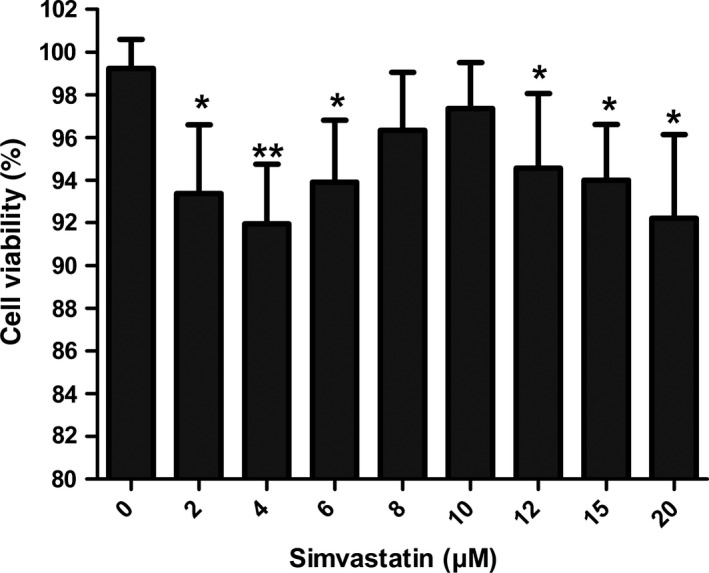
Selection of concentrations of simvastatin (Simv) for treatment. Spinal cord neurons were treated with different concentrations of Simv for 24 h after which cell viability was detected by the reduction in 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide to a purple formazan product assay. *p < 0.05, **p < 0.01 compared with the control group. n = 4
Inhibition of the Wnt/β‐catenin signaling pathway reduces the anti‐apoptotic effect of Simv in primary spinal cord neurons
An injury and apoptosis model was established by exposing spinal cord neurons to LPS, and this model was used to elucidate the mechanisms involved in Simv‐related neuroprotection after injury. As expected, LPS‐induced apoptosis increased the levels of the pro‐apoptotic proteins caspase‐3, caspase‐9, and Bax, but decreased the level of the anti‐apoptotic protein Bcl‐2 (Fig. 8a). These expression patterns were enhanced in the LPS‐induced apoptotic cells after siRNA transfection, which silenced the β‐catenin gene. By contrast, the pro‐apoptotic proteins were significantly down‐regulated and Bcl‐2 was significantly up‐regulated in the LPS‐induced apoptotic cells treated with Simv. However, the beneficial effects of Simv were evidently reversed when the β‐catenin gene was silenced (Fig. 8b–e). Similar expression patterns of neuronal apoptosis were also observed after the Wnt/β‐catenin signaling pathway was inhibited with XAV939 (Fig. 9).
Figure 8.

Inhibition of the Wnt/β‐catenin signaling pathway reduces the anti‐apoptotic effect of simvastatin in the primary spinal cord neurons. (a) Western blot was utilized to detect active caspase‐3, active caspase‐9, Bax, and Bcl‐2 protein expression in the five experimental groups. (b–e) Lipopolysaccharide (LPS)‐induced apoptosis increased the expression of active caspase‐3, caspase‐9, and Bax. The expression patterns were more evident after silencing the β‐catenin gene with siRNA, whereas Simv treatment significantly reduced the expression patterns of pro‐apoptotic proteins while elevating Bcl‐2 expression. The effects of Simv were reversed after silencing the β‐catenin gene with siRNA. NeuN was used as the loading control. Simv, simvastatin; **p < 0.01, ***p < 0.001, compared with LPS alone and siRNA‐treated groups. n = 4 per group.
Figure 9.

Anti‐apoptotic effect of simvastatin in the primary spinal cord neurons was reduced following the suppression of the Wnt/β‐catenin signaling pathway. (a) The expression levels of Bcl‐2, Bax, active caspase‐9, and caspase‐3 in primary spinal cord neurons were detected using western blot analysis. (b–e) Simv observably restrained the apoptosis of spinal cord neurons after lipopolysaccharide (LPS) treatment, whereas the beneficial anti‐apoptotic effect of Simv on spinal cord neurons was destroyed after the inhibition of the Wnt/β‐catenin signaling pathway by the Wnt antagonist XAV939. NeuN was used as the loading control. Simv, simvastatin; *p < 0.05, **p < 0.01, ***p < 0.001, compared with LPS alone and XAV939‐treated groups. n = 4 per group.
Discussion
In this study, Simv treatment increased the protein expression levels of p‐LRP‐6 and β‐catenin in the spinal cord neurons, enhanced the mRNA expression levels of LEF‐1 and TCF‐1, and decreased the expression of p‐β‐catenin in the spinal cord neurons after SCI. Simv treatment increased the number of motor neurons, reduced the spinal cord lesion size in rats, and promoted functional recovery in the rats after SCI. Furthermore, Simv inhibited neuronal apoptosis, but this inhibition was reversed when the Wnt/β‐catenin signaling pathway was suppressed. The results suggest that the neuroprotective effects and functional recovery associated with Simv after SCI are mediated by the Wnt/β‐catenin signaling pathway.
Simv reduces the risk of major vascular events, increases the migration of bone marrow stromal cells, decreases the expression of chondroitin sulfate proteoglycan, and promotes neurite outgrowth (Holmberg et al. 2006, 2008; Han et al. 2012). Recent studies have also demonstrated the neuroprotective effects and improved functional recovery associated with Simv in various CNS diseases. However, reports regarding Simv efficacy in neuroprotection and functional recovery after SCI conflict (Lee et al. 2010; Mann et al. 2010).
In this study, the Simv‐treated rats exhibited increased quantities of motor neurons in the spinal cord anterior horn and reduced lesion size after SCI compared with the vehicle‐treated rats. Furthermore, behavioral analysis showed that the Simv‐treated rats after SCI increased in locomotor function. These results agree with studies indicating the neuroprotective, functional, and pathological recovery effects associated with Simv treatment after SCI.
Several studies over the past decade have shown that activation of the Wnt signaling pathway contributes to functional recovery and axonal regeneration in the CNS (Liu et al. 2008; Onishi et al. 2014). In particular, recent reports have claimed that activation of the Wnt/β‐catenin signaling pathway after SCI improves neuronal survival, axonal guidance, and neuropathic pain remission (Suh et al. 2011; Gonzalez et al. 2012; Yang et al. 2013; Zhang et al. 2013; Gonzalez‐Fernandez et al. 2014). The Wnts are a family of glycoproteins associated with the canonical Wnt/β‐catenin pathway, the non‐canonical planar cell polarity (Wnt‐JNK) pathway, and the Wnt‐Ca2+ pathway (Fernandez‐Martos et al. 2011). During the activation of the Wnt/β‐catenin signaling pathway, the interaction of a Wnt ligand with Frizzleds and LRP‐5/6 causes LRP‐6 phosphorylation, which stabilizes β‐catenin and induces its dissociation from a protein complex consisting of adenomatous polyposis coli, axin, and glycogen synthase kinase‐3β. This phenomenon inhibits the phosphorylation and increases the accumulation of β‐catenin. β‐catenin is then imported into the nucleus where it binds to the transcription factor TCF/LEF, which regulates cell proliferation, cell apoptosis, or differentiation (Reya and Clevers 2005; Lin et al. 2006; van Amerongen and Nusse 2009; MacDonald et al. 2009). Recent studies have suggested that the therapeutic properties associated with Simv are mediated by the Wnt/β‐catenin signaling pathway (Qiao et al. 2011; Robin et al. 2014). However, the mechanism by which Simv regulates the Wnt/β‐catenin signaling pathway after SCI has not been reported.
Neuronal cell death is a feature of CNS diseases, including necrosis and apoptosis (Sakurai et al. 2003). In particular, the suppression of neural cell apoptosis is crucial for functional and pathological recovery after SCI (Tang et al. 2014; Chen et al. 2016; Zhang et al. 2015). Caspases, Bax, and Bcl‐2 are the primary molecular components involved in apoptosis regulation in neurons. Neuronal injury up‐regulates the pro‐apoptotic proteins Bax, caspase‐3, and caspase‐9, but down‐regulates the anti‐apoptotic protein Bcl‐2 (Yuan and Yankner 2000). In addition, previous reports have demonstrated that suppression of the Wnt/β‐catenin signaling pathway increases apoptotic activity in cells (Wang et al. 2002; Lin et al. 2006; Bilir et al. 2013). The results of this study agree with those of previous studies. In particular, western blot analysis detected the increased expression of LRP‐6 phosphorylation and β‐catenin, and the decreased expression of β‐catenin phosphorylation in the spinal cord neurons in the Simv‐treated rats. RT‐PCR results also showed increased mRNA expression levels of LEF‐1 and TCF‐1 in the Simv‐treated group. This result indicates that the Wnt/β‐catenin signaling pathway in the spinal cord neurons was activated by Simv after SCI. Furthermore, Simv treatment significantly decreased the expression levels of Bax, active caspase‐3, and active caspase‐9 in the spinal cord neurons, and the proportion of TUNEL‐positive neurons, but significantly decreased the expression level of Bcl‐2 in the spinal cord neurons compared with the vehicle treatment. Similarly, Simv significantly down‐regulated the expression levels of caspase‐3, caspase‐9, and Bax proteins, and markedly up‐regulated Bcl‐2 levels in the primary spinal cord neurons. Nevertheless, the protective effect of Simv was inhibited when the Wnt/β‐catenin signaling pathway was suppressed with β‐catenin siRNA and XAV939. Overall, these results illustrate that the neuroprotective effects associated with Simv in SCI are mediated by the Wnt/β‐catenin signaling pathway.
This study is the first to determine that Simv inhibits neural cell apoptosis, reduces tissue damage, and improves functional recovery after SCI by activating the Wnt/β‐catenin signaling pathway. These results provide a novel molecular mechanism by which Simv promotes neuroprotection and may have clinical relevance for the future treatment of SCI. Nevertheless, this study only covers the effect of Simv on spinal cord neurons. Further investigation is needed to determine the effects of Simv on different neural cell types of the CNS after SCI and to elucidate other possible molecular mechanisms involved in Simv‐associated neuroprotection through both in vivo and in vitro experiments.
Supporting information
Figure S1. Silencing β‐catenin gene with siRNA decreased the expression level of β‐catenin protein in the primary spinal cord neurons. (a) The β‐catenin protein expression was detected with western blot analysis in different groups. (b) Compared with the control siRNA group, the β‐catenin protein expression was obviously inhibited following silencing β‐catenin gene with siRNA. β‐actin was used as the loading control. ***p < 0.001 compared to control siRNA group. n = 3 per group.
Acknowledgments and conflict of interest disclosure
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 81171799, 81471854). We thank the other researchers for the valuable technical assistance in this work. The authors have declared that they have no conflict of interests for this work and project.
All experiments were conducted in compliance with the ARRIVE guidelines.
References
- van Amerongen R. and Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214. [DOI] [PubMed] [Google Scholar]
- Basso D. M., Beattie M. S. and Bresnahan J. C. (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21. [DOI] [PubMed] [Google Scholar]
- Beattie M. S., Hermann G. E., Rogers R. C. and Bresnahan J. C. (2002) Cell death in models of spinal cord injury. Prog. Brain Res. 137, 37–47. [DOI] [PubMed] [Google Scholar]
- Bilir B., Kucuk O. and Moreno C. S. (2013) Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple‐negative breast cancer cells. J. Transl. Med. 11, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen X., Huang X. et al (2016) Soluble epoxide hydrolase inhibition provides multi‐target therapeutic effects in rats after spinal cord injury. Mol. Neurobiol. 53, 1565–1578. [DOI] [PubMed] [Google Scholar]
- Ching J. K., Ju J. S., Pittman S. K., Margeta M. and Weihl C. C. (2013) Increased autophagy accelerates colchicine‐induced muscle toxicity. Autophagy 9, 2115–2125. [DOI] [PubMed] [Google Scholar]
- Courtine G., van den Brand R. and Musienko P. (2011) Spinal cord injury: time to move. Lancet 377, 1896–1898. [DOI] [PubMed] [Google Scholar]
- Crisby M. (2003) Modulation of the inflammatory process by statins. Drugs of today (Barcelona, Spain: 1998), 39, 137–143. [DOI] [PubMed] [Google Scholar]
- Esposito E., Rinaldi B., Mazzon E., Donniacuo M., Impellizzeri D., Paterniti I., Capuano A., Bramanti P. and Cuzzocrea S. (2012) Anti‐inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR‐alpha. J. Neuroinflammation 9, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Martos C. M., Gonzalez‐Fernandez C., Gonzalez P., Maqueda A., Arenas E. and Rodriguez F. J. (2011) Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS ONE 6, e27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P., Fernandez‐Martos C. M., Gonzalez‐Fernandez C., Arenas E. and Rodriguez F. J. (2012) Spatio‐temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS ONE 7, e50793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Fernandez C., Fernandez‐Martos C. M., Shields S. D., Arenas E. and Javier Rodriguez F. (2014) Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J. Neurotrauma 31, 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi N. R., Al‐Amran F., Yousif M. and Zamil S. T. (2013) Antiapoptotic effect of simvastatin ameliorates myocardial ischemia/reperfusion injury. ISRN Pharmacol. 2013, 815094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Yang N., Xu Y., Zhu J., Chen Z., Liu Z., Dang G. and Song C. (2011) Simvastatin treatment improves functional recovery after experimental spinal cord injury by upregulating the expression of BDNF and GDNF. Neurosci. Lett. 487, 255–259. [DOI] [PubMed] [Google Scholar]
- Han X., Yang N., Cui Y., Xu Y., Dang G. and Song C. (2012) Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat. Neurosci. Lett. 521, 136–141. [DOI] [PubMed] [Google Scholar]
- Holmberg E., Nordstrom T., Gross M., Kluge B., Zhang S. X. and Doolen S. (2006) Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J. Neurotrauma 23, 1366–1378. [DOI] [PubMed] [Google Scholar]
- Holmberg E., Zhang S. X., Sarmiere P. D., Kluge B. R., White J. T. and Doolen S. (2008) Statins decrease chondroitin sulfate proteoglycan expression and acute astrocyte activation in central nervous system injury. Exp. Neurol. 214, 78–86. [DOI] [PubMed] [Google Scholar]
- Hsu H. C., Liu Y. S., Tseng K. C., Tan B. C., Chen S. J. and Chen H. C. (2014) LGR5 regulates survival through mitochondria‐mediated apoptosis and by targeting the Wnt/beta‐catenin signaling pathway in colorectal cancer cells. Cell. Signal. 26, 2333–2342. [DOI] [PubMed] [Google Scholar]
- Huang S. M., Mishina Y. M., Liu S. et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Tigchelaar S., Liu J., Stammers A. M., Streijger F., Tetzlaff W. and Kwon B. K. (2010) Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp. Neurol. 225, 219–230. [DOI] [PubMed] [Google Scholar]
- Li B., Zeng M., He W., Huang X., Luo L., Zhang H. and Deng D. Y. (2015) Ghrelin protects alveolar macrophages against lipopolysaccharide‐induced apoptosis through growth hormone secretagogue receptor 1a‐dependent c‐Jun N‐terminal kinase and Wnt/beta‐catenin signaling and suppresses lung inflammation. Endocrinology 156, 203–217. [DOI] [PubMed] [Google Scholar]
- Lin C. L., Wang J. Y., Huang Y. T., Kuo Y. H., Surendran K. and Wang F. S. (2006) Wnt/beta‐catenin signaling modulates survival of high glucose‐stressed mesangial cells. J. Am. Soc. Nephrol. 17, 2812–2820. [DOI] [PubMed] [Google Scholar]
- Lin C. L., Cheng H., Tung C. W., Huang W. J., Chang P. J., Yang J. T. and Wang J. Y. (2008) Simvastatin reverses high glucose‐induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am. J. Nephrol. 28, 290–297. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Lu C. C., Kerman R., Steward O., Xu X. M. and Zou Y. (2008) Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 28, 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K. and He X. (2009) Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangili O. C., Moron Gagliardi A. C., Mangili L. C., Mesquita C. H., Machado Cesar L. A., Tanaka A., Schaefer E. J., Maranhao R. C. and Santos R. D. (2014) Favorable effects of ezetimibe alone or in association with simvastatin on the removal from plasma of chylomicrons in coronary heart disease subjects. Atherosclerosis 233, 319–325. [DOI] [PubMed] [Google Scholar]
- Mann C. M., Lee J. H., Hillyer J., Stammers A. M., Tetzlaff W. and Kwon B. K. (2010) Lack of robust neurologic benefits with simvastatin or atorvastatin treatment after acute thoracic spinal cord contusion injury. Exp. Neurol. 221, 285–295. [DOI] [PubMed] [Google Scholar]
- Onishi K., Hollis E. and Zou Y. (2014) Axon guidance and injury‐lessons from Wnts and Wnt signaling. Curr. Opin. Neurobiol. 27, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L. J., Kang K. L. and Heo J. S. (2011) Simvastatin promotes osteogenic differentiation of mouse embryonic stem cells via canonical Wnt/beta‐catenin signaling. Mol. Cells 32, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T. and Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850. [DOI] [PubMed] [Google Scholar]
- Robin N. C., Agoston Z., Biechele T. L., James R. G., Berndt J. D. and Moon R. T. (2014) Simvastatin promotes adult hippocampal neurogenesis by enhancing Wnt/beta‐catenin signaling. Stem Cell Reports 2, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Tsuchida M., Umehara S., Kohno T., Yamamoto H. and Hayashi J. (2011) Reduction of spinal cord ischemia/reperfusion injury with simvastatin in rats. Anest. Analg. 113, 565–571. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Nagata T., Abe K., Horinouchi T., Itoyama Y. and Tabayashi K. (2003) Survival and death‐promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J. Thorac. Cardiovasc. Surg. 125, 370–377. [DOI] [PubMed] [Google Scholar]
- Su R., Mei X., Wang Y. and Zhang L. (2012) Regulation of zinc transporter 1 expression in dorsal horn of spinal cord after acute spinal cord injury of rats by dietary zinc. Biol. Trace Elem. Res. 149, 219–226. [DOI] [PubMed] [Google Scholar]
- Suh H. I., Min J., Choi K. H., Kim S. W., Kim K. S. and Jeon S. R. (2011) Axonal regeneration effects of Wnt3a‐secreting fibroblast transplantation in spinal cord‐injured rats. Acta Neurochir. 153, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Sun L., Pan J., Peng Y. et al (2013) Anabolic steroids reduce spinal cord injury‐related bone loss in rats associated with increased Wnt signaling. J. Spinal Cord Med. 36, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P., Hou H., Zhang L., Lan X., Mao Z., Liu D., He C., Du H. and Zhang L. (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol. Neurobiol. 49, 276–287. [DOI] [PubMed] [Google Scholar]
- Wang X., Xiao Y., Mou Y., Zhao Y., Blankesteijn W. M. and Hall J. L. (2002) A role for the beta‐catenin/T‐cell factor signaling cascade in vascular remodeling. Circ. Res. 90, 340–347. [DOI] [PubMed] [Google Scholar]
- Yacoub A., Hajec M. C., Stanger R., Wan W., Young H. and Mathern B. E. (2014) Neuroprotective effects of perflurocarbon (oxycyte) after contusive spinal cord injury. J. Neurotrauma 31, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wu Y., Zheng L., Zhang C., Yang J., Shi M., Feng D., Wu Z. and Wang Y. Z. (2013) Conditioned medium of Wnt/beta‐catenin signaling‐activated olfactory ensheathing cells promotes synaptogenesis and neurite growth in vitro. Cell. Mol. Neurobiol. 33, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. and Yankner B. A. (2000) Apoptosis in the nervous system. Nature 407, 802–809. [DOI] [PubMed] [Google Scholar]
- Zhang Y. K., Huang Z. J., Liu S., Liu Y. P., Song A. A. and Song X. J. (2013) WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J. Clin. Investig. 123, 2268–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li J., Lea R., Vleminckx K. and Amaya E. (2014) Fezf2 promotes neuronal differentiation through localised activation of Wnt/beta‐catenin signalling during forebrain development. Development 141, 4794–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cui Z., Feng G. et al (2015) RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int. J. Biochem. Cell Biol. 60, 43–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Silencing β‐catenin gene with siRNA decreased the expression level of β‐catenin protein in the primary spinal cord neurons. (a) The β‐catenin protein expression was detected with western blot analysis in different groups. (b) Compared with the control siRNA group, the β‐catenin protein expression was obviously inhibited following silencing β‐catenin gene with siRNA. β‐actin was used as the loading control. ***p < 0.001 compared to control siRNA group. n = 3 per group.


