Abstract
What are the fuels of the future? Seven representative carbon‐ and nitrogen‐based fuels are evaluated on an energy basis in a power‐to‐fuel‐to‐power analysis as possible future chemical hydrogen‐storage media. It is intriguing to consider that a nitrogen economy, where hydrogen obtained from water splitting is chemically stored on abundant nitrogen in the form of a nontoxic and safe nitrogen‐based alternative fuel, is energetically feasible.
Keywords: alternative fuels, chemical hydrogen storage, energy conversion, nitrogen, nitrogen-based fuels
1. Introduction
Fossil fuels, formed over hundreds of millions of years, are fundamental building blocks of our civilization: they have made the remarkable prosperity of our society possible, and affected the lives of billions of people. Nevertheless, these fuels have devastating effects on our environment and climate. The extraction and combustion of fossil fuels at an ever‐increasing rate inevitably releases tremendous amounts of CO2 and various pollutants into the atmosphere. Recently, the U.N. Intergovernmental Panel on Climate Change reported that human activity is responsible for the Earth's climate change with a probability of 95 %–100 %.1 In fact, the average global temperature across land and oceans in 2015 was the highest in the 1880–2015 period, surpassing the previous highest record set only in 2014.2
With increasing global greenhouse gas emissions, there is urgency to pursue all measures to develop and deploy carbon‐neutral energy technologies. Therefore, one of the most significant scientific challenges our society must address during the course of the 21st century is the establishment of a secure, economical, and sustainable global energy system. Developing synthetic fuels on a global scale is a key enabling element for clean energy technologies.3, 4 This, in turn, depends on finding effective economic technologies for hydrogen production from seawater. Once such technologies are available, chemical storage of hydrogen will be required due to the extremely low volumetric energy density of pure hydrogen and the potential safety and infrastructure cost issues related to its distribution on a global scale. Large‐scale chemical hydrogen storage can be achieved in the form of a fuel via carbon or nitrogen as the main hydrogen carriers using CO2 5, 6 or N2 7, 8, 9 to produce carbon‐ or nitrogen‐based fuels, respectively. CO2 and N2 could both be obtained from the atmosphere. While the large‐scale separation of about 400 ppm CO2 from the atmosphere10 is a complex engineering challenge,11 the global accessibility and abundance of nitrogen (78.09 vol % of dry air at sea level)12 might enable the large‐scale production of ammonia and its fertilizer derivatives which could be utilized as fuels, “fertilizing” the future energy portfolio. Despite their potential, nitrogen‐based fuels have been missing in previous assessments of future storage systems.13
Herein, we address the question of how to effectively chemically store hydrogen obtained from renewable sources. The main objective of this work is to evaluate the two alternative routes for stationary chemical hydrogen storage: carbon and nitrogen chemistries. We describe metrics by which chemical hydrogen storage via carbon or nitrogen can be critically compared. Finally, we briefly discuss previous experimental studies of a model nitrogen‐based alternative fuel.14, 15, 16, 17, 18, 19, 20, 21
2. The Methanol Economy
The methanol economy,5, 6, 22, 23, 24, 25, 26, 27, 28 an excellent representative of a carbon‐based system, suggests synthesizing methanol for energy storage and distribution through CO2 hydrogenation. Methanol is currently produced worldwide from synthesis gas consisting of 1 CO/2 H2. The hydrogenation of CO2 into CO (reverse water gas shift reaction) consumes an additional mole of H2 per mole of C atoms. Given that H2 is produced from water splitting, the overall process is represented by Reaction (R1), illustrating the stoichiometry of the required water as well.5, 27 Methanol can be combusted without any additives,6, 29 as a mixture (e.g., with gasoline), or utilized in a fuel cell.30 It is also a superior fuel for electric power generation in gas turbines.31, 32
| (1) |
Methanol could be converted into other energetic species such as dimethyl ether (DME), which is produced from methanol by dehydration. Producing almost no soot emissions upon combustion, DME is an excellent diesel‐fuel substitute which also has a high cetane number and a low auto‐ignition temperature.33 Additional liquid organic hydrogen carriers could also be synthesized from methanol.34
3. The Nitrogen Economy
The nitrogen economy is a proposed future system in which nitrogen‐based fuels can be used as a means of energy storage and high‐pressure gas generation.
The simplest nitrogen‐based fuel is ammonia, as described by the ammonia economy.7, 35, 36 The first commercial synthesis of ammonia from its elements was achieved in 1913 with the Haber–Bosch process.37, 38 Today, ammonia is the second largest synthetic inorganic commodity produced worldwide.39 Currently, about 80 % of the global ammonia production is used by the fertilizer industry, most commonly to produce urea and ammonium nitrate (AN).40
The ammonia synthesis reaction is carried out at pressures of 7–30 MPa and temperatures ranging from 400 to 500 °C.39, 41 Given that in future H2 will be derived from water splitting, the overall process of ammonia production is represented by Reaction (R2).39, 41 Ammonia can be used in internal combustion engines and diesel engines with little modification,42, 43, 44 as well as gas turbines45, 46 and rocket engines.47
| (2) |
Ammonia production using renewable intermittent energy is technically feasible with current technologies.48, 49 Moreover, various ammonia‐derived nitrogen‐based fuels have been previously suggested, such as: AN and AN‐based compositions,50, 51, 52, 53, 54, 55, 56 aqueous hydroxylammonium nitrate,57, 58 ammonium dinitramide,59, 60, 61, 62 and aqueous AN with ammonium hydroxide or urea.14 While the methanol economy addresses both the energy and the broad chemical sectors,63, 64 the nitrogen economy addresses the energy and fertilizer sectors. In the context of this Essay only the energy sector is considered.
4. Carbon‐ Versus Nitrogen‐Based Fuels
4.1. Feedstock
Reactions (R1) and (R2) are comparable since both require three moles of water for the hydrolysis of one mole of reactant (CO2 or N2, respectively). Nonetheless, one mole of CO2 yields only one mole of methanol using only two‐thirds of the available hydrogen. On the other hand, two moles of ammonia are produced per mole of N2, without wasting any of the hydrogen feedstock.
The required CO2 feedstock for the production of carbon‐based fuels can be derived either from the atmosphere or by capturing emissions from anthropogenic sources. Anthropogenic sources are more CO2‐rich, typically containing 5–15 vol % CO2.27, 65 Since collecting CO2 directly from billions of small units (i.e., vehicles and small generators) is nonprobable,66 the two realistic CO2 sources are flue gas (from industrial or power plants) and the atmosphere.67 The atmosphere, a large and challenging CO2 source, encompasses the emissions from all emitters, large and small alike (“direct air capture”).5, 6, 65, 66, 67, 68, 69
To establish a common basis for the comparison of the different chemical hydrogen storage routes, we will focus herein on the capture of both CO2 and N2 from the atmosphere as feedstock. Nevertheless, the alternative of separating CO2 from anthropogenic sources (i.e., flue gas) will also be addressed.
The minimum energy required for separating a gas mixture, W min, can be evaluated from entropy considerations assuming ideal gases. Equation (E(3)) simplifies the calculation under the condition that the captured gas is pure and entirely removed from the system (R is the universal gas constant, T is the absolute temperature, y is the mole fraction of the desired gas in the mixture, and m is the molar mass of that gas).70 The minimum energy required for separating N2 from air is about 12 % of the corresponding value for the same mass of CO2 (0.060 GJ t−1 vs. 0.497 GJ t−1).
| (3) |
A realistic estimation of the required energy for the separation of CO2 from the atmosphere can be obtained from previously conducted energy analyses and technology assessments. It is thus estimated that 6.6 GJ of equivalent work is required per ton of CO2 (adopted from literature70 and calculated using Equation (SE1) in the Supporting Information). In addition, the energy requirement for cryogenic air separation plants is estimated at 0.22 GJ of equivalent work per ton of N2.12 It is interesting to note that the realistic separation energy requirement for N2 from air is even lower than the theoretical thermodynamic minimal separation energy for CO2 from air.
4.2. Evaluated Fuels
The seven synthetic fuels assessed herein are: methane, methanol, DME, ammonia, aqueous ammonium hydroxide urea (AHU), aqueous ammonium hydroxide ammonium nitrate (AAN), and aqueous urea ammonium nitrate (UAN). The first three are carbon‐based, ammonia and aqueous AAN are nitrogen‐based, and aqueous AHU and aqueous UAN are low‐carbon nitrogen‐based fuels. The composition of the aqueous AHU, AAN, and UAN fuels, as well as their desirable combustion reactions, are presented by Reactions (R3)–(R5).
| (4) |
| (5) |
| (6) |
Methane, produced using the Sabatier reaction,71 is the simplest carbon‐based fuel (i.e., the smallest molecule with the smallest functional groups). The simplest oxidant variant of methane, methanol, is also a well‐studied and important alternative fuel, and it is the simplest carbon‐based fuel that is liquid at standard conditions. Furthermore, DME is the simplest ether derived from MeOH. Both MeOH and DME have been previously suggested as alternative fuels for various applications, as mentioned above.
Ammonia is the simplest form of a nitrogen‐based fuel, and it is the principal precursor of many nitrogen‐based compounds. Ammonia oxidation through the Ostwald process72 produces nitric acid, which yields AN when reacted with ammonia. As a global commodity AN is manufactured in millions of tons annually, mainly as a fertilizer.73 It is worth noting that an aqueous AN solution is chemically stable and non‐explosive, thus safe to transport, handle, and store.15, 74
Both aqueous AN‐based fuels (i.e., aqueous AAN and UAN) are monofuels since they contain the oxidizer as well as the reducer in the same solution. Consequently, no external oxidizer such as air is required for their combustion [Reactions (R4) and (R5)]. In the aqueous AAN case, ammonia in the form of ammonium hydroxide is a reducer that can react with the net oxidizing AN, while in the aqueous UAN case, urea is the reducer. Urea is a nitrogenous organic compound industrially manufactured worldwide on the order of millions of tons annually, primarily (>90 %) as a fertilizer as well.75 Aqueous AHU is not a monofuel, and contains only the reducer species; thus it requires an external oxidizer such as atmospheric oxygen to combust [Reaction (R3)].
Other synthetic nitrogen‐based fuels could also be suggested, such as aqueous ammonium carbonate, aqueous ammonium acetate, aqueous ammonium carbamate, aqueous ammonium formate, aqueous urea, and methylamine. For reasons of simplicity, only the selected fuels are evaluated herein.
4.3. Fuel Evaluation
For evaluating the selected fuels on an energy basis, a power‐to‐fuel‐to‐power (PFP) index is defined herein as the ratio of the available output power from a fuel's combustion to the energy required for its production (by water splitting, air separation, and synthesis) and distribution. The PFPatm index definition is given by Equation (E(7)), where η combustion is the combustion efficiency (the fraction of a fuel's heating value that is converted to power), and the atmosphere is the feedstock for both N2 and CO2. The material streams entering the system boundaries are water and air, while the material outlet streams are oxygen from water splitting, the byproducts of air separation, water from the fuel's synthesis, and the combustion effluent (Figure 1).
| (7) |
Figure 1.
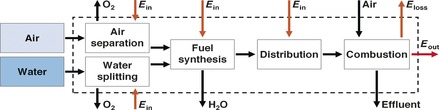
Material and energy streams of the PFP system. Process boundaries are marked by dashed lines.
The analysis was conducted under minor reasonable assumptions (Table S1) and using an equivalent work as a common energy basis [Eq. (SE1)]. The combustion efficiency for each of the fuels was estimated based on the combustion efficiency of methane, an already well‐established and optimized fuel for gas turbines (Table S2). The energy required for distributing the fuels was calculated based on a distribution distance of 1600 km (Table S3). Studies on CO2 utilization suggest that this distance most likely presents a worst‐case scenario. Still, the impact of the energy required for transportation is relatively low (see below).67, 76, 77
The calculated PFPatm indices vary in the range of 12 %–35 % (Table 1). The various energy requirement values for Equation (E2) are given in Table 1 in dimensionless form for ease of comparison across different fuels and in Table S4 in a standard form (i.e., GJ per ton fuel). The dimensionless form was obtained by dividing the required energy by the respective fuel's energy density. The relevance of this approach to the PFPatm index can be seen by dividing both the numerator and the denominator in Equation (E2) by the energy density; the PFPatm index is thus a function of η and the dimensionless form of the various required energies.
Table 1.
PFPatm indices of the seven assessed alternative fuels.
| Fuel | Air separation[a,b] | Water splitting[a,c] | Synthesis energy[a,d] | Distribution[a,e] | Energy density[f] [GJ t−1] | η combustion [g] | PFPatm [h] | |
|---|---|---|---|---|---|---|---|---|
| methane | 0.326 | 1.64 | 0.02280 | 0.027 | 55.5 | 54.1 %82, 83, 84, 85 | 27 % | |
| MeOH | 0.382 | 1.44 | 0.20281 | 0.005 | 23.7 | 54 % | 27 % | |
| DME | 0.398 | 1.50 | 0.274[i] | 0.005 | 31.7 | 50 % | 23 % | |
| ammonia | 0.008 | 1.43 | 0.071[j] | 0.008 | 22.5 | 53 % | 35 % | |
| aq. AHU | 0.138 | 1.58 | 0.162[i] | 0.011 | 9.2 | 50 % | 27 % | |
| aq. AAN | 0.018 | 3.12 | 0.235[i] | 0.023 | 3.7 | 47 % | 14 % | |
| aq. UAN | 0.235 | 3.27 | 0.376[i] | 0.022 | 3.3 | 48 % | 12 % |
[a] Energy values are in an equivalent work basis (see text). [b] Required energy for separating N2, CO2, or both from the atmosphere as feedstock.12, 70 [c] Based on a future prediction for central grid electrolysis evaluated as 180.72 GJ (t H2)−1.86 [d] Values represent state‐of‐the‐art required synthesis energy. [e] Calculated as in Table S3. [f] Taken as high heating value. [g] Calculated as in Table S2. [h] Calculated according to Equation (E2). [i] See Sections 8 and 9 of the Supporting Information for detailed calculations. [j] Average literature value.48, 49, 87
The dimensionless energy required for air separation is relatively high for the carbon‐based fuels due to the energy‐intense separation of atmospheric CO2. On the other hand, the dimensionless energy required for hydrogen generation by water splitting is particularly high for the two aqueous AN‐based fuels. This is a consequence of their relatively low hydrogen‐utilization efficiency (Table S5). The ammonium species in aqueous AN‐based fuels is an excellent approach for chemical hydrogen storage since its N−H bonds are similar to those in ammonia, yet its aqueous form is safe to handle.15, 74 On the other hand, in a large‐scale synthesis process the nitrate species originate from ammonia‐derived HNO3.72 In other words, hydrogen atoms are used for producing the oxidizing nitrate species, and not all are eventually stored in the final fuel form. While the hydrogen‐utilization efficiency for AN itself is 67 %, the values for aqueous AAN and UAN are 75 % and 67 %, respectively (Table S5).
Nevertheless, methane and DME both have the lowest hydrogen‐utilization efficiency of 50 % since the Sabatier reaction consumes 8 hydrogen atoms per synthesized CH4 molecule (Table S5). However, due to the relatively high energy density of both fuels, their dimensionless energy requirement for hydrogen generation is lower than the respective values for the AN‐based fuels (Table 1). Ammonia is the only fuel in the present analysis with a 100 % hydrogen‐utilization efficiency (Table S5), due to the excellent atom economy of the Haber–Bosch process.39 It is noted that the energy required for distributing the different fuels plays only a minor role in the PFP index calculation (Table 1). The PFPatm index of ammonia is particularly high (35 %); next are methane, MeOH, and aqueous AHU—all with an index of 27 %. DME has an index of 23 %, and aqueous AAN and UAN have indices of 14 % and 12 %, respectively (Table 1). It is noted that the PFPatm indices are sensitive to the fuel's combustion efficiency [Eq. (E2)]. While the above notion holds for all fuels, it is of particular significance for the aqueous AN‐based monofuels, for which the estimated combustion efficiency is considered conservative, since they do not require a gas‐compression stage prior to combustion, and can be combusted at relatively high pressures (on the order of 10 MPa).14, 16 It is noted, however, that the PFPatm indices of these fuels could be competitive with those of carbon‐based fuels only at relatively high efficiencies which approach the Carnot limit (Figure S1 d). Further research to better estimate the combustion efficiency of these fuels is required.
In addition to the separation of CO2 and N2 from the atmosphere (Figure 1), separating CO2 from flue gas was also considered. The corresponding PFPflue index was determined, altering the required separation energy accordingly. The PFPflue index (Table 2) was calculated according to Equation (E(8)). An estimate for the energy required for large‐scale CO2 separation from flue gas (e.g., 13.3 vol %) of 0.74 GJ t−1 was used.78, 79 Moreover, an additional energy requirement for transporting the captured CO2 from various distributed sources to a central fuel‐synthesis facility was incorporated into the calculations [Eq. (E3)]. Since at 10 MPa and ambient temperature CO2 is liquid, the energy required for transporting the CO2 was calculated in the same manner as for the liquid fuels (Table S3). The energy values in Table 2 are in a dimensionless form, while standard data are given in Table S6.
| (8) |
Table 2.
PFPflue indices of the seven assessed alternative fuels.
| Fuel | Separation[a] | CO2 transport[b] | PFPflue[c] |
|---|---|---|---|
| methane | 0.037 | 0.006 | 31 % |
| MeOH | 0.043 | 0.007 | 32 % |
| DME | 0.045 | 0.007 | 28 % |
| ammonia | 0.008 | – | 35 % |
| aq. AHU | 0.023 | 0.002 | 28 % |
| aq. AAN | 0.018 | – | 14 % |
| aq. UAN | 0.043 | 0.004 | 13 % |
The PFPflue indices of all carbon‐containing fuels are higher than their respective PFPatm index (Tables 1, 2). The indices of ammonia and aqueous AAN are not affected. The improvement in the PFP indices is moderate (4 to 5 percentage points) for all carbon‐containing fuels, except for aqueous AHU and UAN which exhibited an even less significant increase due to their low carbon content (ca. 5 wt % and ca. 3 wt %, respectively).
A sensitivity analysis was conducted to evaluate the significance of the identified differences in the PFP indices (Table S7, Figure S1). Methane, MeOH, and DME were found to be more sensitive to the required energy for CO2 separation both from air and flue gas than aqueous AHU and UAN, due to the difference in carbon content. All nitrogen‐based fuels had a similar sensitivity to the required energy for atmospheric N2 separation. Nitrogen‐based fuels were more sensitive to a change in the required energy for water splitting than carbon‐based fuels; nevertheless, methane had a relatively high sensitivity coefficient as well. As a result, more efficient future water‐splitting technologies will give a slight energetic advantage to the nitrogen‐based fuels. In this case, methane and aqueous AHU will have similar PFP indices, higher than the PFP index of MeOH (Figure S1 e).
With only one exception, the order in which the seven selected fuels are rated according to the PFP scales does not change when the parameters are altered (Figure S1). The exception occurs if the required energy for atmospheric CO2 separation from air were to decrease from the current level of 6.6 to below 6.3 GJ per ton CO2. In this case, the PFPatm index of methane and MeOH would be higher (near 30 %) than that of aqueous AHU which would remain at 27 % (Figure S1 a).
Other factors in addition to the PFP index are of course important in evaluating synthetic fuels, and should also be taken into account by decision‐makers. These factors include the fuel's techno‐economic and environmental life‐cycle assessments along with infrastructural aspects, its interaction and compatibility with other sectors (i.e., the chemical and fertilizer sectors), toxicity, handling safety, and chemical stability.
5. Closing the Loop
Recent studies of aqueous UAN, a model nitrogen‐based fuel, were previously extensively reviewed elsewhere.88 The continuous combustion feasibility of aqueous UAN was demonstrated14, 15, 16 and the fuel was shown to be safe to handle and store, as well as chemically stable at ambient conditions.15 A thermal analysis of the fuel was performed at ambient and high pressures,17, 18 and its combustion chemistry was unveiled.14, 17, 18, 19, 20 Different catalysts for post‐combustion pollutant abatement were suggested and evaluated.89 Moreover, metal‐corrosion resistance was studied for the fuel's reaction conditions.21 The aqueous UAN fuel possesses an acceptable volumetric energy density for stationary power‐generation applications of 4.4 GJ m−3, equivalent to 10 MPa compressed natural gas. This fuel can theoretically produce an environmentally friendly effluent gas consisting of 73.0 % H2O, 21.6 % N2, and 5.4 % CO2 (mole basis) upon combustion [Reaction (R5)].
The combustion pressure was found to significantly decrease pollutant levels and also increase the N2 yield (Figure 2). At a combustion pressure of 25 MPa and a fuel flow rate of 10 mL min−1, the N2 yield approached 99.9 % (Figure 2 B), while the overall NOx emission level (i.e., NO2, NO, and N2O) was 127 mg MJ−1 (equivalent to 1.85 mmol NO per 1 mol AN),14 significantly below U.S. Environmental Protection Agency's regulatory standard for new stationary natural gas power generation turbines, which is 290 mg MJ−1 (equivalent to 4.22 mmol NO per 1 mol AN).90 Levels of CO and NH3 were 8 and 17 mg MJ−1, respectively.14 While the above results were obtained without implementing a catalyst, catalysis was shown to be effective for abatement of all pollutants, in particular NH3.88, 89 Certainly, the combustion performance of more‐promising nitrogen‐based fuels (Table 1) should be investigated.
Figure 2.
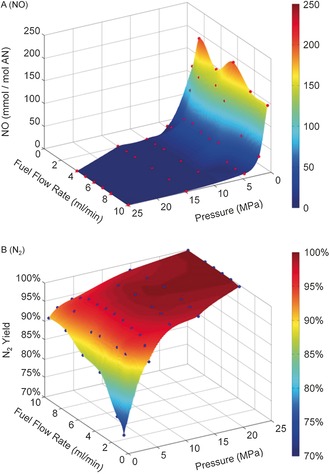
Representative effluent species during continuous combustion of aqueous UAN. A) Measured emission levels of NO as a representative NOx species. Red dots indicate experimental data. B) Determined N2 yield. Blue dots represent calculated values. The x and y axes of the N2 yield were inverted to better demonstrate the effect graphically. RSC Adv. 2014, 4, 10051; reproduced by permission of the Royal Society of Chemistry. Only selected parts of the original image are shown.
6. Conclusions
Large‐scale storage and distribution of future renewable hydrogen mass‐produced by water splitting (rather than from natural gas) will probably be accomplished in the form of chemical fuels via carbon and\or nitrogen as the main hydrogen carriers. The present study compares carbon‐ and nitrogen‐based fuels on an energy basis as chemical hydrogen‐storage media for stationary power generation by providing metrics by which these alternatives can be critically compared and by gleaning updated literature data. A power‐to‐fuel‐to‐power (PFP) index was defined and used to evaluate selected fuels for two cases: a) both CO2 and N2 are obtained from the atmosphere (PFPatm), and b) CO2 is obtained from flue‐gas separation, while N2 is obtained from the atmosphere (PFPflue).
Seven synthetic fuels were evaluated: methane, methanol, dimethyl ether (DME), ammonia, aqueous ammonium hydroxide urea (AHU), aqueous ammonium hydroxide ammonium nitrate (AAN), and aqueous urea ammonium nitrate (UAN). For the case where the CO2 source is the atmosphere, ammonia had an index of 35 %, while methane, MeOH, and aqueous AHU had similar PFPatm indices of 27 %. DME was rated next with a PFPatm value of 23 %, and the aqueous AN‐based monofuels (i.e., AAN and UAN) had PFPatm indices of 12 %–14 %. However, it should be noted that the PFP index is sensitive to the combustion efficiency, which was conservatively estimated for these AN‐based fuels (47 % and 48 %, respectively). Therefore, it is of interest to better assess the combustion efficiency of the AN‐based fuels in an in‐depth thermodynamic analysis.
For the case where the CO2 source is flue gas, ammonia had the highest PFPflue index of 35 %, while MeOH, methane, aqueous AHU, and DME had PFPflue indices of 32 %, 31 %, 28 %, and 25 %, respectively.
Ammonia has the lowest required energy for feedstock separation, even when compared to carbon‐based fuels in the case of flue‐gas‐derived CO2. The normalized energy required for water splitting is highest for the AN‐based fuels. The normalized energies required for fuel synthesis are particularly low for the simple molecules, methane and ammonia.
We showed that a nitrogen economy, where renewable hydrogen is chemically stored on abundant nitrogen in the form of a nontoxic and safe nitrogen‐based alternative fuel, is energetically feasible, and that novel nitrogen‐based fuels are comparable on an energy‐return basis to existing carbon‐based fuels. Incorporating nitrogen‐based fuels as part of a future energy mix will enrich and “fertilize” our energy portfolio.
Finally, aqueous UAN, a model nitrogen‐based fuel, was shown to be safe to handle, and its stable combustion was achieved with lower NOx emissions than U.S. Environmental Protection Agency's regulation standard. Conceptually, it is intriguing to think of a future where atmospheric nitrogen becomes the storage hub for renewable hydrogen that will eventually be mass produced in a sustainable way from water.
Biographical Information
Alon Grinberg Dana received his B.Sc. in Biochemical Engineering from the Technion in 2010, and his Ph.D. in Prof. Grader's research group at the Grand Technion Energy Program in 2015. He then continued to a Postdoctoral Researcher position at the Massachusetts Institute of Technology (MIT). His Ph.D. dissertation focused on combustion of nitrogen‐based fuels, and his research interests are at the crossroads of chemical engineering and applied energy research, in particular novel alternative fuels and computational chemistry.

Biographical Information
Oren Elishav received his B.Sc. in Biochemical Engineering from the Technion in 2014, and then he joined Prof. Grader's research group as an M.Sc. student. His work is focused on the utility of nitrogen‐based fuels, and his research interests are the characterization of the fuel ignition parameters and the design of the production process of novel alternative fuels.

Biographical Information
André Bardow studied at RWTH Aachen University, Germany, and Carnegie Mellon University, USA. In 2004, he received his Ph.D. from RWTH. In 2005 and 2006, he was a Postdoc at ETH Zurich, Switzerland, before becoming Assistant and later Associate Professor in the Department of Process & Energy at TU Delft, The Netherlands. In 2010, he returned to RWTH as Professor for Technical Thermodynamics. His research aims at integrating life‐cycle thinking into engineering design for sustainable energy technologies and chemical industries.

Biographical Information
Gennady E. Shter received his M.Sc. from the Kuibyshev Polytechnical Institute, USSR, in 1971, and his Ph.D. from the Kurnakov Institute of General and Inorganic Chemistry of the USSR Academy of Sciences in 1977. He then held a position as an Associate Professor at the Kuibyshev Medical University starting in 1979. Since 1991 he has been a Senior Research Fellow at the Chemical Engineering Department, Technion.

Biographical Information
Gideon S. Grader received his B.Sc. from Berkeley, USA, in 1982, and his Ph.D. from Caltech, USA, in 1986. He joined Bell Laboratories as a member of technical staff in 1986. He then joined the Technion in 1989, where he has been a Professor of Chemical Engineering since 2000. Between 2007 and 2015 he was the head of the GTEP, and since 2016 he has been the Dean of the Wolfson Department of Chemical Engineering, Technion. His research activities include sol–gel ceramics for catalysis and combustion of alternative fuels.

Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We acknowledge the generous support of Ed Satell, Philadelphia, PA, and the Nancy and Stephen Grand Technion Energy Program (GTEP), the Arturo Gruenbaum Chair in Material Engineering, and the Committee for Planning and Budgeting of the Council for Higher Education under the framework of the KAMEA Program. A.B. gratefully acknowledges support by the German Academic Exchange Service (DAAD) through its thematic network “ACalNet” funded by the German Federal Ministry of Education and Research (BMBF).
A. Grinberg Dana, O. Elishav, A. Bardow, G. E. Shter, G. S. Grader, Angew. Chem. Int. Ed. 2016, 55, 8798.
References
- 1.N. L. Bindoff, P. A. Stott (Intergovernmental Panel on Climate Change), Climate Change 2013: The Physical Science Basis, Chapter 10, 2013.
- 2.NOAA National Centers for Environmental Information, State of the Climate: Global Analysis for September 2015, http://www.ncdc.noaa.gov/sotc/global/201509 (accessed February 2015).
- 3. Turner J. A., Science 1999, 285, 687–689. [DOI] [PubMed] [Google Scholar]
- 4. Lewis N. S., Science 2007, 315, 798–801. [DOI] [PubMed] [Google Scholar]
- 5. Steinberg M., Dang V.-D., Energy Convers. 1977, 17, 97–112. [Google Scholar]
- 6. Olah G. A., Angew. Chem. Int. Ed. 2005, 44, 2636–2639; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 2692–2696. [Google Scholar]
- 7. L. Green Jr. , Int. J. Hydrogen Energy 1982, 7, 355–359. [Google Scholar]
- 8. MacKenzie J. J., Avery W. H., Proc. Intersoc. Energy Convers. Eng. Conf. 1996, 31, 1761–1766. [Google Scholar]
- 9. Zamfirescu C., Dincer I., J. Power Sources 2008, 185, 459–465. [Google Scholar]
- 10.Carbon Dioxide Information Analysis Center, http://cdiac.ornl.gov/ (accessed February 2015).
- 11. House K. Z., Baclig A. C., Ranjan M., van Nierop E. A., Wilcox J., Herzog H. J., Proc. Natl. Acad. Sci. USA 2011, 108, 20428–20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castle W. F., Int. J. Refrig. 2002, 25, 158–172. [Google Scholar]
- 13. Sternberg A., Bardow A., Energy Environ. Sci. 2015, 8, 389–400. [Google Scholar]
- 14. Grinberg Dana A., Shter G. E., Grader G. S., RSC Adv. 2014, 4, 10051–10059. [Google Scholar]
- 15. Grinberg Dana A., Shter G. E., Grader G. S., Energy Technol. 2015, 3, 976–981. [Google Scholar]
- 16.A. Grinberg Dana, B. Mosevitzky, G. Tvil, M. Epstein, G. E. Shter, G. S. Grader, Energy Fuels 2016; DOI: 10.1021/acs.energyfuels.6b00115.
- 17. Grinberg Dana A., Shter G. E., Grader G. S., RSC Adv. 2014, 4, 34836–34848. [Google Scholar]
- 18. Grinberg Dana A., Tvil G., Winter L., Shter G. E., Grader G. S., Fuel 2015, 159, 500–507. [Google Scholar]
- 19.B. Mosevitzky, A. Grinberg Dana, G. E. Shter, G. S. Grader, Proc. 7th Eur. Combust. Meet 2015; DOI: 10.13140/RG.2.1.4490.7364.
- 20.B. Mosevitzky, A. Grinberg Dana, G. E. Shter, G. S. Grader, Comb. Flame 2016; DOI: 10.1016/j.combustflame.2016.01.030.
- 21. Grinberg Dana A., Starostin M., Shter G. E., Buk A., Dinner O., Grader G. S., Oxid. Met. 2014, 82, 491–508. [Google Scholar]
- 22. Olah G. A., Catal. Lett. 2004, 93, 1–2. [Google Scholar]
- 23. Olah G. A., Goeppert A., Prakash G. K. S., Beyond Oil and Gas: The Methanol Economy, Wiley-VCH, Weinheim, 2006. [Google Scholar]
- 24. Reed T., Lerner R., Science 1973, 182, 1299–1304. [DOI] [PubMed] [Google Scholar]
- 25. Perry J. H., Perry C. P., Methanol: Bridge to a Renewable Energy Future, University Press of America, Lanham, Maryland, 1990. [Google Scholar]
- 26. Institute American Petroleum, Institute World Resources, Methanol As An Alternative Fuel Choice: An Assessment, International Energy Program, Foreign Policy Institute, the Paul H. Nitze School of Advanced International Studies, the Johns Hopkins University, 1990. [Google Scholar]
- 27. Duthie J. M., Whittington H. W., IEEE Power Eng. Soc. Summer Meet. 2002, 1, 145–150. [Google Scholar]
- 28.L. Bromberg, W. K. Cheng (Sloan Automotive Laboratory, MIT), Methanol as an alternative transportation fuel in the US: Options for sustainable and/or energy-secure transportation, 2010.
- 29.L. Bromberg, D. Cohn, SAE Tech. Pap. Ser 2010; DOI: 10.4271/2010-01-2199.
- 30. Wasmus S., Küver A., J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar]
- 31.J. Temchin, Analysis of Market Characteristics for Conversion of Liquid Fueled Turbines to Methanol, The Methanol Foundation and Methanex, Inc., 2003.
- 32. General Electric Position Paper: Feasibility of Methanol as Gas Turbine Fuel, February 2001.
- 33. Arcoumanis C., Bae C., Crookes R., Kinoshita E., Fuel 2008, 87, 1014–1030. [Google Scholar]
- 34. Teichmann D., Arlt W., Wasserscheid P., Freymann R., Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar]
- 35. Johnston H., Fuel Cell Rev. 2005, 2, 5–6. [Google Scholar]
- 36. Lan R., Irvine J. T. S., Tao S., Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar]
- 37.“The Haber-Bosch heritage: The ammonia production technology”: M. App, Proc. 50th Anniversary of the IFA Technical Conference, 1997.
- 38. Appl M. in Ammonia, 1. Introduction—Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2011, p. 107. [Google Scholar]; DOI: 10.1002/14356007.a02_143.pub3.
- 39. A M. Appl in mmonia, 2. Production Processes—Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2011. [Google Scholar]; DOI: 10.1002/14356007.o02_o11.
- 40.C. Egenhofer, L. Schrefler, Final Report for a Study on composition and Drivers of Energy Prices and Costs in Energy Intensive Industries: The Case of the Chemical Industry—Ammonia, Center for European Policy Study, Brussels, 2014.
- 41. PRO/II Casebook: Ammonia Synthesis, Simulation Sciences Inc., Fullerton, CA, USA, 1992.
- 42. Westlye F. R., Ivarsson A., Schramm J., Fuel 2013, 111, 239–247. [Google Scholar]
- 43.E. S. Starkman, G. E. James, H. K. Newhall, SAE Tech. Pap. Ser 1967; DOI: 10.4271/670946.
- 44.E. S. Starkman, H. K. Newhall, R. Sutton, T. Maguire, L. Farbar, SAE Tech. Pap. Ser 1966; DOI: 10.4271/660155.
- 45.H. K. Newhall, E. S. Starkman, SAE Techn. Pap. Ser 1966; DOI: 10.4271/660768.
- 46.M. G. Bull, Development of an ammonia-burning gas turbine engine, Solar Turbines International, San Diego, CA, USA, 1968.
- 47.D. R. Jenkins, Hypersonics before the shuttle: A concise history of the X-15 research airplane, NASA Publication SP-2000-4518, 2000.
- 48.J. R. Bartels, M.Sc. Thesis, Iowa State University, 2008.
- 49. Tunå P., Hulteberg C., Ahlgren S., Environ. Prog. Sustainable Energy 2014, 33, 1290–1297. [Google Scholar]
- 50.J. Taylor, G. P. Sillitto in The Use of Ammonium Nitrate as a Solid Fuel to Provide Gas for Propulsive Purposes, Symposium on Combustion and Flame and Explosion Phenomena 1949, 3, 572–579.
- 51. Andersen W. H., Bills K. W., Mishuck E., Moe G., Schultz R. D., Combust. Flame 1959, 3, 301–317. [Google Scholar]
- 52. Oommen C., Jain S. R., J. Hazard. Mater. 1999, 67, 253–281. [DOI] [PubMed] [Google Scholar]
- 53. Sinditskii V. P., Egorshev V. Y., Tomasi D., Deluca L. T., J. Propul. Power 2008, 24, 1068–1078. [Google Scholar]
- 54. Oxley J. C., Kaushik S. M., Gilson N. S., Thermochim. Acta 1989, 153, 269–286. [Google Scholar]
- 55. Kondrikov B. N., Annikov V. E., Egorshev V. Y., DeLuca L., Bronzi C., J. Propul. Power 1999, 15, 763–771. [Google Scholar]
- 56. Izato Y., Miyake A., Date S., Propellants Explos. Pyrotech. 2013, 38, 129–135. [Google Scholar]
- 57. Mueller K. F., Cziesla M. J., US Pat., 5 223 057, 1993.
- 58. Amrousse R., Katsumi T., Sulaiman T., Das B. R., Kumagai H., Maeda K., Hori K., Int. J. Energy Mater. Chem. Propul. 2012, 11, 241–257. [Google Scholar]
- 59. Brill T. B., Brush P. J., Patil D. G., Combust. Flame 1993, 92, 178–186. [Google Scholar]
- 60. Vyazovkin S., Wight C. A., J. Phys. Chem. A 1997, 101, 5653–5658. [DOI] [PubMed] [Google Scholar]
- 61. Matsunaga H., Habu H., Miyake A., J. Therm. Anal. Calorim. 2014, 116, 1227–1232. [Google Scholar]
- 62. Fujisato K., Habu H., Miyake A., Hori K., Vorozhtsov A. B., Propellants Explos. Pyrotech. 2014, 39, 518–525. [Google Scholar]
- 63.F. Asinger, Methanol—Chemie- und Energierohstoff, Springer, Berlin, Heidelberg, 1986; DOI:; 10.1007/978-3-642-70763-6.
- 64.M. Bertau, H. Offermanns, L. Plass, F. Schmidt, H.-J. Wernicke, Methanol: The Basic Chemical and Energy Feedstock of the Future, Springer, Berlin, Heidelberg, 2014; DOI: 10.1007/978-3-642-39709-7.
- 65. Goeppert A., Czaun M., Prakash G. K. S., Olah G. A., Energy Environ. Sci. 2012, 5, 7833–7853. [Google Scholar]
- 66.“The Century-Scale Problem of Carbon Management”: Socolow R. H. in The Carbon Dioxide Dilemma: Promising Technologies and Policies, National Academies Press, 2003, p. 11–12. [Google Scholar]
- 67. von der Assen N., Müller L. J., Steingrube A., Voll P., Bardow A., Environ. Sci. Technol. 2016, 50, 1093–1101. [DOI] [PubMed] [Google Scholar]
- 68. Zeman F., Environ. Sci. Technol. 2007, 41, 7558–7563. [DOI] [PubMed] [Google Scholar]
- 69. Olah G. A., Goeppert A., Prakash G. K. S., J. Org. Chem. 2009, 74, 487–498. [DOI] [PubMed] [Google Scholar]
- 70. Socolow R. H., A Technology Assessment for the APS Panel on Public Affairs, American Physical Society, College Park, 2011. [Google Scholar]
- 71. Müller K., Städter M., Rachow F., Hoffmannbeck D., Schmeißer D., Environ. Earth Sci. 2013, 70, 3771–3778. [Google Scholar]
- 72. Ostwald W., Br. Pat., 190 200 698 (A), 1902.
- 73. Zapp K. H. in Ammonium Compounds—Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. [Google Scholar]; DOI: 10.1002/14356007.a02_243.
- 74. Medard L. A., Accidental Explosions, Vol. 2, Types of Explosive Substances, Wiley, Chichester, 1989, Chap. 23. [Google Scholar]
- 75. Meessen J. H. in Urea – Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2010. [Google Scholar]; DOI: 10.1002/14356007.a27_333.pub2.
- 76. Middleton R. S., Clarens A. F., Liu X., Bielicki J. M., Levine J. S., Environ. Sci. Technol. 2014, 48, 11713–11720. [DOI] [PubMed] [Google Scholar]
- 77. Hasan M. M. F., Boukouvala F., First E. L., Floudas C. A., Ind. Eng. Chem. Res. 2014, 53, 7489–7506. [Google Scholar]
- 78. Belaissaoui B., Cabot G., Cabot M.-S., Wilson D., Favre E., Chem. Eng. Sci. 2013, 97, 256–263. [Google Scholar]
- 79. Boot-Handford M. E., et al., Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar]
- 80. Müller B., Müller K., Teichmann D., Arlt W., Chem. Ing. Tech. 2011, 83, 2002–2013. [Google Scholar]; DOI: 10.1002/cite.201100113.
- 81. Rihko-Struckmann L. K., Peschel A., Hanke-Rauschenbach R., Sundmacher K., Ind. Eng. Chem. Res. 2010, 49, 11073–11078. [Google Scholar]
- 82.Mitsubishi Heavy Industries, LTD., MHI Achieves 1600 °C Turbine Inlet Temperature in Test Operation of World's Highest Thermal Efficiency “J-Series” Gas Turbine, Press information No. 1435, May 2011, http://www.mhi-global.com/news/story/1105261435.html (accessed February 2015).
- 83.Gas turbine SGT5-8000H—World's most powerful 50-Hz gas turbine with a capacity of 400 MW, SIEMENS, http://www.energy.siemens.com/hq/en/fossil-power-generation/gas-turbines/sgt5-8000h.htm (accessed February 2015).
- 84.P. Chiesa, E. Macchi in ASME Turbo Expo 2002: Power for Land, Sea, and Air, pp. 987–1002. American Society of Mechanical Engineers, 2002.
- 85. Beér J. M., Prog. Energy Combust. Sci. 2007, 33, 107–134. [Google Scholar]
- 86.U.S. Department of Energy, Hydrogen and Fuel Cell Program, Future Central Hydrogen Production from PEM Electrolysis, Version 3.1, http://www.hydrogen.energy.gov/h2a_production.html, (accessed February 2015).
- 87.J. Gosnell, Efficient ammonia production, presented at the Ammonia—The Key to Hydrogen Economy Conference, Argonne, IL, USA, 2005.
- 88. Grinberg Dana A., Shter G. E., Grader G. S., Energy Technol. 2015, 4, 7–18. [Google Scholar]
- 89. Tvil G., Grinberg Dana A., Mosevitzky B., Shter G. E., Grader G. S., US Provisional Patent application No. 62/187 861, EFT ID: 22810502, 2015.
- 90.U.S. Environmental Protection Agency, Standards of Performance for Stationary Combustion Turbines, Final Rule, Federal Register Vol. 71, no. 129, p. 38505, 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


