Abstract
Aims
To evaluate the efficacy and safety of dulaglutide 1.5 and 0.75 mg in elderly patients (aged ≥65 years) with type 2 diabetes (T2D) in six phase III clinical trials.
Methods
Patients were grouped into two age groups: ≥65 and <65 years. Pooled analysis for glycated haemoglobin (HbA1c) change from baseline, percentage of patients achieving HbA1c targets, and gastrointestinal tolerability were evaluated at 26 weeks for each dulaglutide dose. Change in weight from baseline and rates of hypoglycaemia were evaluated for each individual study.
Results
A total of 958 of 5171 (18.5%) patients were aged ≥65 years. The reductions in HbA1c were similar between age groups for dulaglutide 1.5 mg‐treated patients {least squares [LS] mean for patients aged ≥65 years: −1.24 [95% confidence interval (CI) −1.36, −1.12] and for patients aged <65 years: −1.29 [95% CI −1.38, −1.20]} and for dulaglutide 0.75 mg‐treated patients [LS mean for patients aged ≥65 years: −1.16 (95% CI −1.29, −1.03) and for patients aged <65 years: −1.10 (95% CI −1.19, −1.01)] at 26 weeks. The percentages of patients who achieved HbA1c targets of <7, <8 or <9% were also similar in the two groups with both dulaglutide doses. Patients aged ≥65 years had similar weight change to patients aged <65 years. Severe hypoglycaemic events were infrequent. A similar incidence of gastrointestinal adverse events was observed in each age group with both dulaglutide doses.
Conclusion
Both dulaglutide doses were well tolerated, with similar efficacy in patients with T2D aged ≥65 years to those aged <65 years. Dulaglutide can be considered a safe and effective treatment option for use in older adults.
Keywords: dulaglutide, type 2 diabetes
Introduction
As individuals age, the occurrence of diabetes increases, and it is estimated that within the next 15 years, >82 million patients with type 2 diabetes (T2D) in developed countries will be aged ≥65 years 1. Caution should be exercised when choosing therapy for T2D in older patients because of comorbidities such as renal impairment, neuropathy and cognitive dysfunction, and treatment regimens, when instituted, should take into consideration the risks of hypoglycaemia, heart failure, renal dysfunction, bone fractures and drug interactions 2, 3. Functional impairments, including cognitive decline, peripheral neuropathy, vision and hearing impairments, muscle atrophy, poor posture and balance problems, should also be taken into account when choosing a treatment option 4, 5, 6, 7, 8, 9, 10. Amongst all these concerns, however, prevention of hypoglycaemia is the most important because the consequences of low blood glucose can be catastrophic for the elderly 11. While hypoglycaemia should be avoided whenever possible, suboptimum treatment of hyperglycaemia resulting in prolonged high blood glucose levels can in itself worsen cognitive impairment, renal dysfunction and neuropathy. With this in mind, guidelines recommend choosing drugs with a low risk of hypoglycaemia, but also recommend higher glycated haemoglobin (HbA1c) targets of <8 or even <9% 7, 12, 13 compared with the non‐elderly populations, for whom a target of <7% is considered optimal 2, 3.
Sulphonylureas, because of their association with unregulated insulin release, increase the risk of hypoglycaemia 14, 15 and are not a good choice for the elderly. Insulin therapy, while effective, has a very high potential for causing hypoglycaemia and can be challenging in older patients who may have poor vision, arthritis or cognitive dysfunction 16.
Incretin‐based therapies including dipeptidyl peptidase‐4 inhibitors and glucagon‐like peptide‐1 (GLP‐1) receptor agonists stimulate insulin secretion in a glucose‐dependent manner resulting in a lower risk of hypoglycaemia when used as monotherapy or in combination with agents that do not increase insulin levels 17, 18, 19, 20, and could therefore be a good alternative for the elderly.
Dulaglutide is a human GLP‐1 receptor agonist, with a half‐life of ∼5 days allowing once‐weekly dosing 21, 22. It is administered with a single‐use pen with no requirement for reconstitution or dialing of a dose 23. It is not renally excreted and pharmacokinetic studies have shown that neither age nor renal function affect its actions, thus no dose adjustment is required in these settings 24.
Dulaglutide has been studied in six phase III clinical trials in 5171 adult patients aged 19–87 years across the T2D treatment continuum: as monotherapy; as add‐on to one or two oral antihyperglycaemic medications; and in combination with prandial insulin. The results of these trials have shown that treatment with dulaglutide 1.5 mg was superior in achieving glycaemic control in head‐to‐head comparisons at the primary endpoint with metformin 25, sitagliptin 26, insulin glargine 27, 28, and exenatide twice daily 29; and non‐inferior to liraglutide 30; however, these phase III published clinical articles do not report the effect of dulaglutide specifically for patients aged ≥65 years.
The results reported in the present analysis include the pooled and individual study data on the efficacy and safety of dulaglutide 1.5 and 0.75 mg, administered once weekly in patients aged ≥65 years, from the six Assessment of Weekly AdministRation of LY2189265 [dulaglutide] in Diabetes (AWARD) clinical trials 25, 26, 27, 28, 29, 30. These are then compared with the efficacy and safety data of both the doses in patients aged <65 years.
Materials and Methods
Design of the AWARD Clinical Trial Programme
All the AWARD trials were designed to evaluate the efficacy and safety of dulaglutide in adult patients with T2D, with primary endpoints of 26 or 52 weeks, depending on the individual study 25, 26, 27, 28, 29, 30. The objectives of these trials were to evaluate the superiority of HbA1c reduction from baseline compared with placebo and the non‐inferiority/superiority to active comparators (Table S1, Supporting Information).
Statistical Analysis
In the present analysis we assessed the efficacy and safety of dulaglutide 1.5 mg and dulaglutide 0.75 mg in patients with T2D aged ≥65 and <65 years. For the overall analysis, elderly patients were defined as those aged ≥65 years 7; however, given the increasing prevalence of patients aged ≥75 years, efficacy in HbA1c change from baseline was also analysed for this age group. The analysis was performed on the intention‐to‐treat population (randomized patients who received at least one dose of study medication). Analyses of efficacy measures and hypoglycaemia excluded observations after start of rescue therapy. All analyses were conducted at 26 weeks because it was the common time point for all of the AWARD studies. Efficacy measures of HbA1c change from baseline and percent of patients achieving HbA1c goals of <7, <8 and <9% were analysed using pooled data from the six trials for each dulaglutide dose. The analysis of pooled data for change in HbA1c was conducted using analysis of covariance (ancova), including study, country, treatment, age group and age group by treatment interaction, and with baseline value as covariate. Analyses of change in HbA1c and weight for individual studies were carried out using ancova, including country, treatment, age group, age group by treatment interaction and study‐specific stratification factors, and with baseline value as covariate. The last observation was carried forward for missing data. Documented symptomatic hypoglycaemia (plasma glucose ≤3.9 mmol/l) and severe hypoglycaemia (defined as requiring assistance) were analysed by individual study only. Summative data of all gastrointestinal adverse events were pooled by dulaglutide treatment (1.5 and 0.75 mg) across studies.
Results
Baseline Characteristics
Across the six phase III studies, a total of 5171 patients (age ≥65 years, n = 958; age <65 years, n = 4213) were included in this analysis, of whom 1719 received dulaglutide 1.5 mg and 1417 received dulaglutide 0.75 mg treatment. Overall, 93 of the patients (1.8%) were aged ≥75 years (dulaglutide 1.5 mg, n = 23; dulaglutide 0.75 mg, n = 25; comparator and placebo arms, n = 45). The mean age of all patients was 56.2 ± 9.9 years, 2553 patients (49%) were female, and the mean body mass index was 32.4 ± 5.2 kg/m2. The mean duration of diabetes was 8.0 ± 6.2 years and the mean baseline HbA1c was 8.1 ± 1.1%. A summary of pooled baseline characteristics and patient demographics with dulaglutide 1.5 mg or dulaglutide 0.75 mg by age group is shown in Table 1. The difference in mean age between groups was ∼17 years for both dulaglutide doses, there were slightly fewer women in the ≥65 years age group, patients aged ≥65 years weighed slightly less than those aged <65 years, patients aged ≥65 years had a longer duration of diabetes (3–4 years longer), and patients aged ≥65 years were more likely to have used insulin.
Table 1.
Baseline characteristics and demographics.
| Variable | Dulaglutide 1.5 mg* | Dulaglutide 0.75 mg† | ||
|---|---|---|---|---|
| Age ≥65 years | Age <65 years | Age ≥65 years | Age <65 years | |
| n = 318 | n = 1401 | n = 258 | n = 1159 | |
| Female, n (%) | 138 (43.4) | 716 (51.1) | 121 (46.9) | 592 (51.1) |
| Age, years | 69.8 (3.7) | 53.1 (8.1) | 69.9 (3.7) | 53.4 (8.0) |
| Weight, kg | 87.5 (16.3) | 91.7 (19.2) | 88.5 (17.5) | 90.6 (19.3) |
| BMI, kg/m2 | 31.5 (4.8) | 32.7 (5.3) | 32.0 (5.0) | 32.4 (5.4) |
| HbA1c, % | 8.0 (1.0) | 8.1 (1.1) | 8.0 (1.1) | 8.1 (1.1) |
| FBG, mmol/l | 9.4 (2.8) | 9.1 (2.9) | 8.9 (2.6) | 9.0 (2.8) |
| Duration of diabetes, years | 11.3 (7.9) | 7.2 (5.6) | 10.5 (7.2) | 7.6 (5.8) |
| Previous oral antidiabetic medication use, n (%) | ||||
| No oral antidiabetic medication | 37 (11.6) | 274 (19.6) | 42 (16.3) | 261 (22.5) |
| 1 oral antidiabetic medication | 111 (34.9) | 550 (39.3) | 67 (26.0) | 311 (26.8) |
| >1 oral antidiabetic medication | 93 (29.2) | 359 (25.6) | 73 (28.3) | 370 (31.9) |
| Insulin + oral antidiabetic medication(s) | 77 (24.2) | 218 (15.6) | 76 (29.5) | 217 (18.7) |
BMI, body mass index; FBG, fasting blood glucose; HbAlc, glycated haemoglobin.
All data are presented as mean (standard deviation) unless otherwise indicated. No formal statistical test of baseline differences was performed.
Pooled data from AWARD 1 through 6 clinical trials.
Pooled data from AWARD 1 through 5 clinical trials.
Efficacy
Change in HbA1c from Baseline
At 26 weeks, both older (≥65 years) and younger (<65 years) patients experienced similar HbA1c reduction from baseline in the pooled analysis both for dulaglutide 1.5 mg [least squares (LS) mean for age group ≥65 years: −1.24; (95% CI −1.36, −1.12); LS mean for age group <65 years: −1.29 (95% CI −1.38, −1.20) and for dulaglutide 0.75 mg [LS mean for age group ≥65 years: −1.16; (95% CI −1.29, −1.03) and for age group <65 years: −1.10 (95% CI −1.19, −1.01); Figure 1]. The individual study analysis for HbA1c change from baseline to 26 weeks (Figure 2) showed a similar HbA1c reduction to that in the pooled analysis (Figure 1). A similar HbA1c reduction from baseline with the pooled analysis for dulaglutide 1.5 mg [LS mean −1.27 (95% CI −1.63, −0.92)] and dulaglutide 0.75 mg [LS mean −1.21 (95% CI −1.56, −0.87)] was observed for patients aged ≥75 years.
Figure 1.
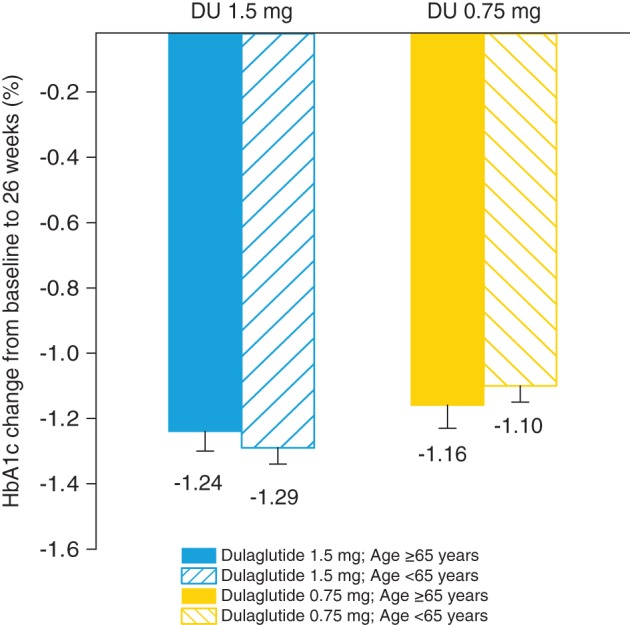
Pooled analysis of glycated haemoglobin (HbA1c) change from baseline to 26 weeks. HbA1c change from baseline to 26 weeks for pooled analysis from AWARD‐1 through AWARD‐6 for dulaglutide 1.5 mg and AWARD‐1 through AWARD‐5 for dulaglutide 0.75 mg. Data presented as least squares means and standard errors. DU, dulaglutide.
Figure 2.
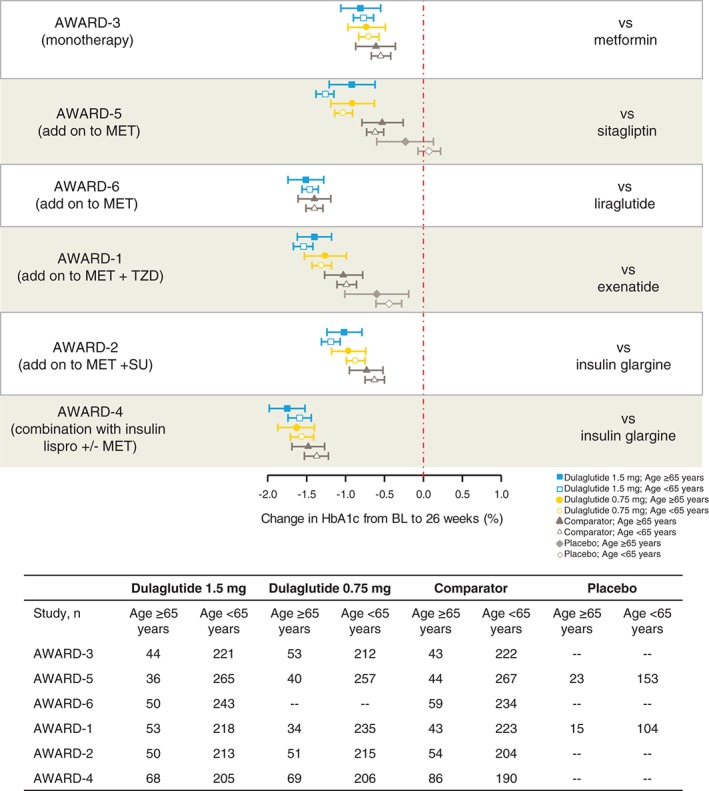
Glycated haemoglobin (HbA1c) change from baseline to 26 weeks by individual study. Data presented as LS means and 95% CIs. AWARD, Assessment of Weekly AdministRation of LY2189265 (dulaglutide) in Diabetes; BL, baseline; HbAlc, glycated haemoglobin; MET, metformin; SU, sulphonylurea; TZD, thiazolidinedione; yrs, years.
Percentage Achieving HbA1c Targets <7,<8 and <9%
At 26 weeks, the percentage of patients who achieved the HbA1c targets of <7, <8 or <9% was similar in the older and younger patients with both dulaglutide doses from the pooled analysis (Figure S1A–C, Supporting Information). For patients aged ≥65 years versus those aged <65 years the results were as follows: HbA1c <7%: dulaglutide 1.5 mg: 67.8 versus 65.4% and dulaglutide 0.75 mg: 64.0% versus 58.7%; HbA1c <8%: dulaglutide 1.5 mg: 89.4% versus 87.3% and dulaglutide 0.75 mg: 87.4% versus 84.4%; HbA1c <9%: dulaglutide 1.5 mg: 96.0% versus 96.2% and dulaglutide 0.75 mg: 96.8% versus 94.7%. Data from the individual study analysis support the pooled data (not shown) 25, 26, 27, 28, 29, 30.
Weight Change from Baseline
Individual study analysis from baseline to 26 weeks showed a similar effect on weight for dulaglutide 1.5 mg or dulaglutide 0.75 mg in older and younger patients (Figure 3).
Figure 3.

Change in weight from baseline to 26 weeks by individual study. Data presented as least squares means and 95% confidence intervals. AWARD, Assessment of Weekly AdministRation of LY2189265 (dulaglutide) in Diabetes; BL, baseline; MET, metformin; SU, sulphonylurea; TZD, thiazolidinedione.
Safety
Hypoglycaemia
The incidence of documented symptomatic, asymptomatic and nocturnal hypoglycaemia (plasma glucose ≤3.9 mmol/l) with dulaglutide was low and similar for older and younger patients across all non‐insulin comparator studies (AWARD‐1, ‐3, ‐5 and ‐6; Table 2). For the insulin comparator studies (AWARD‐2 and ‐4), higher incidences of documented symptomatic, asymptomatic and nocturnal hypoglycaemia were observed with dulaglutide treatments; however, results were similar for either age group (Table 2). Severe hypoglycaemia was infrequent with a total of 25 episodes across the 6 trials:12 episodes occurred in dulaglutide‐treated patients (7 in the 1.5‐mg dose group; 5 in the 0.75‐mg dose group), 1 occurred in a patient treated with exenatide twice daily in AWARD‐1, and 12 episodes occurred in insulin glargine‐treated patients in AWARD‐2 and ‐4. Five of the 12 episodes with dulaglutide were in patients aged ≥65 years (3 in the 1.5‐mg dose group and 2 in the 0.75‐mg dose group), and all were observed in AWARD‐4 with patients on concomitant therapy of insulin lispro with/without metformin. The seven episodes in patients aged <65 years were: dulaglutide 1.5 mg, one patient in AWARD‐2 and three patients in AWARD‐4; dulaglutide 0.75 mg, three patients in AWARD‐4.
Table 2.
Number (%) of patients reporting hypoglycaemia (plasma glucose ≤3.9 mmol/l) at 26 weeks.
| Study (concomitant therapy) Type of hypoglycaemia | Age ≥65 years | Age <65 years | ||||
|---|---|---|---|---|---|---|
| N* | n† (%) | Rate/year‡ | N* | n† (%) | Rate/year‡ | |
| AWARD‐1 (metformin + pioglitazone) | ||||||
| Documented symptomatic | ||||||
| Dulaglutide 1.5 mg | 54 | 2 (3.7) | 0.07 | 225 | 12 (5.3) | 0.25 |
| Dulaglutide 0.75 mg | 37 | 1 (2.7) | 0.05 | 243 | 12 (4.9) | 0.20 |
| Exenatide twice daily | 45 | 4 (8.9) | 0.54 | 231 | 27 (11.7) | 1.17 |
| Placebo | 20 | 0 (0.0) | 0.00 | 121 | 2 (1.7) | 0.07 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 54 | 5 (9.3) | 0.26 | 225 | 11 (4.9) | 0.17 |
| Dulaglutide 0.75 mg | 37 | 3 (8.1) | 0.21 | 243 | 13 (5.3) | 0.75 |
| Exenatide twice daily | 45 | 4 (8.9) | 0.27 | 231 | 18 (7.8) | 0.39 |
| Placebo | 20 | 1 (5.0) | 1.30 | 121 | 2 (1.7) | 0.08 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 54 | 2 (3.7) | 0.11 | 225 | 2 (0.9) | 0.05 |
| Dulaglutide 0.75 mg | 37 | 2 (5.4) | 0.79 | 243 | 5 (2.1) | 0.09 |
| Exenatide twice daily | 45 | 2 (4.4) | 0.13 | 231 | 14 (6.1) | 0.25 |
| Placebo | 20 | 1 (5.0) | 1.30 | 121 | 1 (0.8) | 0.08 |
| AWARD‐3 (none) | ||||||
| Documented symptomatic | ||||||
| Dulaglutide 1.5 mg | 45 | 2 (4.4) | 3.29 | 224 | 7 (3.1) | 0.08 |
| Dulaglutide 0.75 mg | 54 | 2 (3.7) | 0.15 | 216 | 11 (5.1) | 0.18 |
| Metformin | 44 | 1 (2.3) | 0.05 | 224 | 9 (4.0) | 0.10 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 45 | 2 (4.4) | 0.34 | 224 | 14 (6.3) | 0.38 |
| Dulaglutide 0.75 mg | 54 | 5 (9.3) | 0.48 | 216 | 12 (5.6) | 0.30 |
| Metformin | 44 | 4 (9.1) | 0.51 | 224 | 12 (5.4) | 0.13 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 45 | 0 (0.0) | 0.00 | 224 | 3 (1.3) | 0.03 |
| Dulaglutide 0.75 mg | 54 | 3 (5.6) | 0.11 | 216 | 4 (1.9) | 0.08 |
| Metformin | 44 | 1 (2.3) | 0.05 | 224 | 2 (0.9) | 0.04 |
| AWARD‐5 (metformin) | ||||||
| Documented Symptomatic | ||||||
| Dulaglutide 1.5 mg | 37 | 2 (5.4) | 0.21 | 267 | 15 (5.6) | 0.27 |
| Dulaglutide 0.75 mg | 40 | 1 (2.5) | 0.15 | 262 | 7 (2.7) | 0.12 |
| Sitagliptin | 44 | 1 (2.3) | 0.05 | 271 | 9 (3.3) | 0.11 |
| Placebo | 23 | 0 (0.0) | 0.00 | 154 | 2 (1.3) | 0.09 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 37 | 0 (0.0) | 0.00 | 267 | 5 (1.9) | 0.09 |
| Dulaglutide 0.75 mg | 40 | 0 (0.0) | 0.00 | 262 | 5 (1.9) | 0.06 |
| Sitagliptin | 44 | 0 (0.0) | 0.00 | 271 | 0 (0.0) | 0.00 |
| Placebo | 23 | 0 (0.0) | 0.00 | 154 | 0 (0.0) | 0.00 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 37 | 1 (2.7) | 0.05 | 267 | 6 (2.2) | 0.11 |
| Dulaglutide 0.75 mg | 40 | 0 (0.0) | 0.00 | 262 | 5 (1.9) | 0.07 |
| Sitagliptin | 44 | 0 (0.0) | 0.00 | 271 | 2 (0.7) | 0.03 |
| Placebo | 23 | 0 (0.0) | 0.00 | 154 | 0 (0.0) | 0.00 |
| AWARD‐6 (metformin) | ||||||
| Documented symptomatic | ||||||
| Dulaglutide 1.5 mg | 51 | 0 (0.0) | 0.00 | 248 | 8 (3.2) | 0.14 |
| Liraglutide 1.8 mg | 60 | 1 (1.7) | 0.33 | 240 | 7 (2.9) | 0.27 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 51 | 0 (0.00) | 0.00 | 248 | 20 (8.1) | 0.24 |
| Liraglutide 1.8 mg | 60 | 2 (3.3) | 0.07 | 240 | 8 (3.3) | 0.14 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 51 | 0 (0.00) | 0.00 | 248 | 4 (1.6) | 0.08 |
| Liraglutide 1.8 mg | 60 | 2 (3.3) | 0.41 | 240 | 4 (1.7) | 0.08 |
| AWARD‐2 (metformin + glimiperide) | ||||||
| Documented symptomatic | ||||||
| Dulaglutide 1.5 mg | 54 | 14 (25.9) | 2.32 | 219 | 68 (31.1) | 2.35 |
| Dulaglutide 0.75 mg | 51 | 18 (35.3) | 2.97 | 221 | 71 (32.1) | 2.42 |
| Insulin glargine | 56 | 17 (30.4) | 2.47 | 206 | 84 (40.8) | 3.96 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 54 | 19 (35.2) | 4.29 | 219 | 85 (38.8) | 3.64 |
| Dulaglutide 0.75 mg | 51 | 19 (37.3) | 4.56 | 221 | 79 (35.7) | 3.35 |
| Insulin glargine | 56 | 23 (41.1) | 5.34 | 206 | 83 (40.3) | 4.68 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 54 | 6 (11.1) | 0.51 | 219 | 48 (21.9) | 1.37 |
| Dulaglutide 0.75 mg | 51 | 9 (17.6) | 1.36 | 221 | 42 (19.0) | 0.86 |
| Insulin glargine | 56 | 13 (23.2) | 2.15 | 206 | 50 (24.3) | 1.78 |
| AWARD‐4 (insulin lispro ± metformin) | ||||||
| Documented symptomatic | ||||||
| Dulaglutide 1.5 mg | 77 | 55 (71.4) | 29.40 | 218 | 174 (79.8) | 33.11 |
| Dulaglutide 0.75 mg | 76 | 57 (75.0) | 32.75 | 217 | 185 (85.3) | 40.96 |
| Insulin glargine | 90 | 72 (80.0) | 45.59 | 206 | 171 (83.0) | 44.54 |
| Asymptomatic | ||||||
| Dulaglutide 1.5 mg | 77 | 40 (51.9) | 10.42 | 218 | 135 (61.9) | 10.18 |
| Dulaglutide 0.75 mg | 76 | 46 (60.5) | 16.59 | 217 | 134 (61.8) | 11.55 |
| Insulin glargine | 90 | 56 (62.2) | 15.55 | 206 | 143 (69.4) | 17.23 |
| Nocturnal | ||||||
| Dulaglutide 1.5 mg | 77 | 28 (36.4) | 2.56 | 218 | 109 (50.0) | 4.18 |
| Dulaglutide 0.75 mg | 76 | 30 (39.5) | 4.15 | 217 | 102 (47.0) | 4.87 |
| Insulin glargine | 90 | 52 (57.8) | 8.86 | 206 | 129 (62.6) | 9.37 |
N equals the number of patients in that group.
n equals the number of patients that had at least one hypoglycaemic event in that group.
Rate is defined as events/patient/year.
Adverse Events
Treatment‐emergent adverse events were similar across age groups for pooled results of dulaglutide 1.5 mg‐treated patients [those aged ≥65 years, 217 of 318 (68.2%) and those aged <65 years, 917 of 1401 (65.5%)] and pooled results of dulaglutide 0.75 mg‐treated patients [those aged ≥65 years, 159 of 258 (61.6%) and those aged <65 years, 762 of 1159 (65.7%)]. Overall, the percentage of patients reporting gastrointestinal adverse events was similar in each age group with both dulaglutide doses (nausea: dulaglutide 1.5 mg, ≥65 years = 22.6% and <65 years = 20.3%; dulaglutide 0.75 mg, ≥65 years = 13.2% and <65 years = 12.3%; diarrhoea: dulaglutide 1.5 mg, ≥65 years = 14.2% and <65 years = 11.6%; dulaglutide 0.75 mg, ≥65 years = 8.9% and <65 years = 8.9%; and vomiting: dulaglutide 1.5 mg, ≥65 years = 10.4% and <65 years = 10.1%; dulaglutide 0.75 mg, ≥65 years = 8.5% and <65 years =6.3%). Nausea was the most common gastrointestinal adverse event with onset highest in the first 2 weeks of treatment (dulaglutide 1.5 mg, ≥65 years = 16.4% and <65 years = 15.5%; dulaglutide 0.75 mg, ≥65 years = 8.5% and <65 years = 8.4%); and rapid decline thereafter, with no differences observed between age groups.
Discussion
In the present analysis, the efficacy measure of HbA1c change from baseline was analysed using pooled data from six AWARD clinical trials for each dulaglutide dose as well as by individual study for patients aged ≥65 years and those <65 years. The range of HbA1c reduction from baseline to the primary endpoint with dulaglutide treatment was 0.8–1.6% for dulaglutide 1.5 mg 25, 26, 27, 28, 29, 30 and 0.7–1.6% for dulaglutide 0.75 mg 25, 26, 27, 28, 29. This post hoc analysis, based on both the pooled and individual study data, showed that reduction in HbA1c with both dulaglutide doses was similar in patients who were aged ≥65 years and those who were aged <65 years.
The current standards for treating T2D recommend an HbA1c of <7% for healthy adults, but less stringent goals, such as <8–9%, are recommended for those with limited life expectancy and/or comorbid illness 7, 12, 13. All three HbA1c target goals were therefore evaluated in the present analysis. We found that with both dulaglutide doses, the percentage of patients achieving HbA1c targets of <7, <8 and <9% in each age group was similar, and in the ranges achieved in the overall AWARD clinical trial programme, where the percentage of patients achieving HbA1c target goal of <7% ranged from 53 to 78% for dulaglutide 1.5 mg 25, 26, 27, 28, 29, 30, and from 37 to 69% for dulaglutide 0.75 mg 25, 26, 27, 28, 29.
Dulaglutide 1.5 mg treatment resulted in weight loss in both older and younger patients for AWARD‐1 through to AWARD‐6 clinical trials. Dulaglutide 0.75 mg treatment also resulted in similar weight loss in both age groups for AWARD‐2, ‐3, and ‐5; however, a small weight gain was observed in AWARD‐4 for both age groups. This was probably a result of the very‐high‐dose insulin lispro concomitant therapy used in that study 27. In AWARD‐1, dulaglutide 0.75 mg treatment resulted in a small weight loss in the older patients, and a small weight gain in the younger patients, probably as a result of pioglitazone concomitant therapy in that study 29; treatment with thiazolidinedione has been previously reported to result in weight gain 31. In the overall AWARD clinical trial programme, change (LS mean) in body weight from baseline to the primary endpoint ranged from −0.9 to −3.0 kg for dulaglutide 1.5 mg 25, 26, 27, 28, 29, 30, and from +0.2 to −2.6 kg for dulaglutide 0.75 mg 25, 26, 27, 28, 29. This post hoc analysis in the elderly suggest that the results on weight change are in line with those seen in the overall study population.
From a safety perspective, the incidence of documented symptomatic, asymptomatic and nocturnal hypoglycaemic (plasma glucose ≤3.9 mmol/l) events were similar across age groups, and were low when patients were not on concomitant sulphonylurea or insulin therapy. As seen with other incretin agents, when combined with sulphonylurea or insulin, the risk of hypoglycaemia increases 16, 32. Events of severe hypoglycaemia were, however, very infrequent.
Lastly, pooled analyses from all the studies showed that gastrointestinal adverse events were similar in each age group. Nausea, the most common adverse event, was transient; with the highest rates in the first 2 weeks, rapidly declining thereafter. The dulaglutide results from this analysis are consistent with other published studies that show no difference in efficacy and safety of GLP‐1 receptor agonist use in older patients with T2D compared with younger patients 18, 19, 33.
It has been reported that patients with T2D have a higher incidence of cognitive decline 34 and T2D is associated with an increased risk of dementia and Alzheimer's disease development 34. High glucose levels in themselves are also thought to have detrimental effects on the aging brain and may be associated with an increased risk of dementia in populations both with and without diabetes 35. Conversely, stringent glycaemic control in elderly patients may result in hypoglycaemia, which may also have detrimental effects on cognitive function 36 and cognitive impairment in itself also increases the risk of hypoglycaemia. It is therefore important to consider a treatment regimen that not only is effective in HbA1c reduction but also has demonstrated low incidences of hypoglycaemia.
It is recommended that older adults with mild to moderate cognitive impairment achieve an HbA1c target of <8% whilst those with severe cognitive impairment should aim for an HbA1c target of <8.5% 7. In the present analysis, we have shown that treatment of patients aged ≥65 years with dulaglutide resulted in a high percentage of patients achieving HbA1c targets of <8% (87.4–89.4%) and <9% (96.0–96.8%) with a low risk of hypoglycaemia, especially when not used with sulphonylurea or insulin. Also, as mentioned previously, older adults may have poor vision, arthritis or cognitive dysfunction, which may make dose calculations and administration of injectable medications difficult. Results from a study with the dulaglutide single‐use pen showed that injection success was achieved by 99.3% of patients aged <65 years and by 98.6% of patients aged ≥65 years 23.
There are several limitations that should be considered when interpreting these results. This was a post hoc analysis with a small number of elderly patients in each individual study, very few of whom were aged ≥75 years, the fastest growing segment of the aging population. In addition, the focus of this analysis was at 26 weeks because of the varying duration of the AWARD trials. Given the heterogeneity of concomitant therapy across the AWARD clinical trial programme, with concomitant sulphonylurea and insulin therapy increasing the risk of hypoglycaemia (AWARD 2 and 4) 16, 27, 28, 32 and pioglitazone attenuating weight loss (AWARD 1) 29, 31, pooling of all studies would not have been the best representation of the clinical results for weight change and risk for hypoglycaemia.
In conclusion, given the increasing prevalence of T2D in older adults, continued evaluation of diabetes medications for efficacy and safety in this population are necessary. The results of this analysis show that treatment with both dulaglutide doses improves glycaemic control, decreases body weight (or results in less weight gain), has a low risk of hypoglycaemia, and similar incidence of gastrointestinal adverse events in patients aged ≥65 years and those aged <65 years and can be considered a safe and effective treatment option for use in older adults.
Conflict of Interest
I. P. is a speaker's bureau participant for Eli Lilly and Company. M. Y., V. T., O.V. and R. J. are employees of Eli Lilly and Company and own stock in the company. M. B. has no conflict of interest to report.
M. B. and I. P. participated in interpretation of the statistical analysis and helped to draft the manuscript. M. Y. planned and performed the statistical analysis, provided interpretation of the statistical analysis, and helped to draft the manuscript. V. T. and R. J. participated in design of the study, provided interpretation of the statistical analysis, and helped to draft the manuscript. O. V. participated in interpretation of the statistical analysis, and helped to draft the manuscript. All authors read and approved the final manuscript, and agreed to be accountable for all aspects of the work.
Supporting information
Figure S1. Percent to Goal HbA1c.
Table S1. Overview of AWARD‐1 through AWARD‐6 clinical trials.
Acknowledgements
This work was sponsored by Eli Lilly and Company Additional details of the AWARD studies [A Study in Patients with Type 2 Diabetes Mellitus (AWARD‐1); A Study in Patients With Type 2 Diabetes Mellitus (AWARD‐2); A Study in Patients With Type 2 Diabetes Mellitus (AWARD‐3); A Study in Patients With Type 2 Diabetes Mellitus (AWARD‐4); A Study of LY2189265 Compared to Sitagliptin in Patients With Type 2 Diabetes Mellitus on Metformin' (AWARD‐5); and A Study Comparing the Effect of Dulaglutide With Liraglutide in Type 2 Diabetes (AWARD‐6)] can be found at http://clinicaltrials.gov under the numbers: NCT01064687; NCT01075282; NCT01126580; NCT01191268; NCT00734474; and NCT01624259, respectively.
The authors wish to thank Ian Sturdy and Yixun Wu for statistical analysis support.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Gregg EW, Yaffe K, Cauley JA et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med 2000; 160: 174–180. [DOI] [PubMed] [Google Scholar]
- 5. Gregg EW, Mangione CM, Cauley JA et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25: 61–67. [DOI] [PubMed] [Google Scholar]
- 6. Gregg EW, Sorlie P, Paulose‐Ram R et al. Prevalence of lower‐extremity disease in the US adult population > =40 years of age with and without diabetes: 1999‐2000 national health and nutrition examination survey. Diabetes Care 2004; 27: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 7. Kirkman MS, Briscoe VJ, Clark N et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60: 2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil 2004; 85: 245–252. [DOI] [PubMed] [Google Scholar]
- 9. Richardson JK, Thies S, Ashton‐Miller JA. An exploration of step time variability on smooth and irregular surfaces in older persons with neuropathy. Clin Biomech (Bristol, Avon) 2008; 23: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson JK, Thies SB, DeMott TK, Ashton‐Miller JA. Gait analysis in a challenging environment differentiates between fallers and nonfallers among older patients with peripheral neuropathy. Arch Phys Med Rehabil 2005; 86: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 11. Halter JB, Morrow LA. Use of sulfonylurea drugs in elderly patients. Diabetes Care 1990; 13: 86–92. [Google Scholar]
- 12. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care 2012; 35(Suppl. 1): S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinclair AJ, Paolisso G, Castro M, Bourdel‐Marchasson I, Gadsby R, Rodriguez ML. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37 Suppl. 3: S27–S38. [DOI] [PubMed] [Google Scholar]
- 14. Bryan J, Crane A, Vila‐Carriles WH, Babenko AP, Aguilar‐Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des 2005; 11: 2699–2716. [DOI] [PubMed] [Google Scholar]
- 15. Kahn SE, Haffner SM, Heise MA et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 16. Ober SK, Watts S, Lawrence RH. Insulin use in elderly diabetic patients. Clin Interv Aging 2006; 1: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bode B. Liraglutide: a review of the first once‐daily GLP‐1 receptor agonist. Am J Manag Care 2011; 17: S59–S70. [PubMed] [Google Scholar]
- 18. Pencek R, Blickensderfer A, Li Y, Brunell SC, Chen S. Exenatide once weekly for the treatment of type 2 diabetes: effectiveness and tolerability in patient subpopulations. Int J Clin Pract 2012; 66: 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raccah D, Miossec P, Esposito V, Niemoeller E, Cho M, Gerich J. Efficacy and safety of lixisenatide in elderly (>/=65 years old) and very elderly (>/=75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev 2015; 31: 204–211. [DOI] [PubMed] [Google Scholar]
- 20. Russo E, Penno G, Del Prato S. Managing diabetic patients with moderate or severe renal impairment using DPP‐4 inhibitors: focus on vildagliptin. Diabetes Metab Syndr Obes 2013; 6: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrington P, Chien JY, Showalter HD et al. A 5‐week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long‐acting glucagon‐like peptide‐1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 426–433. [DOI] [PubMed] [Google Scholar]
- 22. Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long‐acting glucagon‐like peptide‐1 analogue, showed a dose‐dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab 2011; 13: 434–438. [DOI] [PubMed] [Google Scholar]
- 23. Matfin G, Van Brunt K, Zimmermann AG, Threlkeld R, Ignaut DA. Safe and effective use of the once weekly dulaglutide single‐dose pen in injection‐naive patients with type 2 diabetes. J Diabetes Sci Technol 2015; 9: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trulicity [Summary of Product Characteristics]. Houten, The Netherlands: Eli Lilly and Company; 2014.
- 25. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 26. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blonde L, Jendle J, Gross J et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet 2015; 385: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 28. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care 2015; 38: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 29. Wysham C, Blevins T, Arakaki R et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 30. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 31. Fonseca V, McDuffie R, Calles J et al. Determinants of weight gain in the action to control cardiovascular risk in diabetes trial. Diabetes Care 2013; 36: 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15: 75–80. [DOI] [PubMed] [Google Scholar]
- 33. Bode BW, Brett J, Falahati A, Pratley RE. Comparison of the efficacy and tolerability profile of liraglutide, a once‐daily human GLP‐1 analog, in patients with type 2 diabetes >/=65 and <65 years of age: a pooled analysis from phase III studies. Am J Geriatr Pharmacother 2011; 9: 423–433. [DOI] [PubMed] [Google Scholar]
- 34. Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer's disease. World J Diabetes 2014; 5: 889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med 2013; 369: 1863–1864. [DOI] [PubMed] [Google Scholar]
- 36. Dominguez LJ, Paolisso G, Barbagallo M. Glucose control in the older patient: from intensive, to effective and safe. Aging Clin Exp Res 2010; 22: 274–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percent to Goal HbA1c.
Table S1. Overview of AWARD‐1 through AWARD‐6 clinical trials.


