Abstract
Background
Stereotactic radiotherapy (SRT) is an emerging technique for treating tumors in animals.
Objectives
To assess the outcome of dogs with suspected intracranial trigeminal nerve peripheral nerve sheath tumors (PNST) treated with SRT.
Animals
Eight dogs with presumptive PNST.
Methods
This was a retrospective study of dogs identified by searching UC Davis Veterinary Medical Teaching Hospital medical records for dogs treated with SRT for a presumed PNST. Presumptive diagnosis was based on magnetic resonance imaging. SRT was delivered in 3 dose fractions of 8 Gray (Gy) on consecutive days or every other day to a total dose of 24 Gy.
Results
Median disease‐specific survival was 745 days (range: 99–1375 days, n = 6). No signs of acute adverse effects of radiation treatment were recorded. Late radiation effects versus tumor progression could not be confirmed histopathologically because of few animals undergoing necropsy.
Conclusions and Clinical Importance
This study provides preliminary evidence that dogs with PNST benefit from SRT in terms of long‐term survival. The treatment appears to be well tolerated and requires fewer anesthetic events for animals compared to full‐course radiation.
Keywords: Radiation, Stereotactic radiosurgery, Stereotactic radiotherapy, Trigeminal, Veterinary
Abbreviations
- 3D
three dimensional
- CT
computed tomography
- CTV
clinical target volume
- DICOM
Digital Imaging and Communications in Medicine
- DRR
digitally reconstructed radiograph
- GTV
gross tumor volume
- IMRT
intensity‐modulated radiotherapy
- MRI
magnetic resonance imaging
- MU
monitor unit
- MV
megavoltage
- PNST
peripheral nerve sheath tumor
- PTV
planning target volume
- SRT
stereotactic radiotherapy
- T1W
T1‐weighted
- T2W
T2‐weighted
- TaPos
target positioner
Radiation therapy and surgery are mainstays of treatment for brain tumors in dogs. Historically, radiation protocols involved anesthetized treatments over several weeks, large regions of normal brain receiving high radiation dose, or both.1, 2, 3, 4 Various conventional veterinary radiation protocols for brain tumors prescribe a total dose of 45–54 Gray (Gy) with 2.5–3 Gy given in multiple fractions (treatments) to deliver the prescribed dose.1, 2, 4 Currently, most definitive protocols are delivered Monday–Friday over 3–4 weeks. Historically every other day schedules were common.1, 2, 3 Palliative protocols involve weekly doses of radiation, for example, 38 Gy over 5 weeks (5–9 Gy per fraction).5 Although chemotherapy has been used, a survival benefit has not been demonstrated.6
Stereotactic radiotherapy (SRT) delivers high doses of radiation to tumors in 1–5 fractions. Instead of sparing normal tissue through fractionation, the organs at risk are spared by avoidance.7, 8 SRT is most appropriate for small, noninvasive malignancies or benign neoplastic lesions, and it effectively ablates the tumor tissue. In contrast to conventional definitive treatments that use small fraction doses to minimize damage to normal tissue in or near the radiation field, stereotactic fields have steep dose gradients that minimize the irradiated brain volume and safely allow for higher dose per fraction.9
Stereotactic radiotherapy can be delivered with a linear accelerator via a multileaf collimator (MLC) system or a cone‐based system for beam collimation. A frameless SRT system with a body support sled has been developed, including a stereotactic box affixed to the sled at the head to eliminate the need for an affixed head frame, and a set of cones that attach to the machine head, creating circular fields of a defined diameter for beam delivery.10
Intracranial peripheral nerve sheath tumors (PNST) are relatively uncommon tumors in dogs.11 Clinically, dogs with intracranial PNST have one or more of the following clinical signs: ipsilateral masticatory muscle atrophy, loss of facial sensation, and Horner's syndrome. Signs from intracranial brainstem compression can also occur.11 Only one study has exclusively assessed a group of suspected intracranial PNST causing unilateral trigeminal nerve dysfunction. In this study, 3 dogs received surgery and 7 had no treatment. One dog survived 27 months after surgery. Survival time ranged from 5 to 21 months in those left untreated, and some were euthanized at diagnosis.12 In another study, 51 dogs with various intracranial tumors received SRT treatment. Four of these dogs had suspected trigeminal nerve tumors and received a median dose of 13.75 Gy (range 12.5–17.5 Gy). Three of the dogs had a median survival of 881 days and had follow‐up imaging revealing tumor reduction. One dog was lost to follow‐up.13
To date there is little information on radiation for intracranial PNSTs. The goal of this study was to assess survival in dogs receiving SRT for suspected trigeminal nerve tumors treated with a cone‐based linear accelerator system, which allows for fewer anesthetic events and reduces the volume of normal brain in the field.
Methods
This study was a retrospective review of medical records for dogs treated at the UC Davis William R. Pritchard Veterinary Medical Teaching Hospital between 2009 and 2013. Animals were cared for in accordance with hospital policies. Some of these dogs were also included in a separate publication (JVIM‐SA‐15‐370). Dogs were included that underwent SRT therapy for a suspected trigeminal nerve tumor based on magnetic resonance imaging (MRI). Cerebrospinal fluid analyses were available for 5/8 dogs: 3/8 did not reveal abnormalities, 1 revealed minimal lymphocytic pleocytosis, and 1 revealed slight mixed leukocytosis. Abdominal ultrasound was performed in 5/8 dogs, which did not reveal abnormalities except in 1 dog that had 2 small splenic nodules and mild hepatomegaly. Thoracic radiographs were available for and did not reveal abnormalities in 4/8 cases.
Initial MR images were obtained from referring facilities and did not have standardized protocols. T1, T2, and T1 contrast‐enhanced images were available for all studies. Repeat MRI was performed approximately 12 and 24 weeks after treatment. Repeat MRI was performed with a 1.5T system1 with dogs positioned in sternal recumbency. Sequences included noncontrast transverse T1, T2, PD FLAIR, and contrast (0.2 mL/kg)2 T1 transverse and sagittal images.
Computed Tomography (CT) imaging was acquired with a helical CT scanner.3 Briefly, animals were anesthetized by means of propofol4 and maintained on a constant rate infusion to effect. All dogs had endotracheal intubation and were ventilated with 100% oxygen. Animals were positioned in sternal recumbency in the stereotactic head frame, head cushion, and body cushion. The vacuum‐locked bag5 was used from the shoulders caudally to position the dog in the commercially available frameless stereotactic mask system sled.6 A commercially available customized head support pillow7 was also used. The thermoplastic mask8 was molded to the dog's head to immobilize the skull. The stereotactic target positioner box was locked onto the sled, and the CT origin coordinates were aligned to the box with the CT positioning lasers.10
A noncontrast CT with 120 kV and 150 mA with 1 mm collimation was performed. Contrast‐enhanced images with 1 mm collimation were acquired with iodinated contrast medium9 at a dose of 740 mg I/kg. The scans encompassed the entire skull, and the field of view included the positioning frame for noncontrast images.
The oval foramen was measured at the largest diameter on each side with the CT images, and a ratio of affected to unaffected diameter was calculated. The oval foramen was considered enlarged if the ratio was >1:1. Tumors were classified as having extracranial nerve extension if a mass was visible along 1 or more branches outside the skull. Enlargement of the nerve roots was also noted.
After the CT scan was completed, Digital Imaging and Communications in Medicine (DICOM) images were imported into the treatment planning system.10 The CT images were localized based on Z‐bars located on the stereotactic box; the Z‐bars serve to identify the unique position of each CT slice as it relates to the stereotactic box. The MRI images were fused to the CT images by planning software. Relevant anatomic volumes were contoured. The gross tumor volume (GTV) included all visible tumor or suspect tumor‐related contrast enhancement on CT and MRI, and the clinical target volume (CTV) was defined as the GTV without any additional margin. The planning target volume (PTV) was created by adding a 2 mm margin around the CTV to account for uncertainties of mechanical positioning (e.g., beam geometry, collimator leaf width, and light‐radiation field coincidence), quality of portal imaging, and interfraction and intrafraction movement of the dog's head.14, 15 Relevant organs at risk (OAR) were contoured, including the brain, brain minus PTV (region of brain not included in the PTV), brainstem, eyes, and inner ears, based on clinician preferences for plan optimization (Fig 1A). A radiation plan was created with 1 or more isocenters with varying numbers and lengths of arcs, and tissue heterogeneity correction was employed. When more than 1 isocenter or more than 1 arc were used, the isocenters and arcs were differently weighted to optimize the radiation dose distribution in the target volume and minimize radiation exposure to OAR. Skin bolus was not used. Where possible, the mean dose to one or both inner ears was limited to <14 Gy, and the mean dose to the brainstem was limited to <12.5 Gy.16 Dose volume histograms were used to assess for plan quality, and plans were normalized to ideally achieve 90–95% of the PTV receiving 100% of the prescribed dose (prescribed dose = 24 Gy; 8 Gy fractions) (Fig 1B).
Figure 1.
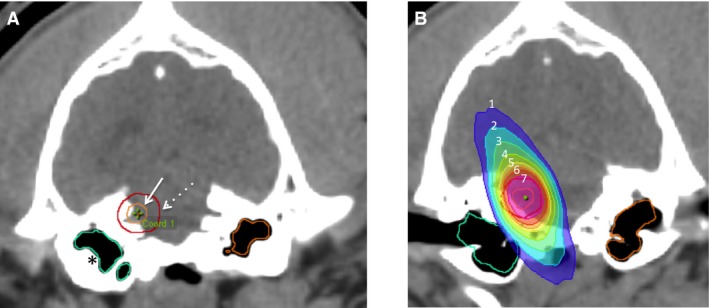
Example contouring and treatment isodose distribution. (A) GTV (solid arrow), PTV expansion (dashed arrow), and inner ear contours (asterisks). (B) Isodose distribution for the radiation plan (isodose lines represent percentage of prescribed dose: 1: 30%, 2: 40%, 3: 80%, 4: 90%, 5: 95%, 6: 100%, 7: 107%).
After the plan was approved by the clinician, a quality assurance test was created using a tissue equivalent virtual water plastic phantom molded from a canine head and embedded with a canine skull. The phantom had previously been cut dorsally, dividing the head into 2 approximately equal coronal halves (Fig 2A,B). Radiochromic, self‐developing film11 was placed in between the 2 halves. A hole bored through the phantom 1 cm above the film plane was used to place a chamber12 for point dose measurement (Fig 2C). The plan was calculated for and delivered to the phantom, and the dose delivered relative to the calculated dose was assessed by quality assurance software13 (Fig 2D,E). The point dose calibration was acquired by use of a 10 × 10 cm field receiving 100 monitor units (MU) at Dmax in a solid water phantom. Film gamma values, which are an assessment of accuracy of the delivered radiation dose, that were <3% and 2–3 mm different, and point dose values <2% different from treatment planning system‐calculated values were acceptable.
Figure 2.
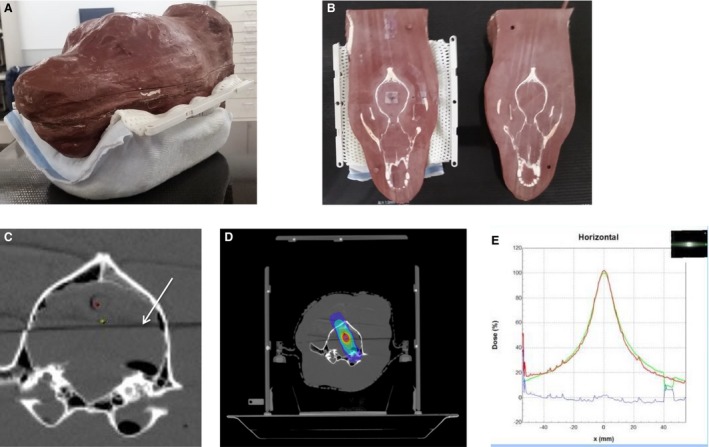
Quality assurance testing, and example contouring and treatment isodose distribution. (A) Tissue equivalent plastic quality assurance phantom. (B) Phantom is cut in half dorsally, revealing the embedded skull. Radiosensitive film is sandwiched between the 2 halves for testing. (C) Axial CT image demonstrating bore for ion chamber (red dot), plan isocenter (green dot), and plane where the phantom is cut in half for film placement for quality assurance (white arrow). (D) Example of a radiation plan transferred to the phantom system, with the same isodose cloud lines as seen in Fig 1. (E) Graph comparing x‐ray–sensitive film dose profile from phantom quality assurance testing to the planned dose. The dose profiles overlap very closely.
Two orthogonal‐view digitally reconstructed radiographs (DRR) were created for each planned isocenter with a 4 × 4 cm setup field placed around the treatment isocenter at 0 (dorsal port) and 90 (right port) for image comparison on treatment days. The images were transferred to the electronic portal imaging software program14 for treatment. BrainLab TaPos (target positioner) grids were computed, printed, and attached to the stereotactic box for head positioning.
In preparation for treatment, the dogs were anesthetized in the same manner as with CT imaging. Anesthetized dogs were placed in the positioning device by the attending radiation oncologist. The linear accelerator laser crosshairs were aligned to the lateral stereotactic box crosshairs for the CT origin. The dorsal box crosshairs were aligned to the machine isocenter crosshairs to affix the box properly to the head frame and the treatment couch. The couch was then moved to the first isocenter for treatment based on the TaPos.
Two orthogonal digital images were acquired with the electronic megavoltage portal‐imaging device and a 6 MV beam.15 Window and leveling values were adjusted to best visualize the bony landmarks on the images. The images were compared with the DRR by measuring the distance between the setup field isocenter and a bony structure on each of the orthogonal images with a digital measuring tool. The couch was adjusted in the cranial‐caudal, lateral, and dorsal‐ventral directions to match the planned isocenter to the machine isocenter by moving the couch the distances measured. Dogs that required adjustment >2 mm were considered poorly positioned; these dogs were repositioned in the mask, realigned to the lasers, another setup image was compared to the DRR, and the couch was adjusted as needed. Once in the correct position, the dog was treated. All dogs were treated with 6 MV photons delivered by a linear accelerator16 with coplanar arcs delivered to a single isocenter or noncoplanar arcs with multiple isocenters. The subsequent treatments were repeated on 2 consecutive days or every 48 hours for 2 more treatments.
All dogs were placed on approximately 0.5 mg/kg PO daily prednisone before or on the first treatment day. The dogs had recheck visits 2 weeks after radiation, then phone calls or recheck visits were performed every 2 weeks thereafter, with 20–50% reductions in prednisone dose at each contact until the dogs were no longer on prednisone. A grant was available for 6 dogs to have MRI imaging approximately 3 months after SRT treatment. Three dogs also received MRI imaging approximately 6 months after treatment. One dog had further MRI imaging 1 year after treatment. The dogs were followed up either with phone calls or recheck visits until death or until last contact before publication submission.
All graphs and statistical analyses were made by commercially available statistics programs.17 The Kaplan‐Meier method was used to calculate median survival times and 95% confidence intervals. To look for estimated differences in survival between groups, a log rank test was used. Disease‐specific survival involved censoring of dogs that died as a result of accident‐related death. A P value <.05 was considered statistically significant.
Results
Eight dogs undergoing SRT therapy for suspected intracranial peripheral nerve sheath tumors met the inclusion criteria. Five dogs were purebred, including 1 Belgian malinois, 1 basenji, 1 Australian shepherd, and 2 golden retrievers. Three dogs were mixed breed, including 1 poodle/cocker spaniel mix and 2 pit bull terrier mixes. Five dogs were female spayed, 2 dogs were male neutered, and 1 dog was male intact, with a median age of 9.5 years at diagnosis (range 5–15 years). The median weight was 29.4 kg (range 8–41.7 kg). All dogs had contrast‐enhancing trigeminal nerve masses: 5 dogs (63%) had left and 3 dogs (37%) had right trigeminal involvement. No biopsies were performed.
One dog had previously been diagnosed with myxomatous mitral regurgitation and bilateral cataracts, 1 had a history of complete excision of a mast cell tumor, 1 one had mild aortic valve stenosis. No abnormalities were detected in the serum of 6/8 dogs, with the other 2 dogs having mildly elevated BUN (1), moderately elevated ALT (1), and low T4 (1). No abnormalities were detected on CBC in 5/8 dogs, with 3/8 dogs having either mild eosinophilia (1) or mild nonregenerative anemia (2). Four of 8 dogs had thoracic radiographs before treatment, which did not reveal abnormalities. Abdominal ultrasound was performed in 5/8 dogs; 4 did not reveal any abnormalities, and 1 revealed hepatomegaly with mottled hepatic appearance consistent with prednisone administration, and 2 hyperechoic splenic nodules suggestive of myelolipomas. Five dogs had a cerebrospinal fluid tap: 3 did not reveal abnormalities, 1 had mild lymphocytic pleocytosis, and 1 had mild, mixed leukocytosis.
All dogs had some degree of temporalis or masseter muscle atrophy during the study. Four dogs presented with enophthalmos, 2 presented with anisocoria, and 2 presented with reduced corneal sensation. At least one of the dogs had the following clinical signs: reduced postural reflexes, proprioceptive placement deficits, altered mentation, reduced facial sensation, reduced palpebral reflex, and reduced PLR.
All dogs had an MRI and CT scan before treatment and began treatment within 7 days of the CT scan. Six dogs began treatment within 3 weeks of diagnosis by MRI imaging, 1 dog began treatment within 5 weeks, and 1 began treatment within 9 weeks.
Based on radiation planning contouring, the treatment volumes were as follows: median GTV = 1.89 cm3, mean GTV = 2.45 cm3 (range 0.143–4.738 cm); median PTV = 5.82 cm3, mean PTV = 6.36 cm3 (range 0.749–11.04 cm3). Treatment plans used 1–4 isocenters and 2–11 arcs of radiation with cone field diameter ranging from 10 to 25 mm. PTV coverage for all plans ranged from 75.5% to 97.2% of the PTV receiving 100% of the prescribed dose, based on limiting the dose to normal organs at risk. The minimum dose to any PTV was 11.12 Gy, and the maximum dose to any PTV was 47.54 Gy. The mean PTV dose ranged from 25.92 to 30.73 Gy for all plans. There were no treatment interruptions or deviations from protocol. More detailed dose summaries are listed in Tables S1 and S2 as recommended by the Veterinary Radiation Therapy Oncology Group for treatment reporting.17
Four dogs were treated with 8 Gy × 3 fractions on consecutive days, whereas 4 dogs received 8 Gy × 3 fractions every other day. One dog was reported to have inappetance transiently after treatment. No other immediate adverse effects were noted. Six dogs had a recheck MRI approximately 3 months after SRT. Three dogs had a follow‐up MRI 6 months after SRT.
All images acquired before and after treatment were reviewed by a single radiologist (Fig 3). The imaging examinations included initial MRI (8) and CT (8), a second MRI (8), a second CT (3), and a third MRI (4). Muscle atrophy of the regional musculature was seen in 7/8 dogs on presentation, and the remaining dog developed atrophy on the second MRI. Of the dogs with initial muscle atrophy, 1/7 resolved after SRT therapy; however, the atrophy returned by the third MRI.
Figure 3.
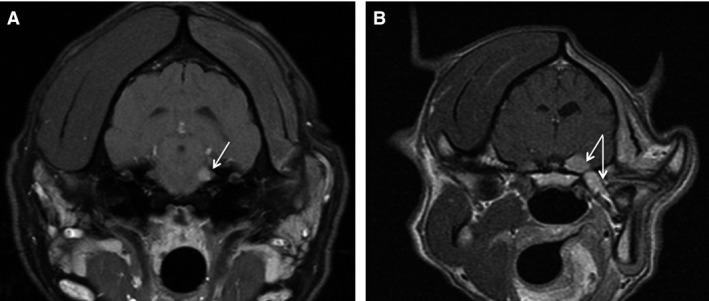
Transverse MRI images of the brain. (A) Dog #7 presented with moderate muscle atrophy. Fat‐saturated contrast T1 MRI images revealed a small extra‐axial lesion in the region of the left trigeminal nerve. After treatment, the mass was no longer visible on MRI (not shown). (B) Dog # 1 presented with severe muscle atrophy. Contrast‐enhanced T1 images MRI revealed a left extra‐axial contrast‐enhancing mass with involvement of the extracranial trigeminal nerve extending to the left mandibular branch. The dog was euthanized at 855 days after seizure and diagnosis of sarcoma in the mouth that was possible progression.
The imaging characteristics were as follows: 5/8 masses were T1 isointense and 3/8 were T1 hypointense; 6/8 masses were hyperintense on T2 and FLAIR sequences; and 1/8 were each isointense and mixed intensity on T2 and FLAIR sequences. All masses were strongly, uniformly contrast enhancing on contrast‐enhanced MR images. In total, 3/8 masses had peripheral enhancement after SRT treatment.
All dogs had intracranial masses in the region of the cerebellopontine angle with variable compression of the brainstem. Three of 8 dogs had enlargement of the oval foramen on the first CT (ratios 1.37:1, 1.89:1, 1.71:1). The trigeminal nerve terminates in 3 major branches outside of the cranial vault: the mandibular, maxillary,and ophthalmic nerves.11, 18 Of the dogs with extracranial involvement, 2/3 dogs had extracranial masses along the mandibular branch on presentation, and 1/3 dogs developed a mass of that branch on the second MRI. The mandibular branch was enlarged in 4/8 dogs, the maxillary branch was enlarged in 3/8 dogs, and the ophthalmic branch was enlarged in 1/8 dogs. Initially 1 dog had brain edema on MRI, but 4/8 dogs had brain edema after SRT therapy. Seven of 8 dogs had brainstem compression by the mass. The dog without brainstem compression had a mass that was not visualized on MRI after treatment. One dog had bulla effusion on the ipsilateral side before treatment, and 2 more dogs developed bulla effusion after treatment. The mass size increased on a subsequent MRI in 3/8 dogs, remained static in size in 1/8, became smaller in 3/8, and had variable regions of increase and decrease in size in 1/8 dogs. Other imaging findings after treatment included multifocal small hemorrhages (n = 1), meningeal extension (n = 1), and an intra‐axial region of edema and enhancement separate from the trigeminal nerve tumor (n = 1).
Regarding clinical outcome, 1 dog was euthanized at 99 days after SRT after being hit by a car, and necropsy revealed necrosis in the region of the tumor with no obvious identifiable tumor. A second dog also had an accident‐related death 257 days after treatment, with no necropsy performed. Four dogs developed seizures that contributed to the decision to euthanize with seizure onset reported at 235, 340, and 855 days after treatment. One of these dogs was euthanized 855 days after treatment when the dog developed seizures; this dog also was diagnosed with a biopsy‐confirmed intraoral sarcoma. Two dogs were euthanized 158 and 324 days after treatment as a result of progression of brainstem neurologic signs and more frequent seizures. One dog was euthanized 745 days after treatment as a result of difficulty controlling seizures. The remaining living dog, at the time of publication, did not have any new clinical brain signs after 1,375 days, although the masseter muscle wasting was more pronounced starting approximately 1,200 days after treatment.
The median disease‐specific survival time was 745 days (95% CI: lower limit 158 days, upper limit 975 days; Fig 4). The median survival time for all dogs without censoring for cause of death was 324 days (95% CI: lower limit 99 days, upper limit 975; Fig 4). There was no statistically significant difference in survival (regardless of censorship) for dogs with the following features: extracranial component (P = .15), enlarged oval foramen on MRI (P = .92), or tumor shrinkage at 3 months based on MRI (P = .52).
Figure 4.
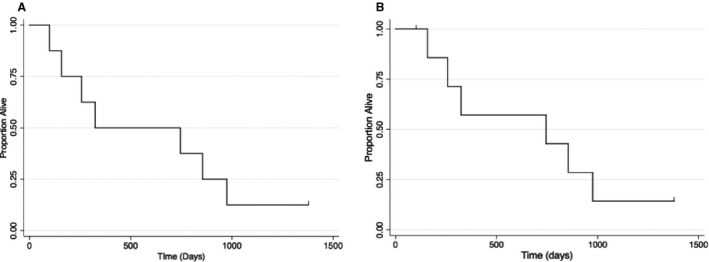
Kaplan‐Meier survival curves. (A) Overall survival for all cases, and (B) disease‐specific survival for dogs receiving SRT therapy for suspected intracranial PNST. Eight dogs were treated with SRT, resulting in an overall median survival of 745 days, and a disease‐specific survival of 324 days. One dog was still alive at the time of publication, and 2 dogs were censored for disease‐specific survival as a result of accident‐related death.
Discussion
Cone‐based SRT was an apparently safe treatment option for suspected intracranial PNST tumors in dogs, with a median disease‐specific survival of 745 days and median survival for all dogs of 324 days. There were few clinically relevant adverse effects seen in this small group of dogs.
Diseases specifically affecting the trigeminal nerve are relatively rare, and tumors are the cause of clinical signs in many cases compared to noncancer causes, with nerve sheath tumors appearing more common than other tumors in this location.12, 19, 20, 21, 22, 23 Although histological differentiation of nerve sheath tumors into the subgroups of schwannoma, neurofibroma, and neurofibrosarcoma is possible, these histological types have a clinically similar presentation and disease course.11 In humans, most peripheral nerve sheath tumors (PNST) are schwannomas and neurofibromas; however, the clinical behavior of intracranial PNST in dogs is more aggressive.11, 24
MRI imaging of PNSTs reveals solitary or lobular masses that are isointense to the brain parenchyma on T1‐weighted (T1W) and T2‐weighted (T2W) images and contrast enhance.23 Typically, there is atrophy of the temporalis and masseter muscles with increased T1W signal.23 Our lesion characteristics were consistent with the previously reported information on suspected intracranial PNST. However, it is not possible to conclude whether the dogs in this study had a particular subgroup of PNST, and necropsy was not available for most dogs. It is possible that some dogs had an infectious or inflammatory lesion with tumor‐like MRI characteristics. Given that biopsy access is difficult in this location, owners often decline biopsy.
The MRI‐based tumor volume enlargement after treatment in some dogs was an unexpected finding. The contrast‐enhancing volume increased in half of dogs, whereas it decreased or was static in the others, with no apparent correlation with outcome. It is possible that these tumors were not responsive to radiation and grew during the time between examinations. However, in human medicine where repeat MRI imaging is commonplace, it is difficult to differentiate tumor progression from pseudprogression caused by tumor necrosis, edema, and secondary inflammation resulting in an apparently larger region of contrast uptake.25 The edema seen after SRT is not specific for a particular pathological process and could be because of tumor progression, tumor necrosis, or radiation treatment.
Several dogs had an enlarged oval foramen or involvement of extracranial nerve components. Most dogs had some degree of brainstem involvement. The number of dogs was too small to make conclusions regarding whether extracranial or brainstem involvement results in worse outcomes. However, the dog with the smallest tumor, which was intracranial and not causing brainstem compression, had the longest survival time. In the dog where the sarcoma extended beyond the radiation field and into the mouth, it remains unclear if this extension was originally microscopic and tumor extension along the nerve branch was as a result of a geographic miss. Although less likely, it is possible that the sarcoma arose secondary to radiation treatment.
Historically, 2–4 conformal fields were used for canine brain tumors treated with radiation. In 1 study, the whole brain comprised the PTV, whereas in other studies the PTV was a 4 × 4 cm field.4, 5 Still, other studies have used a CTV based on suspicion of microscopic disease with a PTV of 0.5 cm.1 When treating with a limited numbers of fields and minimal imaging capabilities, large portions of normal brain must be included in the PTV to provide ample coverage of intracranial tumors. Therefore, a compromise must be made among tumor coverage, risk of normal tissue complications, and dose that can be accurately and precisely delivered to the PTV. Because the brain is a late‐responding tissue, and is therefore sensitive to the dose per fraction, most protocols historically employed small doses per fraction over many treatments to treat a relatively large PTV.
Stereotactic radiotherapy provides an alternative to fractionated protocols by avoiding normal tissues altogether. To achieve normal brain sparing, stereotactic irradiation techniques rely upon onboard imaging to ensure proper positioning before each treatment. Thermoplastic masks, vacuum‐locked moldable bags, dental molds for bite blocks, and nonmigrating fiducials imbedded in tumors aid in replicating the dog and PTV positioning for subsequent treatments.26 External mobilization devices help position the body for treatment, and several immobilization systems have previously been evaluated for radiotherapy of the head and neck in dogs.10, 27, 28, 29, 30, 31, 32
There are several different treatment units and techniques available for delivering stereotactic radiation. Standalone stereotactic systems, such as Gamma Knife, where a stereotactic frame with multiple radioactive cobalt sources is affixed around the head, have been employed in human medicine. Another system, Cyberknife, consists of a linear accelerator mounted on a gantry that allows for treatment from multiple, noncoplanar, angles around the dog. For linear accelerator‐based SRT, rather than using a cone system on a linear accelerator as we had applied in this study, a small leaf‐width MLC can instead be used to create stereotactic fields delivered from multiple angles. Intensity‐modulated radiotherapy, whereby the MLC leaves move to further modulate the fluence of the beam at each angle treated, can also be used to optimize the delivered dose.
Three veterinary studies have described stereotactic methods for intracranial tumors. In 1 study, 3 dogs with either meningioma or oligodendroglioma were treated with SRT with a frame system attached to the dog's head for simulation imaging, and treatment planning and delivery occurred under the same anesthetic event. Dogs were treated with 5–9 noncoplanar arcs resulting in a dose of 10–15 Gy to the 60–80% isodose line, and dogs were positioned with a bite block. Dogs survived 13 months – 4 years after SRT in this case series.9 A more recent SRT publication involving suspect meningiomas by a single isocenter planning technique with intensity‐modulated radiotherapy (IMRT) resulted in a median survival of 561 days for all causes of death.33 Another study treated 51 dogs with various tumors with SRT resulting in a median survival time of 399 days. The median dose was 15 Gy, and dogs were treated to the 60–80% isodose line with 5–21 arcs and 1–8 isocenters.13 As previously mentioned, the median survival for the 3 suspected PNST cases that were not lost to follow‐up in this study was 881 days.
The dogs in this study had an apparently shorter median survival when compared to those from the previously mentioned study involving stereotactic radiation on 3 suspected trigeminal nerve tumors (745 days versus 881 days).13 Although little information is available regarding the survival time with any type of treatment for intracranial PNST, SRT appears to offer a good median survival time. Both early and late clinical effects of radiation appeared uncommon, with seizures and muscle loss seen as possible signs of radiation damage or tumor regrowth. Few dogs were available for necropsy to confirm a presence or absence of radiation effects on the normal brain or tumor regrowth. Two dogs were noted to have ipsilateral bullae effusion on MRI recheck after treatment, but were not clinical for this suspected adverse effect.
Stereotactic radiotherapy techniques not only offer great potential in terms of survival times but they also alleviate some of the logistical and anesthetic challenges in treating dogs with heavily fractionated radiation. Because 20 or more fractions are used for definitive radiotherapy treatments with conformal fields, multiple anesthetic events are required over several weeks. Each event poses a small risk for the animals, and the requirement for fasting each evening before anesthesia might affect nutritional intake. Another problem with conventional radiotherapy is logistical: pet owners might not live near one of the veterinary external beam radiation facilities in the United States, Canada, and Europe.34 Therefore, a recommendation for definitive radiation might prevent some pet owners from pursuing treatment simply because of logistics. Although some studies have assessed more coarsely fractionated protocols with large PTVs that are ultimately more convenient for owners, those protocols carry a higher risk of devastating late radiation adverse effects to brain tissue because of the large amount of normal brain in the radiation field.5 Treatment options like SRT, which might provide similar outcomes to definitive radiation with less anesthetic events, are desirable for treatment of veterinary cancers in general and intracranial tumors in particular.
We began treating dogs every other day (Monday, Wednesday, and Friday) early in our experience with SRT. Once we confirmed that the dogs were not experiencing immediate adverse effects, we treated on consecutive days, which is reflected in the treatment protocols for this study population. Although there is controversy regarding the application of biologic effective dose (BED) calculation to stereotactic protocols, compressing the total dose into fewer days increases the BED, which might improve tumor control.35, 36, 37
Because SRT delivers high doses of radiation to the tumor, it is imperative to minimize the PTV required for treatment to reduce unnecessary dose to neighboring tissues. However, when using planar MV imaging for positioning verification, the PTV must be sufficient to account for uncertainty in positioning based on image detail. We chose a PTV of 2 mm, which is smaller than we require for our head and neck cases with a different immobilization device, and was chosen because of the minimal expected intrafraction motion of the cranial and intracranial structures with the BrainLab positioning system. Certainty in head positioning is now greatly improved with onboard cone‐beam CT (CBCT) imaging available on linear accelerators, and PTV margins could be further reduced with this type of imaging.38
There are limitations to this study. As a relatively uncommon tumor, few dogs with trigeminal tumors are seen and treated with radiation; therefore, our study group was small. There was no control group to indicate the course of disease in untreated dogs with suspected trigeminal nerve tumors. In addition, lack of necropsy information on the dogs makes it difficult to fully assess whether tumor regrowth or late radiation adverse effects occurred, or which tumor type was being treated. MRI imaging of all dogs at multiple time points after radiation would be ideal. However, it remains clear from human studies, where serial MRI is more feasible, that it is difficult to clearly define radiation necrosis versus tumor regrowth, and some dogs can also have suspected radiation necrosis on MRI without apparent clinical signs.39, 40
The use of cone‐based SRT also has certain limitations. When radiation planning requires more than a single cone and isocenter, it is necessary to overlap the edges of fields to maintain tumor coverage. As a result, hotspots occur in the overlapping regions, and these hotspots can approach twice the prescribed dose when the plan has been normalized to provide coverage needed for tumor ablation. Therefore, the conformity index (CI) of a cone‐based planning system, where CI is a quantitative assessment of plan quality and unity suggests that a plan has good PTV coverage, minimal OAR dose, and a homogeneous dose profile, will be low in cases where more than 1 isocenter is employed. As such, we did not use CI as an assessment of plan quality. It is also important to recognize that planning constraints set for one type of stereotactic delivery are not directly applicable to a different planning technique.
In conclusion, cone‐based SRT appears to be a safe treatment option for suspect intracranial PNST cases with the possibility of long‐term survival for some dogs. Further assessment of SRT techniques for intracranial tumors is warranted.
Supporting information
Table S1. Dose to organs at risk (OARs).
Table S2. Radiation planning data: Dose to planning target volume (PTV).
Acknowledgments
The authors acknowledge Sonja Dieterich for her advice on the manuscript. The authors acknowledge Randall Holt, Earl A. Trestrail, Paul Primas, Brooke Jones, Randolph Gordon, and Paul Magee for their assistance in acquiring these data.
This work was not previously presented.
Grant Support: This study was funded in part by the Meadowview Foundation.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was performed at the UC Davis William R. Pritchard Veterinary Medical Teaching Hospital in Davis, CA.
Footnotes
MR Signa LX, General Electric Co., Milwaukee, WI
Magnevist, Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ
Prospeed General Electric Co., Milwaukee, WI or Lightspeed 16 General Electric Co., Milwaukee, WI
Propoflo, Abbott Animal Health, Abbott Park, Illinois
SecureVac, Bionix Development Corporation, Toledo, OH
Brainlab AG, Feldkirchen, Germany
MoldCare pillow, Bionix Development Corporation, Toledo, OH
Brainlab AG, Feldkirchen, Germany
Iopamidol, 370 mg I/ml, Bracco Diagnostics Inc., Princeton, NJ
Iplan version 4.1, BrainLab, Munich, Germany
EBT2, Gafchromic, Ashland, Covington, KY
Standard Imaging, Middleton, WI
FilmQA 2.2.0113, Ashland, Covington, KY
Portal Vision Treatment Acquisition Software Version 7.3, Varian Medical Systems, Palo Alto, CA
Portal Vision aS500 Electronic Portal Imaging Device, Varian Medical Systems, Palo Alto, CA
Clinac 2100, Varian Medical Systems, Palo Alto, CA
STATA 10.0, Stata Corporation, College Station, TX; Microsoft Excel 2008 for Mac, Version 12.1, Microsoft Corporation, Redmond, WA
References
- 1. Keyerleber MA, McEntee MC, Farrelly J, et al. Three‐dimensional conformal radiation therapy alone or in combination with surgery for treatment of canine intracranial meningiomas. Vet Comp Oncol 2015;13(4):385–97. [DOI] [PubMed] [Google Scholar]
- 2. Bley CR, Sumova A, Roos M, Kaser‐Hotz B. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med 2005;19:849–854. [DOI] [PubMed] [Google Scholar]
- 3. Axlund TWMM, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial me‐ ningiomas in dogs: 31 cases (1989–2002). J Am Vet Med Assoc 2002;221:1597–1600. [DOI] [PubMed] [Google Scholar]
- 4. Spugnini EP, Thrall DE, Price GS, et al. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound 2000;41:377–380. [DOI] [PubMed] [Google Scholar]
- 5. Brearley MJ, Jeffery ND, Phillips SM, Dennis R. Hypofractionated radiation therapy of brain masses in dogs: A retrospective analysis of survival of 83 cases (1991–1996). J Vet Intern Med 1999;13:408–412. [DOI] [PubMed] [Google Scholar]
- 6. Van Meervenne S, Verhoeven PS, de Vos J, et al. Comparison between symptomatic treatment and lomustine supplementation in 71 dogs with intracranial, space‐occupying lesions. Vet Comp Oncol 2014;12:67–77. [DOI] [PubMed] [Google Scholar]
- 7. Buatti JM, Bova FJ, Friedman WA, et al. Preliminary experience with frameless stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 1998;42:591–599. [DOI] [PubMed] [Google Scholar]
- 8. Bova FJ, Buatti JM, Friedman WA, et al. The University of Florida frameless high‐precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys 1997;38:875–882. [DOI] [PubMed] [Google Scholar]
- 9. Lester NV, Hopkins AL, Bova FJ, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumors in dogs. J Am Vet Med Assoc 2001;219:1562–1567. [DOI] [PubMed] [Google Scholar]
- 10. Dieterich S, Zwingenberger A, Hansen K, et al. Inter‐ and intrafraction motion for stereotactic radiosurgery in dogs and cats using a modified brainlab frameless stereotactic mask system. Vet Radiol Ultrasound 2015;56(5):563–9. [DOI] [PubMed] [Google Scholar]
- 11. Dewey CW. A Practical Guide to Canine and Feline Neurology, 2nd ed Ames, IA: Wiley‐Blackwell; 2008. [Google Scholar]
- 12. Bagley RS, Wheeler SJ, Klopp L, et al. Clinical features of trigeminal nerve‐sheath tumor in 10 dogs. J Am Anim Hosp Assoc 1998;34:19–25. [DOI] [PubMed] [Google Scholar]
- 13. Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol 2015;13(4):409–23. [DOI] [PubMed] [Google Scholar]
- 14. Hodapp N. The ICRU Report 83: Prescribing, recording and reporting photon‐beam intensity‐modulated radiation therapy (IMRT). Strahlenther Onkol 2012;188:97–99. [DOI] [PubMed] [Google Scholar]
- 15. ICRU . Report 62: Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). Bethesda, MD: ICRU; 1999. [Google Scholar]
- 16. Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keyerleber MA, McEntee MC, Farrelly J, Podgorsak M. Completeness of reporting of radiation therapy planning, dose, and delivery in veterinary radiation oncology manuscripts from 2005 to 2010. Vet Radiol Ultrasound 2012;53:221–230. [DOI] [PubMed] [Google Scholar]
- 18. Evans H, de Lahunta A. Miller's Anatomy of the Dog, 4th ed St. Louis, MO: Elsevier; 2013. [Google Scholar]
- 19. St Clair LE, Safanie AH. Intracranial nerve root tumors; report of a neoplasm (neurofibroma) of the trigeminal nerve in a dog. J Am Vet Med Assoc 1957;131:188–191. [PubMed] [Google Scholar]
- 20. Pumarola M, Anor S, Borras D, Ferrer I. Malignant epithelioid schwannoma affecting the trigeminal nerve of a dog. Vet Pathol 1996;33:434–436. [DOI] [PubMed] [Google Scholar]
- 21. Saunders JH, Poncelet L, Clercx C, et al. Probable trigeminal nerve schwannoma in a dog. Vet Radiol Ultrasound 1998;39:539–542. [DOI] [PubMed] [Google Scholar]
- 22. Cizinauskas S, Lang J, Maier R, et al. Paradoxical vestibular disease with trigeminal nerve‐sheath tumor in a dog. Schweiz Arch Tierheilkd 2001;143:419–425. [PubMed] [Google Scholar]
- 23. Schultz RM, Tucker RL, Gavin PR, et al. Magnetic resonance imaging of acquired trigeminal nerve disorders in six dogs. Vet Radiol Ultrasound 2007;48:101–104. [DOI] [PubMed] [Google Scholar]
- 24. Woertler K. Tumors and tumor‐like lesions of peripheral nerves. Semin Musculoskelet Radiol 2010;14:547–558. [DOI] [PubMed] [Google Scholar]
- 25. Ruzevick J, Kleinberg L, Rigamonti D. Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: Differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev 2014;37:193–201; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubicek LN, Seo S, Chappell RJ, et al. Helical tomotherapy setup variations in canine nasal tumor patients immobilized with a bite block. Vet Radiol Ultrasound 2012;53:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green EM, Forrest LJ, Adams WM. A vacuum‐formable mattress for veterinary radiotherapy positioning: Comparison with conventional methods. Vet Radiol Ultrasound 2003;44:476–479. [DOI] [PubMed] [Google Scholar]
- 28. Hansen KS, Theon AP, Dieterich S, Kent MS. Validation of an Indexed Radiotherapy Head Positioning Device for Use in Dogs and Cats. Vet Radiol Ultrasound 2015;56:448–455. [DOI] [PubMed] [Google Scholar]
- 29. Harmon J, Van Ufflen D, Larue S. Assessment of a radiotherapy patient cranial immobilization device using daily on‐board kilovoltage imaging. Vet Radiol Ultrasound 2009;50: 230–234. [DOI] [PubMed] [Google Scholar]
- 30. Kent MS, Gordon IK, Benavides I, et al. Assessment of the accuracy and precision of a patient immobilization device for radiation therapy in canine head and neck tumors. Vet Radiol Ultrasound 2009;50:550–554. [DOI] [PubMed] [Google Scholar]
- 31. Kippenes H, Gavin PR, Sande RD, et al. Comparison of the accuracy of positioning devices for radiation therapy of canine and feline head tumors. Vet Radiol Ultrasound 2000;41:371–376. [DOI] [PubMed] [Google Scholar]
- 32. Mayer MN, Waldner CL, Elliot KM, Sidhu N. Comparison of interfractional variation in canine head position using palpation and a head‐repositioning device. Vet Radiol Ultrasound 2010;51:472–476. [DOI] [PubMed] [Google Scholar]
- 33. Griffin LR, Nolan MW, Selmic LE, et al. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet Comp Oncol 2014. Dec 18. doi: 10.1111/vco.12129. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 34. Farrelly J, McEntee MC. A survey of veterinary radiation facilities in 2010. Vet Radiol Ultrasound 2014;55(6):638–43. [DOI] [PubMed] [Google Scholar]
- 35. Brown JM, Carlson DJ, Brenner DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non‐small cell lung cancer. In reply to Rao et al. Int J Radiat Oncol Biol Phys 2014;89:693–694. [DOI] [PubMed] [Google Scholar]
- 36. Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014;32:2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Timmerman RD, Solberg T, Kavanagh B, Lo S. Stereotactic techniques for lung cancer treatment In: Pass H, Carbone D, Johnson D, Minna J, Scagliotti G, Turrisi A, eds. Principles & Practice of Lung Cancer: The Official Reference Text of the IASLC, 4th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 38. Yoshikawa H, Harmon JF, Custis JT, Larue SM. Repeatability of a planning target volume expansion protocol for radiation therapy of regional lymph nodes in canine and feline patients with head tumors. Vet Radiol Ultrasound 2012;53:667–672. [DOI] [PubMed] [Google Scholar]
- 39. Chernov MF, Ono Y, Abe K, et al. Differentiation of tumor progression and radiation‐induced effects after intracranial radiosurgery. Acta Neurochir Suppl 2013;116:193–210. [DOI] [PubMed] [Google Scholar]
- 40. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys 2013;87:449–457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dose to organs at risk (OARs).
Table S2. Radiation planning data: Dose to planning target volume (PTV).


