Abstract
Background
Transpulmonary thermodilution (TPTDCO) and calibrated pulse contour analysis (PCACO) are alternatives to pulmonary artery thermodilution cardiac output (PATDCO) measurement.
Hypothesis
Ten mL of ice‐cold thermal indicator (TI 10) would improve the agreement and trending ability between TPTDCO and PATDCO compared to 5 mL of indicator (TI 5) (Phase‐1). The agreement and TA between PCACO and PATDCO would be poor during changes in systemic vascular resistance (SVR) (Phase‐2).
Animals
Eight clinically normal dogs (20.8–31.5 kg).
Methods
Prospective, experimental study. Simultaneous TPTDCO and PATDCO (averaged from 3 repetitions) using TI 5 and TI 10 were obtained during isoflurane anesthesia combined or not with remifentanil or dobutamine (Phase‐1). Triplicate PCACO and PATDCO measurements were recorded during phenylephrine‐induced vasoconstriction and nitroprusside‐induced vasodilation (Phase‐2).
Results
Mean bias (limits of agreement: LOA) (L/min), percentage bias (PB), and percentage error (PE) were 0.62 (−0.11 to 1.35), 16%, and 19% for TI 5; and 0.33 (−0.25 to 0.91), 9%, and 16% for TI 10. Mean bias (LOA), PB, and PE were 0.22 (−0.63 to 1.07), 6%, and 23% during phenylephrine; and 2.12 (0.70–3.55), 43%, and 29% during nitroprusside. Mean angular bias (radial LOA) values were 2° (−10° to 14°) and −1° (−9° to 6°) for TI 5 and TI 10, respectively (Phase‐1), and 38° (5°–71°) (Phase‐2).
Conclusions and Clinical Importance
Although TI 10 slightly improves the agreement and trending ability between TPTDCO and PATDCO in comparison to TI 5, both volumes can be used for TPTDCO in replacement of PATDCO. Vasodilation worsens the agreement between PCACO and PATDCO. Because of PCACO's poor agreement and trending ability with PATDCO during SVR changes, this method has limited clinical application.
Keywords: Indicator dilution cardiac output, Monitoring, Pulse contour analysis
Abbreviations
- AOA
accuracy of agreement
- CO
cardiac output
- CRI
constant rate infusion
- CVP
central venous pressure
- HR
heart rate
- LOA
limits of agreement
- MAP
mean arterial pressure
- PATDCO
pulmonary artery thermodilution cardiac output
- PB
percentage bias
- PCACO
calibrated pulse contour analysis cardiac output
- PE
percentage error
- POAREFxREF
precision of agreement expected if the reference technique was compared to itself
- POATESTxREF
precision of agreement of test versus reference methods
- POM
precision of method
- POMREF
precision of reference method
- POMTEST
precision of test method
- SVR
systemic vascular resistance
- TPTDCO
transpulmonary thermodilution cardiac output
- ΔCO
delta changes in CO
- ΔTblood
delta changes in blood temperature
Cardiac output (CO) monitoring can play an important role during the decision‐making process in critically ill patients that require cardiovascular stabilization. Although pulmonary artery thermodilution cardiac output (PATDCO) has been the clinical gold standard in human medicine since its introduction in the early 1970s,1, 2 placement of a pulmonary artery catheter is an invasive procedure that does not improve survival in critically ill patients and might result in rare (overall incidence 0.1%) but potentially fatal complications, including right ventricular rupture, knotting, and pulmonary artery rupture.3, 4 Although PATDCO is frequently used in animal experimentation, its clinical application in veterinary medicine is restricted.
Transpulmonary thermodilution cardiac output (TPTDCO) is an alternative to PATDCO that has become increasingly popular in human medicine because it does not require pulmonary artery catheterization.2, 5, 6 For both techniques, changes in blood temperature (ΔTblood) induced by the rapid injection of a thermal indicator (ice‐cold or room temperature solution) into the vena cava or right atrium are recorded downstream for CO calculation.2, 5, 6 In PATDCO, the ΔTblood is recorded by a thermistor located in the pulmonary artery, whereas in TPTDCO the ΔTblood is recorded further downstream by a thermistor located in a central artery (usually the femoral artery).2, 5, 6 While placing a femoral catheter produce some risks in veterinary medicine, it appears more attractive then placing a pulmonary artery catheter because it is does not require cardiac catheterization. However, the longer distance between the site of ice‐cold signal injection and the site for measuring ΔTblood results in greater loss of thermal signal in comparison to PATDCO and can lead to CO overestimation.2, 5, 6, 7 This source of error could be particularly important in large animal species and during low CO states. Significant CO overestimation might also occur with lower volumes of thermal indicator because of decreased signal‐to‐noise ratio. Although the agreement between PATDCO and TPTDCO has been studied in humans,7, 8, 9, 10, 11 pigs,12 calves,13 cats,14, 15 and dogs,16, 17 objective interpretation of these reports is difficult because of the absence of clearly defined criteria to determine the clinically acceptable bias and limits of agreement (LOA) between PATDCO and TPTDCO.
Calibrated pulse contour analysis (PCACO) provides continuous CO estimations based on beat‐to‐beat variations in stroke volume calculated from complex analysis of the systolic portion of the arterial pressure waveform.5, 18 After an initial calibration of the system with TPTDCO, continuous PCACO values are provided by the same monitor. Although a good agreement between PCACO and thermodilution CO techniques has been reported in the literature,19, 20, 21, 22 several studies have shown wide LOA between these methods during hemodynamic changes induced by hemorrhage, vasopressor or vasodilator therapy, and volume replacement.23, 24, 25, 26 Inaccurate CO estimations by PCACO might be caused by changes in systemic vascular resistance (SVR) because modifications in vascular tone alter the shape of the arterial blood pressure wave.25
The first hypothesis of the present study was that the use of a higher volume of thermal indicator (10 mL of ice‐cold physiological saline) for TPTDCO would result in better agreement and ability to track changes in the clinical gold standard (PATDCO) in comparison to a lower volume of thermal indicator (5 mL of ice‐cold physiological saline). The second hypothesis was that PCACO would result in poor agreement and trending ability with PATDCO during drug‐induced changes in SVR.
Material and Methods
Animals
This study was approved by the Institutional Animal Care Committee (protocol number 93/2013). Eight clinically normal adult (27–28 months old) English Pointer breed dogs (3 intact nonpregnant females and 5 intact males), weighing 20.8–31.5 kg, were enrolled in this study. Food but not water was withheld 12 hours prior to each experiment. Health status was evaluated by means of CBC, biochemical profile and venous blood gases that were within normal ranges.
Instrumentation
After placing a 20‐gauge catheter1 in a cephalic vein, anesthesia was induced with intravenous propofol2 (6.0 ± 1.0 mg/kg) and maintained with isoflurane.3 End‐tidal isoflurane concentrations were monitored4 and maintained at 1.5 times the minimum alveolar concentration (MAC) (1.78 ± 0.41% at sea level) throughout the study. Isoflurane MAC values were obtained for each individual animal in a preliminary study.
Animals were mechanically ventilated4 with an inspired oxygen fraction of 60% and received intravenous Lactated Ringer's solution (2 mL/kg/h) throughout anesthesia. Tidal volume was set at 12 mL/kg with an inspiration‐to‐expiration ratio of 1 : 2, whereas the respiratory rate was adjusted to maintain PaCO2 between 35 and 45 mmHg.
A 22‐Gauge catheter1, inserted percutaneously into the femoral artery 2.5 cm away from the inguinal fold, was used for the introduction of a wire that served as a guide for the subsequent insertion of a 3‐french, 7 cm long thermistor‐tipped catheter5 into the femoral artery based on the Seldinger technique. The pressure sensing lumen of the femoral artery catheter5 was connected to a pressure transducer6 via noncompliant tubing filled with heparinized (4 UI/mL) physiological saline. The transducer was zeroed at the level of the heart and connected to a monitor7 to display systolic, diastolic, and mean arterial pressure (MAP). Square wave tests were performed by intermittently pulling the fast flush tab of the pressure transducer to ensure that the dynamic response of the system was adequate and 2 wave deflections after the square wave on the screen of the monitor were present. The thermistor located at the tip of the catheter was connected to the same monitor for recording ΔTblood during TPTDCO measurements.
A 7‐french, 110 cm long, double lumen, thermistor‐tipped catheter8 was inserted into a jugular vein through an 8.5 F introducer sheath and advanced until its tip was positioned in the pulmonary artery using pressure waveform guidance. The proximal and distal ports of the catheter were connected to fluid‐filled pressure transducers9 zeroed at the level of the heart for continuous display of central venous pressure (CVP) and pulmonary artery pressure. The thermistor located at the tip of the pulmonary artery catheter was connected to the monitor10 for recording ΔTblood during PATDCO measurements.
Experimental Procedure—Phase‐1
This study was divided in 2 phases (Fig 1A and B). Phase‐1 aimed to evaluate the effect of 2 different volumes of thermal indicator on the agreement and trending ability between TPTDCO and PATDCO. Syringes prefilled with 5 and 10 mL of physiological saline were immersed in an iced bath for at least 1 hour before commencing CO measurements. For each data sampling time, CO was averaged from 3 repeated measurements using 5 mL and 10 mL of ice‐cold (2–5°C) physiological saline. The initial order of thermal indicator volumes was determined at random; this order was inverted during every other subsequent measurement. Each thermal indicator bolus was rapidly injected into the CVP port of the pulmonary artery catheter (<3 seconds) for recording of ΔTblood in the pulmonary artery and in the femoral artery for PATDCO and TPTDCO measurements, respectively. Temperature of the injectate was monitored by 2 in‐line thermistors connected to the PATDCO and TPTDCO monitors. Thermistors were placed in series between the syringe containing the thermal indicator and the CVP port of the pulmonary artery catheter. Computation constants for CO measurements were based on the catheter type and volume/temperature of the thermal indicator, as recommended by the manufacturers.
Figure 1.
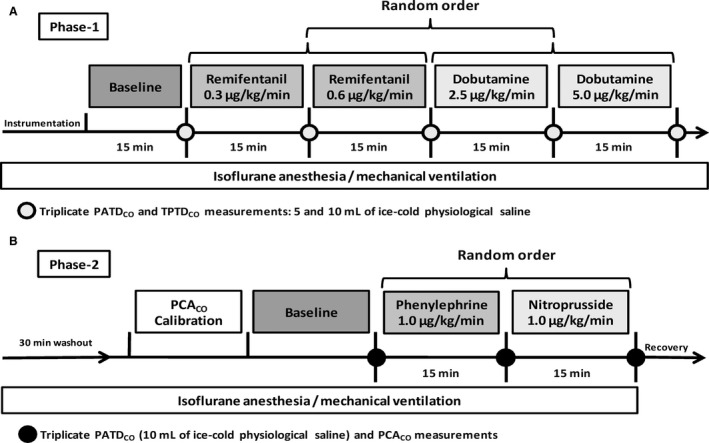
Experimental protocol during Phase‐1 (A) and Phase‐2 (B). PATDCO, pulmonary artery thermodilution; TPTDCO, transpulmonary thermodilution cardiac output; PCACO, calibrated pulse contour analysis cardiac output.
Low normal CO states were recorded during anesthesia with isoflurane alone (1.5 MAC), and isoflurane combined with 2 ascending constant rate infusions (CRIs) of remifentanil11 (0.3 and 0.6 μg/kg/min). High CO states were induced by 2 ascending dobutamine12 CRIs (2.5 and 5.0 μg/kg/min) administered during isoflurane anesthesia. The sequence of drug administration was determined at random. Cardiac output was recorded during anesthesia with isoflurane alone and after each CRI was maintained for at least 15 minutes. After data collection, infusion drugs were stopped and a 15‐minute washout period was allowed before commencing the next CRI.
Experimental Procedure—Phase‐2
After the end of Phase‐1, infusion drugs were interrupted and anesthesia was maintained with 1.5 MAC of isoflurane alone for 30 minutes before commencing Phase‐2, which aimed to evaluate the effects of changes in SVR induced by phenylephrine13 and nitroprusside14 on the agreement and trending ability between PCACO and PATDCO.
The PCACO was calibrated one single time with 1 TPTDCO measurement using 10 mL of ice‐cold physiological saline before conditions of increased and decreased SVR were induced by phenylephrine (1.0 μg/kg/min) and nitroprusside (1.0 μg/kg/min), respectively. Further TPTDCO measurements were not performed during phase‐2 to avoid recalibration of the PCACO system. The order of CRI drugs was determined at random and PATDCO was recorded as the average of 3 repeated measurements using 10 mL of ice‐cold (2–5°C) physiological saline before CRI drugs were administered (baseline) and after each CRI was maintained for 15 minutes. Cardiac output obtained by calibrated pulse contour analysis was recorded simultaneously with each PATDCO measurement and averaged for comparison. After CO data were obtained, a 15‐minute washout period was allowed before starting the next CRI.
Heart rate (HR) (recorded from a lead II electrocardiogram), MAP (recorded from the femoral artery catheter), PATDCO, and SVR [(MAP‐CVP)/PATDCO × 79.9], were recorded at the same times of CO comparisons.
For both phases, CO determinations were accepted only at steady state hemodynamic conditions, defined as HR and MAP recorded immediately before and after each series of CO measurements varying ≤10%.
After the end of data collection, all catheters were removed and a single dose of meloxicam15 (0.2 mg/kg, IV) was administered for analgesia. The sites of femoral artery and pulmonary artery catheter insertion were manually compressed with ice cubes packed in lap sponges for at least 15 minutes after catheter removal.
Statistical Methods
Commercially available software was used for data analysis and graphic generation,16 , 17 , 18 , 19 Cardiac output data recorded during Phase‐1 were analyzed by the Bland Altman method for multiple measurements per subject.16 Data recorded during Phase‐2 were analyzed separately during baseline (prior to drug infusion), phenylephrine, and nitroprusside infusion by the standard Bland Altman method.16 Shapiro‐Wilk normality tests were applied to the bias recorded in each phase. During Phase‐1, the bias (difference between TPTDCO and PATDCO) recorded during injection of 5 and 10 mL of thermal signal was compared by a Wilcoxon matched‐pairs signed rank test (asymmetrical data distribution).17 During Phase‐2, the bias between PCACO and PATDCO recorded during baseline, phenylephrine, and nitroprusside CRI were compared by a one‐way repeated measures ANOVA, followed by a Tukey's post hoc test (symmetrical data distribution).17
During Phase 1 and 2, the percentage bias (PB) was calculated from the mean bias divided by the mean CO (mean of CO values plotted on the X axis of the Bland Altman graphs). The percentage error (PE) was calculated as 1.96 times the SD of the mean bias divided by the same denominator.27 Additionally, the precision of method (POM) of each series of triplicate CO measurements was calculated as 2 times the coefficient of error (CE).28, 29 The CE represents the coefficient of variation divided by the square root of number of replicates (n) (CE = coefficient of variation/√n).28
During Phase‐2, hemodynamic variables recorded at baseline, and during phenylephrine/nitroprusside infusion were compared by an ANOVA for repeated measurements followed by a Tukey's test (P < .05).17 The arterial blood pressure tracing was inspected for the presence of the dicrotic notch during PCACO measurements.
Ability of the test methods to track changes in the reference method was evaluated by 4‐quadrant plot and polar plot analysis.30, 31, 32 Sequential changes in TPTDCO (ΔTPTDCO) or in PCACO (ΔPCACO) were plotted on the y axis, whereas corresponding changes in the reference method (ΔPATDCO) were plotted on x axis.17 After dividing the graphs in 4‐quadrants by the intersection of lines originated from zero in both axes, the concordance rate (%) was calculated as the percentage of data in the upper right/lower left quadrants (data that follow the same trend) in relation to the total number of data points.
Sequential changes in CO (ΔCO) were calculated as arithmetic mean of ΔCO of the test and reference method (ΔTPTDCO + ΔPATDCO/2 during Phase 1, and ΔPCACO + ΔPATDCO/2 during Phase 2). This mean ΔCO is represented as the distance of the vector from the center of the polar plot.31, 32 The ΔCO values were converted to polar coordinates using a spreadsheet.18 , 31 The polar angle was calculated as the angle of divergence of the ΔCO from the line of identity.31, 32 The actual polar plots were generated using a graph drawing software.19 As recommended by Critchley et al.,32 when the mean polar angle was ≤ ±5°, the radial LOA were estimated from the SD of the mean polar angle (±1.96 SD). When the mean angular bias was > ±5°, the radial LOA were estimated from the plot of the inclusion rate against the radial sector size.32
Good, acceptable (or marginal), and poor trending ability was considered if the concordance rates obtained after excluding ΔCO values ≤0.5 L/min were >95%, between 90 and 95%, and <90%, respectivelly.31, 32 Good trending ability based on polar plot analysis was defined by the mean angular bias ≤ ±5° with radial LOA ≤ ±30° obtained after excluding mean ΔCO values ≤0.5 L/min because small changes are likely because of random effects or noise.32
Results
All animals recovered uneventfully from anesthesia. No catheter‐related complications (hematoma, bruising) were observed during the postanesthetic period.
Phase‐1
The time elapsed from placing the catheter introducer until the PATDCO catheter was positioned in the pulmonary artery was 33 ± 25 minutes. The time for placing the TPTDCO catheter in the femoral artery was 8 ± 3 minutes. A total of 39 sets of 3 measurements were performed for TPTDCO and PATDCO. Minimum and maximum PATDCO values (mean ± SD) recorded for 10 mL of thermal indicator during phase‐1 ranged from 1.74 ± 0.21 L/min to 6.73 ± 0.70 L/min.
The mean bias and LOA (±1.96 SD) between TPTDCO and PATDCO were 0.62 (−0.11 to 1.35) L/min and 0.33 (−0.25 to 0.91) L/min for 5 and 10 mL of thermal indicator, respectively (Fig 2A and B). The bias recorded with 10 mL of thermal indicator was significantly smaller than with 5 mL of thermal indicator (P < .0001). The PB and PE values were 16% and 19% for 5 mL of thermal indicator, respectively. The use of 10 mL of injectate slightly decreased the PB to 9% and the PE to 16%.
Figure 2.
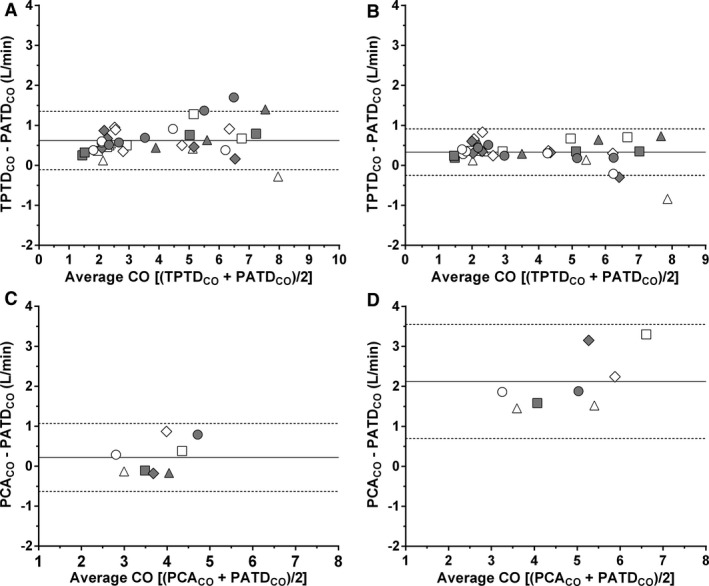
Bland Altman plots for repeated measurements of differences between transpulmonary thermodilution cardiac output (TPTDCO) and pulmonary artery cardiac output (PATDCO) using 5 mL (A) and 10 mL (B) of ice‐cold physiological saline as thermal indicator (Phase‐1); and Bland Altman plots of differences between pulse contour analysis cardiac output (PCACO) and PATDCO during phenylephrine‐induced vasoconstriction (C) and nitroprusside‐induced vasodilation (D) (Phase‐2). Each dog is represented by different geometric figures. Mean biases and limits of agreement (±1.96 SD) are shown by continuous and dashes lines, respectively.
The POM of PATDCO was 4.2% and 3.1% and the POM of TPTDCO was 3.7% and 3.4% for 5 and 10 mL of ice‐cold injectate, respectively.
Analysis of trending ability during Phase‐1 is presented in Table 1 and Figure 3A–D. Concordance rates were >95% regardless of the thermal indicator volume. The mean angular bias and radial LOA (calculated as ±1.96 SD) were < ±5° and < ±30°, respectively, for both volumes of ice‐cold injectate.
Table 1.
Variables derived from 4‐quadrant and polar plot analysis to assess the ability of transpulmonary thermodilution (TPTDCO) and of calibrated pulse contour analysis (PCACO) for estimating changes in cardiac output measured by pulmonary artery thermodilution (PATDCO). Trending analysis was performed using 5 and 10 mL of ice‐cold physiological saline as thermal indicator during simultaneous measurements of TPTDCO and PATDCO. Trending analysis between PCACO and PATDCO was performed using 10 mL of ice‐cold physiological saline solution for the thermodilution method. Only data after excluding mean cardiac output changes ≤0.5 L/min are presented
| Phase‐1: TPTDCO and PATDCO (5 mL of Thermal Indicator) | Phase‐1: TPTDCO and PATDCO (10 mL of Thermal Indicator) | Phase‐2: PCACO and PATDCO | |
|---|---|---|---|
| Four‐quadrant plot analysis | |||
| Measurements in the right quadrant (n) | 23 | 23 | 10 |
| Measurements in the wrong quadrant (n) | 0 | 0 | 6 |
| Concordance rate (%) | 100 | 100 | 63 |
| Polar plot analysis | |||
| Measurements with angular bias ≤30° (n) | 23 | 23 | 3 |
| Measurements with angular bias >30° (n) | 0 | 0 | 8 |
| Mean angular bias (°) | 2 | −1 | 38 |
| Radial limits of agreement (°) | −10 to 14 | −9 to 6 | 5–71 |
Figure 3.
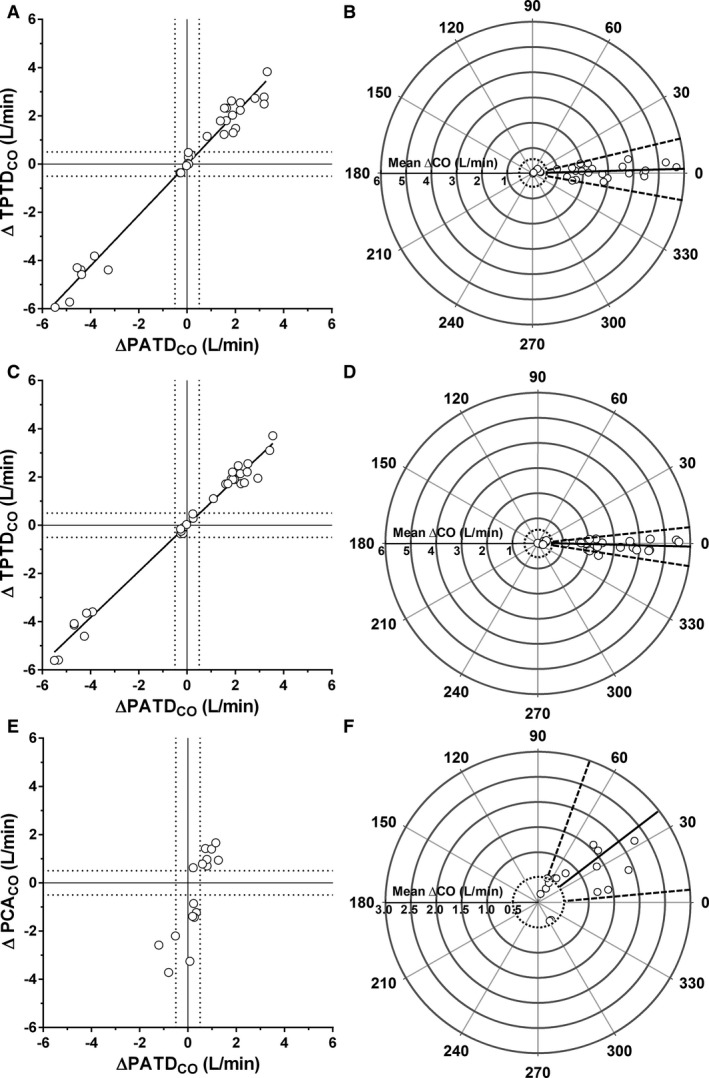
Four‐quadrant and polar plots of changes in transpulmonary thermodilution cardiac output (ΔTPTDCO) in relation to changes in pulmonary artery thermodilution (ΔPATDCO) using 5 mL (A and B) and 10 mL (C and D) of ice‐cold physiological saline as thermal indicator (Phase‐1); and 4‐quadrant and polar plots of changes in pulse contour analysis cardiac output (ΔPCACO) in relation to ΔPATDCO (Phase‐2) during phenylephrine and nitroprusside‐induced changes in systemic vascular resistance (E and F). The exclusion zones (cardiac output changes ≤0.5 L/min) in 4‐quadrant plots (A, C, and E) are shown by the intersection of the dashed lines whereas in the polar plots they are shown by the dashed circles. Mean angular biases and radial limits of agreement of polar plots are shown by continuous and dashed lines, respectively (B, D, and F).
Phase 2
A total of 24 sets of 3 measurements were performed for PCACO and PATDCO comparisons. Compared with baseline, phenylephrine significantly (P < .05) increased SVR and MAP by 44% and 18%, respectively, whereas PATDCO, PCACO and HR were significantly decreased by 18%, 20% and 10%, respectively. Nitroprusside significantly decreased SVR, MAP and PATDCO by 14%, 27% and 15% from baseline, respectively, without significant changes in HR and PCACO (Table 2).
Table 2.
Mean ± SD values of heart rate (HR), pulmonary artery thermodilution cardiac output (PATDCO), pulse contour analysis cardiac output (PCACO), mean arterial pressure (MAP), and systemic vascular resistance (SVR) recorded in 8 isoflurane anesthetized dogs during phase 2 of the study, before drug administration (Baseline), during phenylephrine (1 μg/kg/min), and nitroprusside administration (1 μg/kg/min)
| Variable | Baseline | Phenylephrine | Nitroprusside |
|---|---|---|---|
| HR (beats/min) | 131 ± 12a | 118 ± 8b | 130 ± 8a |
| PATDCO (L/min) | 5.2 ± 0.8a | 4.2 ± 0.6b | 4.4 ± 1.0b |
| PCACO (L/min) | 4.9 ± 0.8a | 3.9 ± 0.8b | 6.0 ± 1.4a |
| MAP (mmHg) | 88 ± 14a | 104 ± 9b | 64 ± 15c |
| SVR (dynes/s/cm5) | 1325 ± 74a | 1906 ± 190b | 1134 ± 153c |
a,b,cMeans followed by different superscript letters are significantly different from each other (Tukey's, P < .05).
The dicrotic notch was clearly identified during phenylephrine CRI in all animals. During nitroprusside CRI, the incisura of the dicrotic notch was unidentifiable at most instances (Fig 4).
Figure 4.
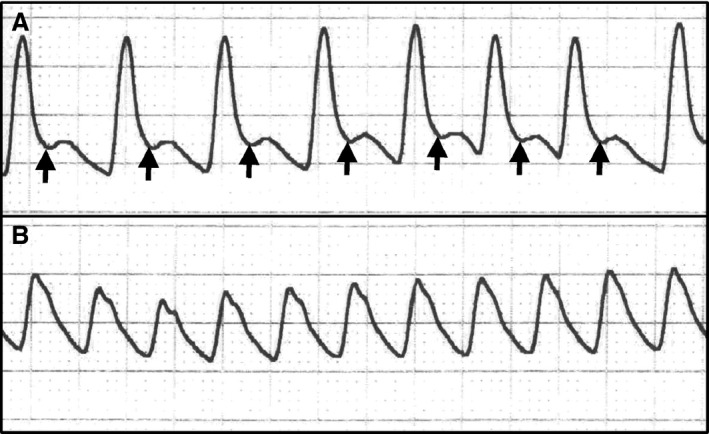
Arterial blood pressure tracings (25 mm/s) obtained from a dog anesthetized with isoflurane during intravenous phenylephrine (1 μg/kg/min) (A) and nitroprusside (1 μg/kg/min) infusions (B). The incisura of the dicrotic notches is shown by arrows.
Mean bias (LOA) between PCACO and PATDCO at baseline were 0.47 (0.18–0.76) L/min, while the PB and PE values at this time were 11% and 6%, respectively. Mean bias (LOA) between PCACO and PATDCO were 0.22 (−0.63 to 1.07) L/min during phenylephrine CRI and 2.12 (0.70–3.55) L/min during nitroprusside infusion (Fig 2C and D). The bias recorded during phenylephrine CRI did not differ from the bias at baseline (P = .15); whereas the bias recorded during nitroprusside CRI was significantly higher in comparison to the bias recorded at baseline (P = .0005) and to the bias recorded during phenylephrine infusion (P = .0006). The PB and PE recorded at baseline was 10% and 6%, respectively. Phenylephrine CRI decreased PB to 6% and increased the PE to 23%; whereas nitroprusside CRI increased the PB to 43% and the PE to 29%. The POM of PATDCO was 3.5% and POM of PCACO was 2.6%.
During Phase‐2, the concordance rate was <90% (Table 1 and Fig 3E). The mean angular bias was > ±5° and the radial LOA was > ±30° (Table 1 and Fig 3F).
Discussion
The main findings of the present study were: (1) The TPTDCO technique can be used in dogs in replacement of PATDCO because it provides good agreement and trending ability over a wide range of CO values. (2) The PCACO has limited clinical application because it does not agree well with PATDCO and has a poor ability to track changes in PATDCO during acute changes in SVR. The slightly better agreement and trending ability observed with 10 mL in comparison to 5 mL of thermal indicator corroborates the recommendation for the use of larger volumes of thermal indicator to improve the reliability of TPTDCO measurements.2, 5 The trend for larger overestimation of PATDCO by TPTDCO with the lower volume of ice‐cold indicator can be explained by greater loss of cold indicator by conductive warming as the solution traverses longer distances between the injection point (vena cava/right atrium) and the site of ΔTblood measurement (pulmonary artery for PATDCO versus femoral artery for TPTDCO).2, 5, 6 Because CO is inversely proportional to the area under the thermodilution curve according to the Stewart‐Hamilton equation, an artifactual decrease in the area under the thermodilution curve because of conductive warming will lead to an overestimation of CO values.2, 5, 6 The worsening of the agreement between PCACO and PATDCO during vasodilation and the poor trending ability between these methods during acute changes in SVR can be explained by the influence of changes in the vasomotor tone on the shape of the arterial pressure wave, which might not be recognized and properly corrected by the PCACO algorhythm.18
In the present study, the use of 10 mL of ice‐cold indicator for TPTDCO measurements resulted in smaller LOA (−0.25 to 0.91 L/min) and in a smaller PE (16%) than the LOA (−0.86 to 0.78 L/min) and an estimated PE of 36% in an earlier study in dogs weighting 12–18 kg using the same volume of ice‐cold indicator.16 In another recent study, PATDCO and TPTDCO measurements with 10 mL ice‐cold injectate in beagle dogs resulted in even wider LOA (−2.37 to 2.29 L/min/m2).17 The authors incorrectly concluded that TPTDCO agreed well with PATDCO in spite of the wide LOA.17 Percentage error estimated from the data presented in that study17 was >50%, likely representing a poor precision of agreement between the test and reference methods (POATESTxREF). The PE, calculated from the Bland Altman plots, is superior to the LOA for estimating the POATESTxREF because its calculation incorporates an estimation of the “true” CO values from the Bland Altman plots (mean CO).27, 29 Although the use of PE values to compare CO studies has been recommended since 1990s,27 several publications evaluating the agreement between TPTDCO and PCACO with PATDCO published thereafter have not reported the PE.8, 9, 10, 11, 12, 14, 16, 17, 18, 19, 23, 24
Although some studies have defined that the acceptable PE of the new method should be ≤30% if it is to be used clinically in replacement for the reference standard,13, 15, 25 this criterion was not adopted in the present study. The use of a predefined PE as a benchmark to determine the interchangeability between the test and the reference methods of CO measurement assumes that the precision of the test method (POMTEST) should be at least equal to the precision of the reference method (POMREF). Based on this assumption, the acceptable PE would represent an estimation of the precision of agreement expected if the reference technique was compared to itself (POAREFxREF), according to the formula: .28, 29 Considering the acceptable PE of ≤30% as a benchmark to determine the interchangeability between methods of CO measurement, TPTDCO and PCACO would be considered interchangeable with PATDCO in the present report. However, the 30% limit is estimated from the precision of single PATDCO measurements as the reference method (20%)27, 28, 29 and was incorrectly used in studies where PATDCO was averaged from triplicate measurements.13, 15, 25 The error of PATDCO can be substantially smaller if values are averaged from repeated measurements and if there is a strict control of factors that increase the variability of repeated PATDCO measurements (absence of arrhythmias, cardiovascular stability, constant injectate temperature, and volume).
More recently, it has been recommended to determine the acceptable PE based on the POMREF calculated for each study (POAREFxREF), as described above, and to compare these values with the PE calculated from the Bland Altman plots, which represents the POATESTxREF.28, 29 If the POATESTxREF was worse than the POAREFxREF, then the POMTEST would have been worse than the POMREF and the interchangeability between the new method and the reference standard would have been rejected.28, 29 However, if the acceptable PE was estimated from the POMREF obtained in our study as it has been recommended,28 the acceptable PE would range from 4 to 6% and the interchangeability between the test method of Phase‐1 (TPTDCO) and the reference standard (PATDCO) would have been paradoxically rejected because the PE derived from the Bland Altman plots was larger (19 and 16% for 5 and 10 mL of thermal indicator, respectively). However, this apparent discrepancy can be explained by the fact that the PE obtained from Bland Altman plots (POATESTxREF) is composed not only by the combination of the POMTEST and the POMREF, but also by the general variability of CO measurements about the true CO values (defined as trueness).29 For the reasons explained above, we determined the POMTEST and POMREF from repeated measurements and did not use the PE estimated from the POMREF to accept or reject the tested methods because calculation of the acceptable PE from the POMREF leads to falsified conclusions about the POMTEST as it does not take into account the method's variability about the true values (trueness).29
The use of 10 mL, rather than 5 mL of thermal injectate, improved the accuracy of agreement (AOA) and the POATESTxREF (smaller PB and PE, respectively) but did not result in obvious differences in POM values. Calculation of the POM requires that the true value is held constant over time during repeated measurements. Considering that an exactly constant true value is impossible to be achieved to allow POM calculation in live animal models,29 we assumed in our study design that the true CO value was relatively constant during each series of triplicate CO measurements because CO values were recorded under conditions of cardiovascular stability (defined as HR and MAP recorded at the beginning and at the end of each triplicate CO measurements differing by ≤10%). The relatively good POM recorded during triplicate CO measurements throughout the study (error <5%) shows that the overall precision of all methods was good and suggests that true CO values were held relatively constant to allow the recording of repeated measurements.
Although both volumes of thermal indicator resulted in good overall agreement and good trending ability between TPTDCO and PATDCO, the use of 10 mL of ice‐cold indicator might be preferred in some instances because it significantly decreased the bias and provided a slightly better POATESTxREF (lower PE) and a slightly lower tendency for TPTDCO to overestimate PATDCO (PB closer to zero). On the other hand, the use of 5 mL boluses of thermal indicator might be favored if there are concerns with fluid overload or if several TPTDCO measurements are required during short periods of time.
The injectate volume ranged from 0.16 to 0.24 mL/kg and from 0.32 to 0.48 mL/kg for 5 and 10 mL of ice‐cold injectate, respectively. These volumes were above the minimum volume recommended for accurate PATDCO measurements in human infants (0.15 mL/kg).33 To minimize the greater loss of thermal signal with the transpulmonary technique, the injectate volumes used for TPTDCO are larger than the volumes recommended for PATDCO in individuals with the same body mass. The volume of ice‐cold injectate used for TPTDCO measurements in human pediatric patients in one report was 1.5 mL plus 0.15 mL/kg.34 Based on the volume used for TPTDCO in human infants,34 3 animals of the present report would have received a slightly larger volume (5.3–6.2 mL of ice‐cold physiological saline) than the fixed 5 mL volume of thermal injectate administered during Phase‐1.
Compared to baseline conditions, phenylephrine administration slightly improved the AOA (PB decreased from 11% to 6%). In spite of a good POMTEST (2.6%) and POMREF (3.5%), the POAREFxTEST was worsened by vasoconstriction (PE increased from 6% at baseline to 23% during phenylephrine). This observation might be at least in part explained by an increase in test method's variability (PCACO) around true CO values (trueness).29
During nitroprusside‐induced vasodilatory states, Bland Altman analysis showed an unacceptable overestimation of PATDCO by PCACO. Compared to baseline, the vasodilatory state induced by nitroprusside significantly increased the mean bias from 0.47 L/min to 2.12 L/min and widened the LOA from ± 0.29 L/min to ± 1.43 L/min. Although the AOA was markedly worsened by nitroprusside (PB: 43%), the POATESTxREF did not seem to be markedly affected when the vasodilatory state (PE: 29%) was compared to the vasoconstrictive state (PE: 23%). However, the PE values recorded during nitroprusside CRI were underestimated because of the poor AOA between methods. Because PCACO values were substantially higher than PATDCO measurements (mean bias 2.12 L/min), there was an artifactual increase in the denominator of the PE formula (mean CO), leading to an underestimation of PE calculated during the vasodilatory state. Therefore, the PE recorded during nitroprusside CRI would have been even larger if the AOA was not markedly deviated from zero.
Because the agreement between methods was not the same as the hemodynamic state changed from high to low SVR, the ability of PCACO to track changes in PATDCO was also poor as shown by the 4‐quadrant plot and polar plot analysis. These observations, confirm previous reports showing that calibrated pulse contour analysis does not provide reliable agreement with PATDCO in individuals that present hemodynamic instability induced by vasodilatory states.25
Analysis of the arterial pressure wave showed that the dicrotic notch was not identifiable during nitroprusside‐induced vasodilation in the present study. Vasodilatory states attenuate the rebound of arterial blood (wave reflection) against the closed aortic valve at the end of the ejection phase and this phenomenon could have blunted or attenuated the dicrotic notch during nitroprusside infusion.35 Failure of the algorithm to detect the end of the ejection phase (dicrotic notch) during nitroprusside‐induced vasodilation could lead to an artifactual increase in the area under the systolic portion of the arterial pressure wave, causing overestimation of CO values.18
A limitation of the TPTDCO method is the requirement for catheterization of a central artery. More peripheral arteries (eg, dorsal metatarsal artery in dogs) would be more practical and carry lower risk of complications, but in one report the TPTDCO monitor failed to detect a 20 mL bolus of ice‐cold physiological saline when a 22 cm long thermodilution catheter was placed in the dorsal metatarsal artery in 8 out of 30 attempts for calibrating the PCACO with TPTDCO.36 Although no catheter‐related complications were observed in the present report, femoral artery catheterization might result in increased risk of bleeding/hematoma and these potential morbidities should be considered if the technique is used in veterinary patients.
Among the study limitations, one should consider the relatively small number of animals evaluated. However, even with this limited number of animals, clear differences in the agreement and trending ability of the 2 tested methods (TPTDCO and PCACO) against the reference technique (PATDCO) were evident. Also, this study had some limitations in the design as comparisons between TPTDCO and PATDCO were not performed during changes in SVR; whereas comparisons between PCACO and PATDCO were not performed over a wide range of CO values. Because hemodynamic conditions induced during Phase‐1 and Phase‐2 differed substantially, results obtained during both phases might not be directly comparable.
Conclusion
The use of a larger volume of thermal indicator (10 mL versus 5 mL of ice‐cold physiological saline) minimizes the trend for TPTDCO to overestimate PATDCO. Although the use of 10 mL of thermal indicator significantly decreases the bias and slightly improves the agreement (AOA and POA) and the trending ability between TPTDCO and PATDCO when compared with a lower volume (5 mL) of ice‐cold indicator, both volumes can be used for TPTDCO measurements in replacement of PATDCO in dogs.
While phenylephrine‐induced vasoconstriction caused a dual effect on the agreement between calibrated PCACO and PATDCO (increased AOA and decreased POA) without significantly changing the bias, nitroprusside‐induced vasodilation worsened the overall agreement between these methods (significantly larger bias, decreased AOA and POA) and caused a significant overestimation of PATDCO by PCACO. Because the agreement and the ability of PCACO to track changes in PATDCO are poor during drug‐induced changes in SVR, this method is of limited clinical usefulness.
Acknowledgments
Funding was provided by “Fundação de Amparo à Pesquisa de São Paulo” (2012/03207‐2). A sincere gratitude goes to Professor Emeritus Wayne McDonell (University of Guelph, Canada) for inspiring the senior author (F.J. Teixeira‐Neto) to accomplish this work.
Conflict of Interest Declaration:
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration:
Authors declare no off‐label use of antimicrobials.
This work was performed at the “Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista, UNESP, Botucatu, Sáo Paulo, Brazil, CEP 18618–970”.
Footnotes
Insyte, Becton Dickinson, Juiz de Fora, MG, Brazil
Propovan, Cristália, São Paulo, Brazil
Isoforine, Cristália, São Paulo, Brazil
Dräger Primus, Drägerwerk AG & Co, Lübeck, Germany
PV2013L07N, Pulsion Medical Systems, Munich, Germany
PV4046, Pulsion Medical Systems, Munich, Germany
PiCCO module/DX‐2020 Monitor, Dixtal Biomédica, São Paulo, SP, Brazil
Model 131HF7, Edwards Lifesciences, Irvine, CA
PX260, Edwards Lifesciences, Irvine, CA
AS/3, Datex‐Ëngstrom, Helsinki, Finland
Ultiva, GlaxoSmithkline, Rio de Janeiro, RJ, Brazil
Dobutrex, Pfizer, Guarulhos, SP, Brazil
Felinefrin, Cristália, Itapira, SP, Brazil
Nitrop, Hypofarma, Ribeirão das Neves, MG, Brazil
Maxicam 0.2%, Ouro Fino, Cravinhos, SP, Brazil
Medcalc 15.8, Medcalc Software bvba, Ostend, Belgium
Prism 6.02, GraphPad, San Diego, CA
Microsoft Excel 2007, Redmond, WA
Sigmaplot 13, Systat Software, San Jose, CA
References
- 1. Ganz W, Donoso R, Marcus HS, et al. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol 1971;27:392–396. [DOI] [PubMed] [Google Scholar]
- 2. Reuter DA, Huang C, Edrich T, et al. Cardiac output monitoring using indicator‐dilution techniques: basics, limits, and perspectives. Anesth Analg 2010;110:799–811. [DOI] [PubMed] [Google Scholar]
- 3. Bossert T, Gummert JF, Bittner HB, et al. Swan‐Ganz catheter‐induced severe complications in cardiac surgery: right ventricular perforation, knotting, and rupture of a pulmonary artery. J Card Surg 2006;21:292–295. [DOI] [PubMed] [Google Scholar]
- 4. Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary‐artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213–2224. [DOI] [PubMed] [Google Scholar]
- 5. Litton E, Morgan M. The PiCCO monitor: a review. Anaesth Intensive Care 2012;40:393–409. [DOI] [PubMed] [Google Scholar]
- 6. Sakka SG, Reuter DA, Perel A. The transpulmonary thermodilution technique. J Clin Monit Comput 2012;26:347–353. [DOI] [PubMed] [Google Scholar]
- 7. Böck JC, Barker BC, Mackersie RC, et al. Cardiac output measurement using femoral artery thermodilution in patients. J Crit Care 1989;4:106–111. [Google Scholar]
- 8. Sakka SG, Reinhart K, Meier‐Hellmann A. Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med 1999;25:843–846. [DOI] [PubMed] [Google Scholar]
- 9. Zöllner C, Haller M, Weis M, et al. Beat‐to‐beat measurement of cardiac output by intravascular pulse contour analysis: a prospective criterion standard study in patients after cardiac surgery. J Cardiothorac Vasc Anesth 2000;14:125–129. [DOI] [PubMed] [Google Scholar]
- 10. Della Rocca G, Costa MG, Pompei L, et al. Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth 2002;88:350–356. [DOI] [PubMed] [Google Scholar]
- 11. Østergaard M, Nielsen J, Rasmussen JP, et al. Cardiac output – pulse contour analysis vs. pulmonary artery thermodilution. Acta Anaesthesiol Scand 2006;50:1044–1049. [DOI] [PubMed] [Google Scholar]
- 12. Rupérez M, López‐Herce J, García C, et al. Comparison between cardiac output measured by the pulmonary arterial thermodilution technique and that measured by the femoral arterial thermodilution technique in a pediatric animal model. Pediatr Cardiol 2004;25:119–123. [DOI] [PubMed] [Google Scholar]
- 13. Kutter AP, Jud Schefer RS, Bircher B, et al. Comparison of pulmonary artery and transpulmonary thermodilution cardiac output measurements in unsedated newborn calves. Vet Anaesth Analg 2015;42:614–622. [DOI] [PubMed] [Google Scholar]
- 14. Beaulieu KE, Kerr CL, McDonell WN. Evaluation of transpulmonary thermodilution as a method to measure cardiac output in anesthetized cats. Can J Vet Res 2009;73:1–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Kutter AP, Bektas RN, Hofer CK, et al. Trending ability and limitations of transpulmonary thermodilution and pulse contour cardiac output measurement in cats as a model for pediatric patients. J Clin Monit Comput 2015;29:377–383. [DOI] [PubMed] [Google Scholar]
- 16. Friedman Z, Berkenstadt H, Margalit N, et al. Cardiac output assessed by arterial thermodilution during exsanguination and fluid resuscitation: experimental validation against a reference technique. Eur J Anaesthesiol 2002;19:337–340. [DOI] [PubMed] [Google Scholar]
- 17. Morgaz J, Granados Mdel M, Muñoz‐Rascón P, et al. Comparison of thermodilution, lithium dilution, and pulse contour analysis for the measurement of cardiac output in 3 different hemodynamic states in dogs. J Vet Emerg Crit Care (San Antonio) 2014;24:562–570. [DOI] [PubMed] [Google Scholar]
- 18. Wesseling KH, Jansen JR, Settels JJ, et al. Computation of aortic flow from pressure in humans using a nonlinear, three‐element model. J Appl Physiol 1993;74:2566–2573. [DOI] [PubMed] [Google Scholar]
- 19. Buhre W, Weyland A, Kazmaier S, et al. Comparison of cardiac output assessed by pulse‐contour analysis and thermodilution in patients undergoing minimally invasive direct coronary artery bypass grafting. J Cardiothorac Vasc Anesth 1999;13:437–440. [DOI] [PubMed] [Google Scholar]
- 20. Goedje O, Hoeke K, Lichtwarck‐Aschoff M, et al. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: comparison with pulmonary arterial thermodilution. Crit Care Med 1999;27:2407–2412. [DOI] [PubMed] [Google Scholar]
- 21. Hamzaoui O, Monnet X, Richard C, et al. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6‐hour calibration‐free period. Crit Care Med 2008;36:434–440. [DOI] [PubMed] [Google Scholar]
- 22. Monnet X, Anguel N, Naudin B, et al. Arterial pressure‐based cardiac output in septic patients: different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit Care 2010;14:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rödig G, Prasser C, Keyl C, et al. Continuous cardiac output measurement: pulse contour analysis vs thermodilution technique in cardiac surgical patients. Br J Anaesth 1999;82:525–530. [DOI] [PubMed] [Google Scholar]
- 24. Bein B, Meybohm P, Cavus E, et al. The reliability of pulse contour‐derived cardiac output during hemorrhage and after vasopressor administration. Anesth Analg 2007;105:107–113. [DOI] [PubMed] [Google Scholar]
- 25. Yamashita K, Nishiyama T, Yokoyama T, et al. The effects of vasodilation on cardiac output measured by PiCCO. J Cardiothorac Vasc Anesth 2008;22:688–692. [DOI] [PubMed] [Google Scholar]
- 26. Muller L, Candela D, Nyonzyma L, et al. Disagreement between pulse contour analysis and transpulmonary thermodilution for cardiac output monitoring after routine therapeutic interventions in ICU patients with acute circulatory failure. Eur J Anaesthesiol 2011;28:664–669. [DOI] [PubMed] [Google Scholar]
- 27. Critchley LA, Critchley JA. A meta‐analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999;15:85–91. [DOI] [PubMed] [Google Scholar]
- 28. Cecconi M, Rhodes A, Poloniecki J, et al. Bench‐to‐bedside review: the importance of the precision of the reference technique in method comparison studies with specific reference to the measurement of cardiac output. Crit Care 2009;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hapfelmeier A, Cecconi M, Saugel B. Cardiac output method comparison studies: the relation of the precision of agreement and the precision of method. J Clin Monit Comput 2016;30:149–155. [DOI] [PubMed] [Google Scholar]
- 30. Perrino AC Jr, O'Connor T, Luther M. Transtracheal doppler cardiac output monitoring: comparison to thermodilution during noncardiac surgery. Anesth Analg 1994;78:1060–1066. [DOI] [PubMed] [Google Scholar]
- 31. Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 2010;111:1180–1192. [DOI] [PubMed] [Google Scholar]
- 32. Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth 2011;25:536–546. [DOI] [PubMed] [Google Scholar]
- 33. Nishikawa T, Dohi S. Errors in the measurement of cardiac output by thermodilution. Can J Anaesth 1993;40:142–153. [DOI] [PubMed] [Google Scholar]
- 34. Linton RA, Jonas MM, Tibby SM, et al. Cardiac output measured by lithium dilution and transpulmonary thermodilution in patients in a paediatric intensive care unit. Intensive Care Med 2000;26:1507–1511. [DOI] [PubMed] [Google Scholar]
- 35. Hoeksel SA, Jansen JR, Blom JA, Schreuder JJ. Detection of dicrotic notch in arterial pressure signals. J Clin Monit 1997;13:309–316. [DOI] [PubMed] [Google Scholar]
- 36. Shih A, Maisenbacher HW, Bandt C, et al. Assessment of cardiac output measurement in dogs by transpulmonary pulse contour analysis. J Vet Emerg Crit Care (San Antonio) 2011;21:321–327. [DOI] [PubMed] [Google Scholar]


