Abstract
Background
The establishment and progression of metastases remains the life‐limiting factor for dogs diagnosed with osteosarcoma (OS). The pattern of metastases is likely regulated through interactions between chemokine receptors and chemokines, and perturbations in these signaling cascades responsible for cytoskeletal organization and directional migration have the potential to alter metastatic cell trafficking behaviors.
Hypothesis
Zoledronate will impair directional migration of OS cells through downregulation of chemokine (C‐X‐C motif) receptor 4 (CXCR4) expression and functionality.
Samples
Nineteen archived tumor specimens and plasma from 20 dogs with OS.
Methods
Prospectively, the expressions of CXCR4 were studied in OS cell lines and spontaneous tumor samples. The effect of zoledronate on CXCR4 expression and functionality was investigated by characterizing responses in 3 OS cell lines. In 19 OS specimens and 20 dogs with OS, changes in CXCR4 expression and circulating CXCR4 concentrations were characterized in response to zoledronate therapy respectively.
Results
All canine OS cells express CXCR4, and zoledronate reduces CXCR4 expression and functionality by 27.7% (P < .0001), through augmented proteasome degradation and reduced prenylation of heterotrimeric G‐proteins in 33% of tumor cell lines evaluated. In OS‐bearing dogs, zoledronate reduces CXCR4 expressions by 40% within the primary tumor compared to untreated controls (P = .03) and also decreases the circulating concentrations of CXCR4 in 18 of 20 dogs with OS.
Conclusions and clinical importance
Zoledronate can alter CXCR4 expression and functionality in OS cells, and consequent perturbations in CXCR4 intracellular signaling cascades might influence patterns of metastases.
Keywords: Aminobisphosphonate, Cell chemotaxis, Pattern of metastases
Abbreviations
- OS
osteosarcoma
- SDF‐1α
stromal derived factor 1 alpha
- FPPS
farnesyl pyrophosphate synthetase
- GGOH
geranylgeraniol
Introduction
The establishment of metastases is an expected clinical sequela in the majority of dogs diagnosed with osteosarcoma (OS), and consequently remains the major cause of death in this affected population of animals. Distinct patterns of metastases are associated with differing tumor histologies, and the pulmonary parenchyma serves as the preferential site for successful metastatic colonization in dogs with OS treated with amputation alone or receiving adjuvant chemotherapy.1, 2, 3 The tropism of OS cells for the lung microenvironment is likely attributed to multiple, nonexclusive biologic processes including the provision of a suitable microenvironment conducive for colonization, mechanical entrapment due to restrictive microvasculature diameters, and host‐tumor specific interactions reliant upon active receptor and ligand signaling.4, 5, 6 In particular, leukocyte trafficking mechanisms mediated through chemokine receptor signaling have received considerable attention as an active strategy subverted by metastatic tumor cells in mediating directional migration toward distant organs.6
Chemokine receptors are serpentine transmembrane receptors that signal through heterotrimeric G‐proteins consisting of α and βγ subunits adhered to the inner plasma membrane leaflet by fatty acid acylation and prenylation respectively.7, 8 Upon binding with cognate chemokine ligands, activated heterotrimeric G‐proteins mediate intracellular signaling through the generation of secondary messengers such as cAMP and calcium.9 Although over 20 different chemokine receptor/ligand pairs have been characterized and demonstrated to participate in directional cell migration, the CXCR4/SDF‐1α axis has been explored most extensively as a druggable pathway in the context of solid tumor metastases.10 Although small molecule inhibitors of CXCR4 signaling have shown considerable promise in preclinical models,11, 12 in addition to having been evaluated in patients diagnosed with advanced solid tumor malignancies including breast cancer,13 the tolerability and clinical benefit of such inhibitors in combination with standard‐of‐care therapeutics has yet to be reported.
Zoledronate is a third generation aminobisphosphonate that potently inhibits osteoclastogenesis, and is used as a first line agent in combination with conventional treatment options to attenuate the development of skeletal metastases associated with diverse solid tumor malignancies, in particular breast cancer.14 Mechanistically, zoledronate's capacity to impede malignant colonization of bone by cancer cells is attributed to both microenvironmental and cell specific effects. Given its potent antiresorptive properties, zoledronate creates an inhospitable tumor microenvironment within osseous tissues, and reduces the success of metastatic colonization by attenuating pathologic osteoclastogenesis.15 Additionally, zoledronate inhibits prenylation‐dependent signaling pathways responsible for key cytoskeletal processes,16 which if perturbed could impair the establishment of distant solid tumor metastases. The role of zoledronate in suppressing cancer cell dissemination remains controversial; however, specific to breast cancer, evidence supporting the antimetastatic effects of zoledronate includes in vitro studies demonstrating the reduction of CXCR4 expression in human breast cancer cells with consequent impaired cell motility and invasion.17 Concordant with these in vitro findings, a subset of women with breast cancer receiving adjuvant zoledronate combined with standard‐of‐care therapy have reduced risk of disease recurrence, which include metastases to skeletal and nonskeletal tissues.18 Collectively, these intriguing findings suggest the possibility that zoledronate might exert some effect on cancer cell metastases through alterations in CXCR4 signaling and consequent motility.
The contribution of chemokine receptors in the behavior of companion animal tumors remains poorly defined; however, emerging evidence suggests their potential role in the biology of aggressive sarcomas, such as hemangiosarcoma and OS.19, 20 Given the metastatic phenotype associated with these particularly high grade sarcomas, strategies that disrupt CXCR4 signaling could alter natural disease progression and potentially improve survival time in this population of dogs. Based upon prior studies demonstrating zoledronate's inhibitory effect on the metastatic properties of breast cancer cells, the purposes of this investigation were to (1) annotate altered expressions of CXCR4 as a consequence of zoledronate exposure in a limited panel of canine OS cell lines; (2) explore potential molecular mechanisms induced by zoledronate in altering CXCR4 expression and functionality; (3) compare CXCR4 expressions at the level of primary tumor and systemic circulation in dogs with OS receiving or not receiving zoledronate; and (4) describe the pattern of metastases observed in a small cohort of dogs treated with zoledronate therapy in the absence of systemic cytotoxic treatment.
Materials and Methods
Reagents and Antibodies
Zoledronate was generously provided by Novartis Pharma AG. Anti‐human CXCR4 antibody1 (ab2074), anti‐human farnesyl pyrophosphate synthetase (FPPS) antibody1 (ab153805), anti‐β actin antibody1 (AC‐15), and anti‐human γ5 antibody2 were purchased from commercial vendors. Reagents AMD31003, IBMX4 , geranylgeraniol4 (GGOH), MG1325, and human recombinant SDF‐1α6 (13511‐H07E‐10) were purchased from commercial vendors. Image IT Fx Signal Enhancer7 (R37107), ProLong Gold Antifade Mount7 (P10144), 4’,6‐diamidino‐2‐phenylindole7 (DAPI), and goat anti‐rabbit Alexa 488 secondary antibody7 (A‐11034) were purchased from Thermo Fisher Scientific.
Cell Protein Collection
Cells were grown to 80–100% confluence with zoledronate 1 μM or 5 μM for 48 hours continuously, and then cells were washed with phosphate buffered saline (PBS). In some studies with proteasome inhibition, K003 cells were pre‐incubated with 1 μM MG132 for 60 minutes, and then exposed to zoledronate 1 μM or 5 μM for 48 hours. After exposure to experimental conditions, cells were trypsinized and centrifuged at 450 g for 5 minutes at 4°C. Cell pellets were homogenized in 1 mL PBS, centrifuged at 1,100 g for 5 minutes at 4°C, resuspended with 100 μL of Mammalian Protein Extraction Reagent7, mixed with protease inhibitor cocktail solution7 for 15 minutes, and then centrifuged at 1,100 g for 10 minutes at 4°C. Protein concentrations of the resultant supernatants were assessed for protein concentrations using a standard assay kit7.
Western Blot Analysis
For investigated protein, 50 μg samples were electrophoresed on 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and block with tris buffered saline‐tween 20 (TBST) with 5% milk for 1 hour at room temperature. Western blot analysis was performed using anti‐human CXCR4 or anti‐human FPPS antibody at a concentration of 1:1000 in TBST with 5% milk, incubated for 1 hour at room temperature. The membrane was then washed 3 times with TBST, probed with the secondary antibody diluted 1:5000 in TBST with 5% milk, and developed using ChemiDoc XRS+ molecular imager system8 . Band volume analysis was done using Image Lab software8. Relative protein expressions were adjusted against β‐actin using anti‐human β‐actin antibody at a concentration of 1:5000 in TBST with 5% milk, incubated for 1 hour at room temperature. Results reported were derived from at least 2 independent experiments.
Confocal Fluorescent Microscopy
Cells were seeded at 104 cells per well and exposed to zoledronate 1 μM or 5 μM for 48 hours in phenol red‐free DMEM in chamber well slides. In some studies with proteasome inhibition, K003 cells were pre‐incubated with 1 μM MG132 for 60 minutes, rinsed with PBS, and then exposed to zoledronate 1 μM or 5 μM for 48 hours. In prenylation rescue studies with exogenous GGOH, K003 cells were co‐incubated with 20 μM GGOH for 48 hours with zoledronate 5 μM. Following exposure to different conditions, cells were washed with phenol red‐free DMEM, and fixed with 4% methanol‐free paraformaldehyde for 10 minutes at room temperature. Cells were permeabilized for 5 minutes at room temperature using 0.1% Triton X‐100, and preblocked with 5 drops of IT signal FX solution for 30 minutes at room temperature. Cells were washed with PBS, blocked with 3% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature and then rinsed with PBS. Either anti‐human CXCR4 or γ5 antibody (1:100 in 3% BSA in PBS) was incubate with fixed cells for 24 hours at 4°C, then counterstained with DAPI (1:100 in 3% BSA in PBS) for 15 minutes. Cells were incubated with Alexa Fluor® 488 goat anti‐rabbit antibody (1:100 in 3% BSA in PBS) for 60 minutes at room temperature while protected from light. Cells were washed with PBS and mounted with 5 drops of ProLong Gold solution, and left to dry at room temperature for 24 hours protected from light. Cells were imaged using a Ziess LSM 700 confocal laser scanning microscope, and image analysis performed with ImageJ software9 . CXCR4 or γ5 stain intensity was derived from 50 individual cell counts/well and expressed as fluorescence intensity per surface area (RFU/μm2). A total of 3 independent experiments were conducted.
Cyclic AMP Assay
Intracellular concentrations of cAMP were measured with a commercial assay10 . K003 cells were seeded at 104 cells per well with zoledronate 1 μM or 5 μM for 24 hours in a 96‐well plate. After preincubation with or without zoledronate, K003 cells were incubated in the absence of serum for 1 hour. Subsequently, cells were incubated with 500 μM of IBMX, a phosphodiesterase inhibitor, for 30 minutes to prevent the degradation of cAMP following either stimulatory (SDF‐1α) or inhibitory (AMD3100) conditions. K003 cells were exposed to various SDF‐1α concentrations (0–300 ng/mL) for 15 minutes with or without concurrent AMD3100 (1 μg/mL). After 15 minutes, stimulatory and inhibitory conditions were removed, cells were lysed, and intracellular cAMP concentrations measured using manufacturer instructions.
Chemotaxis and Migration Assay
K003 cells were pretreated with zoledronate 1 μM or 5 μM for 24 hours in complete media, and then grown under serum‐free conditions for an additional 24 hours. Cells were harvested and rinsed with PBS. Cells were seeded at 5 × 104 cells per 100 μL and loaded into the upper chamber of a 96‐well migration plate. In some wells, K003 cells were co‐incubated with AMD3100 (1 μg/mL). To the lower feeder tray, serum free media with or without SDF‐1α (100 ng/mL) was added. Cells were left undisturbed and allowed to migrate for 6 hours at 37°C. Migrant cells trapped within the intermembranous filter between upper and lower trays were incubated with a fluorescent reporter and subsequently lysed, and fluorescence was measured using manufacturer's kit11 instructions.
In Vitro Zoledronate Cytotoxicity Analysis
For cytotoxicity analysis, an apoptosis detection kit12 was used according to the manufacturer's directions. K003 OS cells were incubated with zoledronate 1 μM or 5 μM for 48 hours. Adherent and nonadherent cells were collected and centrifuged at 450 g for 5 minutes. Cells were resuspended in 100 μL chilled binding buffer and incubated with 5 μL of Annexin‐V FITC and 5 μL of propidium iodide for 15 minutes protected from light. Following incubation, an additional 400 μL binding buffer was added and samples analyzed by using a BD Accuri C6 flow cytometer.
Caspase‐3 Colorimetric Assay
Enzymatic activities of caspase‐3 were determined by a colorimetric assay kit13 according to the manufacturer's protocol. K003 OS cells were incubated with zoledronate 1 μM or 5 μM for 48 hours. Exposure to staurosporine 1 mM for 4 hours served as a positive caspase activating control. Cells were collected, centrifuged into a pellet, and lysed with 25 μL of lysis buffer. Lysates were centrifuged at 1,100 g for 1 minute, then 100 μL lysates per well were transferred into a 96 well plate, and 5 μL of caspase‐3 colorimetric substrate (DEVD‐pNA) was then added for 1–2 hours at 37°C. Colorimetric changes were measured using a microplate reader and normalized against protein concentration.
Immunohistochemistry
Nineteen archived tissue blocks containing OS primary tumors derived from the distal radius (8), proximal humerus (4), distal tibia (3), proximal tibia (2), proximal femur (1), and distal ulna (1) were retrieved from the University of Illinois Veterinary Diagnostic Laboratory for immunohistochemical assessment. Nine specimens were from dogs receiving a standardized palliative protocol inclusive of 20 gray ionizing radiation, oral analgesics (carprofen, tramadol, and gabapentin), and serial zoledronate treatments (median 11 treatments, range 4–16) every 4 weeks prior to limb amputation, while 10 specimens originated from dogs’ naïve to zoledronate exposure and had received variable therapeutic management prior to amputation. Slides were deparaffinized in xylene and rehydrated in ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 15 minutes. Slides were incubated with preheated 0.1% protease at 37°C for 20 minutes, and then rinsed in wash buffer for 2 minutes. Nonspecific staining was minimized with incubation for 10 minutes with Power Block14 , and then blocked for 15 minutes with avidin and biotin block. Slides were incubated with anti‐human CXCR4 antibody (1:100) for 1 hour at room temperature. Slides were incubated with a biotinylated secondary antibody for 20 minutes at room temperature; then washed in buffer before incubation for 20 minutes with a streptavidin–biotinylated horseradish peroxidase complex15, and developed with DAB substrate for 5 minutes. Slides were counterstained with hematoxylin and evaluated by a single investigator (KLW). Negative controls for the samples were processed identically in the absence of the primary antibody. Using ImageJ software, immunohistochemical staining positivity was expressed as normalized pixel intensity per positive cell.
Circulating CXCR4 Assay
In 20 dogs with stage IIb, histologically confirmed OS (Table S1), the plasma concentrations of CXCR4 were quantified using a canine‐specific CXCR4 ELISA kit16 . Paired plasma samples were measured before treatment with zoledronate (Pre‐ZOL) and repeated 28 days following a single intravenous treatment of zoledronate (Post‐ZOL) combined with 20 gray ionizing radiation and standardized oral analgesics (carprofen, tramadol, and gabapentin). Zoledronate was administered as a 15‐minute constant rate infusion at a dosage of 0.1 mg/kg. The assay was performed according to manufacturer instructions.
Descriptive Analysis of OS Metastatic Pattern
In 9 dogs with OS receiving serial zoledronate therapy every 4 weeks prior to the development of advanced metastatic disease burden, complete necropsies were performed to characterize the patterns of metastases identified on gross and microscopic examination.
Statistical Analysis
The distribution of continuous variable data was evaluated using the Shapiro–Wilk test, skewness, kurtosis, and q–q plots. Data were analyzed with parametric methods based upon the achievement of normality assumptions. For CXCR4 expressions, 1‐way ANOVA was used to evaluate for differences among treatment groups with the use of Dunnet's post‐hoc test. For the comparison of 2 data sets, 2‐tailed student t‐test or 2‐tailed paired t‐test was employed for normal distributed data sets respectively. Statistical calculations were performed using a commercial software program17 , and P < .05 was considered statistically significant for all analyses.
Results
Zoledronate Alters CXCR4 Protein Expression in Some OS Cell Lines
After antibody validation (Fig S1A), it was determined that CXCR4 was basally expressed by all 3 immortalized canine OS cell lines utilized in this study, and exposure to biologically achievable concentrations of zoledronate21 for 48 hours resulted in variable regulation of CXCR4 expression (Fig 1A and B). Cell lineage susceptibility to zoledronate‐induced alterations in CXCR4 was imperfectly associated with relative expressions of FPPS, the enzyme target of zoledronate. The Abrams cell line expressed FPPS robustly, while both K003 and HMPOS cell lines demonstrated modest FPPS expressions (Fig S1B). In K003 cells, CXCR4 expression was reduced as demonstrated by qualitative western blot analysis (Fig 1C), demonstrating greater than 50% decrease following incubation with zoledronate. In corroboration, quantitative confocal fluorescent microscopy (Fig 1B and D) identified reductions in normalized CXCR4 fluorescent expression as a function of zoledronate exposure, being 104.6 ± 25.3, 86.3 ± 21.2, and 75.6 ± 18.4 RFU/μm2 for untreated control, 1 μM zoledronate, and 5 μM zoledronate respectively (Fig 1D). Both concentrations of zoledronate reduced CXCR4 expression in K003 cells compared to untreated control, P < .01. Incubation of K003 cells with aqueous vehicle (sterile water) did not affect CXCR4 expression (Fig S2A). Contrary with K003, no consistent change in CXCR4 expression following zoledronate exposure was identified in either Abrams or HMPOS cell lines by western blot analysis or confocal fluorescent microscopy (Fig 1A, C, and D).
Figure 1.
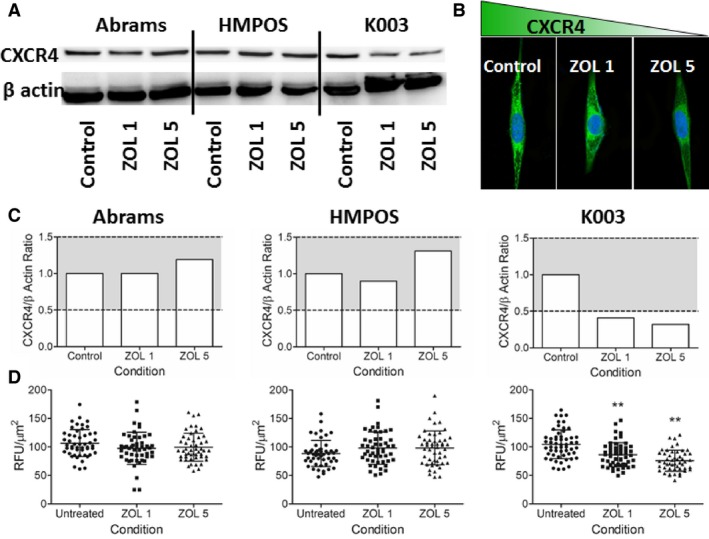
(A) Modulation of CXCR4 expression as a function of zoledronate concentration in 3 canine OS cell lines by western blot analysis. (B) Reduced CXCR4 expression by confocal fluorescent microscopy in K003 cells following exposure to zoledronate for 48 hours. (C‐D) Correlative changes in CXCR4 protein expression represented graphically as normalized values by (C) western blot and (D) confocal fluorescent microscopy. Gray shaded region (C) denotes ± 50% qualitative change in CXCR4 protein expression relative to untreated control cells. (D) Based upon 50 cell counts, quantitative reductions in CXCR4 expression in K003 cells following exposure to low concentrations of zoledronate as assessed by confocal fluorescent microscopy. Data expressed as mean ± SD and significance defined as **P < .01.
Molecular Mechanism of Zoledronate for Reducing CXCR4 in K003 Cells
To explore how zoledronate might regulate CXCR4 expressions in K003 cells, transcriptional and post‐translational mechanisms were investigated. Based on real‐time polymerase chain reaction experiments, no substantive change was identified in the transcriptional regulation of CXCR4 in K003 cells following zoledronate exposure. In comparison with untreated cells, exposure to zoledronate 1 μM or 5 μM resulted in 1.6 and 1.1‐fold increases in transcriptional activities of CXCR4, respectively; findings which suggested the reductions in CXCR4 expression in K003 cells were likely post‐transcriptional in nature. Based upon prior studies characterizing the role of proteasome degradation in chemokine receptor homeostatic recycling,22 the effects of incubating K003 cells with MG132, a potent proteasome inhibitor, were studied by western blot analysis (Fig 2A–B) and confocal fluorescent microscopy (Fig 2C–D). Qualitatively, the addition of MG132 to untreated K003 cells increased CXCR4 expressions above baseline levels by approximately 50%. The co‐addition of MG132 and 5 μM zoledronate robustly augmented the expression of CXCR4 by approximately 2‐fold in comparison with untreated K003 cells. Concordant with western blot analysis, quantitative comparisons derived from confocal fluorescent studies demonstrated MG132's capacity to enhance CXCR4 expressions. Normalized fluorescent intensities for CXCR4 expression in untreated K003 cells (30127.7 ± 6230.7 RFU/μm2) were greater than cells treated with zoledronate at 1 μM (26389.0 ± 7050.3 RFU/μm2; P < .05) and 5 μM (15121.5 ± 4035.2 RFU/μm2; P < .01) respectively. Concurrent incubation of MG132 with K003 cells exposed to either 1 μM (32857.0 ± 7,466.9 RFU/μm2; P < .001) or 5 μM (33401.6 ± 7806.7 RFU/μm2; P < .001) zoledronate completely inhibited any reduction in CXCR4 expression induced with zoledronate alone.
Figure 2.
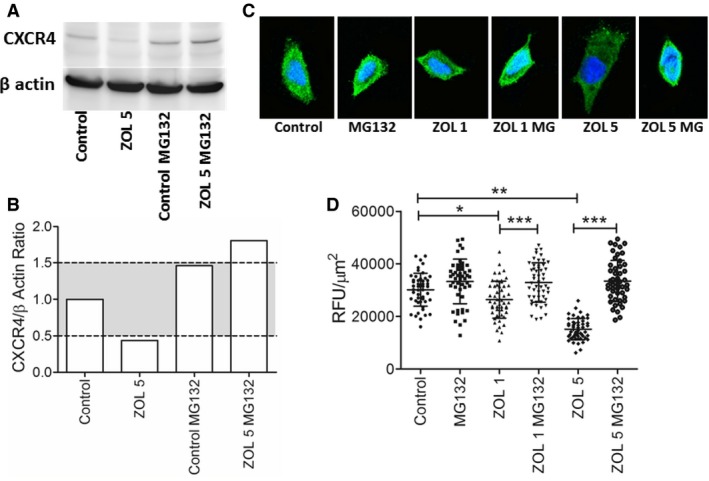
Qualitative modulation of CXCR4 protein expression by zoledronate and proteasome inhibition represented by (A) western blot analysis and (B) normalized graphical presentation where gray shaded region denotes ± 50% change in CXCR4 expression relative to untreated control cells. (C) Visual reduction in CXCR4 fluorescent intensities in K003 cells exposed to low concentrations of zoledronate, and complete normalization of CXCR4 expression with the co‐addition of MG132, a proteasome inhibitor. (D) Based upon a 50 cell count, quantitative changes in CXCR4 expression in K003 cells exposed to zoledronate with or without co‐addition of MG132, a proteasome inhibitor. Data expressed as mean ± SD and significance defined as *P < .05, **P < .01, and ***P < .001.
Zoledronate Impairs cAMP Generation and Consequent Directional Migration
To explore the functional consequences of reduced CXCR4 expression induced by zoledronate in K003 cells, intracellular cAMP and directional cell migration were characterized following exogenous stimulation. In untreated K003 cells, a dose‐dependent increase in intracellular cAMP was elicited with the addition of SDF‐1α (Fig 3A). At the lowest concentration of SDF‐1α (30 ng/mL), K003 cells produced 4.8 ± 0.9 nM cAMP. At this low level of stimulation, co‐incubation of K003 cells with 1 μg/mL of AMD3100 did not significantly attenuate cAMP production (3.2 ± 1.2 nM; P = .14). However, 5 μM zoledronate exposure reduced the production of cAMP (2.3 ± 0.8 nM; P < .05) after stimulation with the lowest level SDF‐1α (30 ng/mL) in comparison with untreated K003 cells. With greater concentrations of exogenous SDF‐1α (100 and 300 ng/mL), both AMD3100 and 5 μM zoledronate exposure blunted cAMP production in comparison to untreated K003 cells (Fig 3A). With 100 ng/mL of SDF‐1α, the concentrations of cAMP produced in untreated, AMD3100, and 5 μM zoledronate exposed K003 cells were 9.3 ± 0.6 nM, 1.9 ± 0.3 nM (P < .01), and 1.9 ± 0.4 nM (P < .01) respectively. Similarly, the concentrations of cAMP elicited by 300 ng/mL of SDF‐1α were different between untreated and treated (AMD3100 and 5 μM zoledronate) K003 cells being 15.1 ± 1.3 nM and (2.2 ± 0.3 nM; P < .01 and 2.3 ± 0.8 nM; P < .01) respectively.
Figure 3.
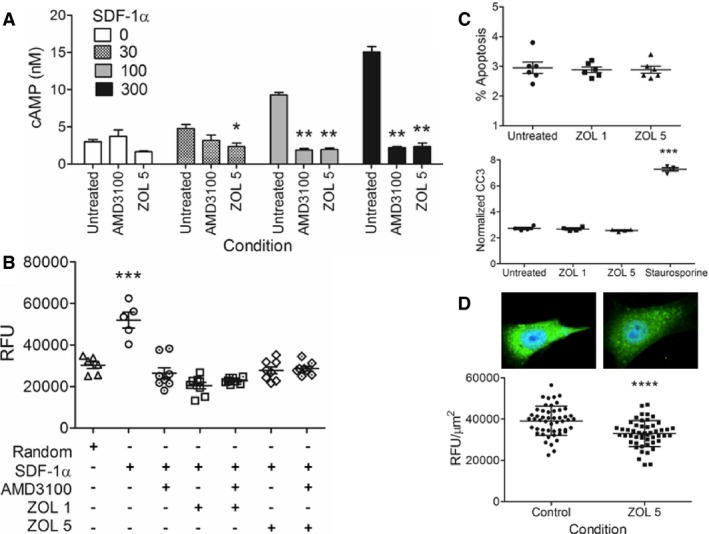
(A) Quantitative differences in cAMP generation in K003 cells stimulated with SDF‐1α (0–300 ng/mL) alone or with inhibitory agents, 1 μg/mL AMD3100 or zoledronate 5 μM. (B) SDF‐1α induced directional cell migration of K003 cells, either untreated or following exposure to inhibitory agents, 1 μg/mL AMD3100, zoledronate (1 or 5 μM), or combination. (C) Percent apoptosis and normalized cleaved caspase‐3 activities in K003 cells either untreated or exposed to low concentrations of zoledronate (1–5 μM). (D) Visual and quantitative (based upon a 50 cell count), expression of γ5 subunit in K003 cells, untreated, or exposed to 20 μM GGOH, 5 μM zoledronate, or combination. Data expressed as mean ± SD and significance defined as *P < .05, **P < .01, ***P < .001, and ****P < .0001.
To assess the impact of reduced intracellular cAMP on the biologic processes involved in cytoskeletal dynamics, quantitative changes in directional migration were studied in K003 cells following exposure to stimulatory and inhibitory conditions. Random and SDF‐1α (100 ng/mL) induced migration of K003 cells was quantitatively different, being 30,260 ± 4,170 RFU and 51,890 ± 8,500 RFU; P < .001 respectively (Fig 3B). Directional migration elicited by SDF‐1α was completely attenuated and comparable to random migration following exposure of K003 cells to all inhibitory conditions, singly or in combination; AMD3100 (26,380 ± 7,510 RFU), 1 μM zoledronate (20,390 ± 4,480 RFU), 1 μM zoledronate + AMD3100 (22,790 ± 1,490 RFU) 5 μM zoledronate (27,730 ± 4,820 RFU), and 5 μM zoledronate + AMD3100 (28,590 ± 3,040 RFU).
Participatory Molecular Mechanisms for Blunted CXCR4 Functionality
The magnitude of impaired CXCR4 secondary messenger generation and consequent migration were unexpected findings given the extent of CXCR4 reduction (~50%) following exposure to zoledronate, and suggested the involvement of additional molecular mechanisms. To determine if early programmed cell death played any role in the observed dysfunction of CXCR4 signaling and activity, apoptosis and cleaved caspase‐3 activities were quantified in untreated and zoledronate treated K003 cells (Fig 3C). After 48 hours of zoledronate exposure (1 or 5 μM), there was no difference in the percentage of apoptotic cells or cleaved caspase‐3 activities in K003 cells exposed to zoledronate when compared to untreated cells. Percent apoptosis in untreated, zoledronate 1 μM, and zoledronate 5 μM exposed cells were 3.0 ± 0.5%, 2.9 ± 0.2%, and 2.9 ± 0.3% respectively. Similarly, cleaved caspase‐3 activities in untreated, zoledronate 1 μM, and zoledronate 5 μM exposed cells were 2.7 ± 0.2 OD/μg, 2.7 ± 0.2 OD/μg, and 2.6 ± 0.1 OD/μg respectively. Expectedly, exposure of K003 cells to staurosporine 1 mM for 4 hours produced increased cleaved caspase‐3 activities, measuring 7.3 ± 0.1 OD/μg (P < .001).
Binding of SDF‐1α to CXCR4 results in heterotrimeric G‐protein activation through dissociation of α and βγ subunits, which are localized to the inner plasma membrane leaflet by fatty acid acylation and prenylation respectively. Given the capacity of zoledronate to inhibit FPPS, an enzyme necessary for protein prenylation, experiments were conducted to determine if reduced prenylation might contribute to loss of CXCR4 functionality. Confocal fluorescent microscopy was utilized to quantitate changes in a surrogate of βγ heterodimers, specifically the γ5 subunit, in K003 cells untreated or exposed to GGOH, zoledronate, or combination. In K003 cells, γ5 subunit expression in untreated cells was 38,670 ± 7,580 RFU/μm2 and was unaffected by co‐incubation with 20 μM GGOH being 39,450 ± 5,070 RFU/μm2. Following exposure to 5 μM zoledronate for 48 hours, γ5 subunit expression was reduced to 29,090 ± 4,420 RFU/μm2, P < .001 (Fig 3D). Expression of γ5 subunit was not affected by incubation with sterile water, the aqueous vehicle of zoledronate (Fig S2B). The observed reductions in membranous γ5 subunit following zoledronate exposure were likely attributed to the inhibition of protein prenylation, as co‐incubation of K003 cells with zoledronate and GGOH, a metabolite of isoprenoid pyrophosphate, completely rescued γ5 subunit membranous expressions, 39,980 ± 7,540 RFU/μm2.
Zoledronate reduces CXCR4 expression within the primary tumor and systemic circulation
To determine if zoledronate could exert any effect on the expressions of CXCR4 in dogs with naturally occurring OS, 19 archived primary bone tumor samples derived from dogs treated with (n = 9) or without zoledronate (n = 10) were retrieved from the University of Illinois Veterinary Diagnostic Laboratory. To compensate for confounding differences in tumor stromal densities and extracellular matrix effects within primary tumor samples, CXCR4 staining intensity area was restricted to and normalized on a per positive cell basis using ImageJ software (Fig 4A, bottom row). Expression of CXCR4 within the primary bone tumor was reduced in dogs receiving zoledronate therapy in comparison to dogs not receiving zoledronate, 16.2 ± 9.0 versus 26.9 ± 10.8 normalized pixel intensity; P = .02 (Fig 4B). Given the observed CXCR4 reductions within primary bone tumors in dogs receiving zoledronate therapy, systemic changes in CXCR4 plasma concentrations before and after zoledronate infusion were evaluated in a separate cohort of 20 OS‐bearing dogs. Eighteen of 20 (90%) treatment naïve dogs with OS achieved an average reduction in circulating CXCR4 concentrations of 54.2 ± 33.8% 4 weeks following standardized palliative therapy inclusive of a single intravenous infusion of zoledronate, P = .02 (Fig 4C).
Figure 4.
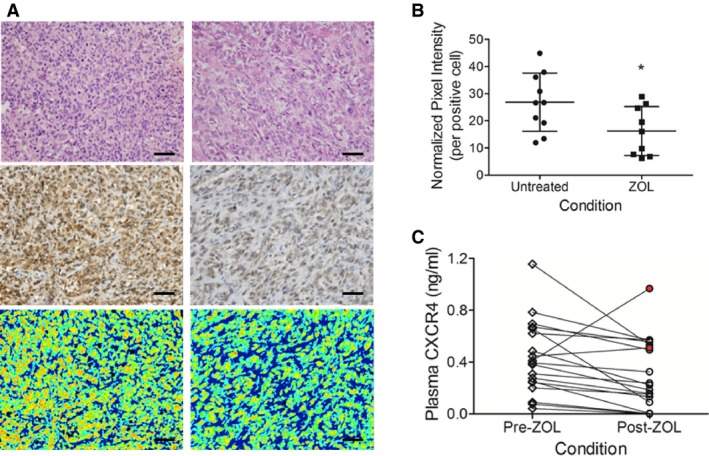
(A) Visual comparison of primary tumor OS cells by hematoxylin and eosin (top row), CXCR4 immunohistochemistry (middle row), and computer‐based fluorescence quantification of CXCR4 (bottom row) from untreated (left panel) and zoledronate‐treated (right panel) dogs. (B) Reductions in CXCR4 expression by computer‐based fluorescence quantification in dogs receiving serial zoledronate therapy prior to limb amputation compared to untreated control dogs. (C) Directional changes in plasma soluble CXCR4 concentrations achieved in dogs 28‐days following treatment with intravenous infusions of zoledronate. Red filled circles identify 2 animals that had elevations in plasma soluble CXCR4 following zoledronate exposure. Data expressed as mean ± SD and significance defined as *P < .05. Scale bar = 100 microns.
Atypical OS metastases pattern in dogs treated with zoledronate
In the same 9 dogs not ever receiving systemic chemotherapy, and only treated with zoledronate combined with ionizing radiation and oral analgesics in which primary tumor CXCR4 expression was reduced in comparison with dogs not receiving zoledronate therapy, full necropsies were performed at the time of death (median 283 days, range 85–445 days), and allowed for patterns of metastases, identified grossly and confirmed histologically, to be described (Table 1). In comparison with historical reports which document lung parenchymal involvement as the primary and sole site of metastatic colonization in approximately 60% of affected animals,1 only 3/9 dogs receiving zoledronate were confirmed to have pulmonary metastases as the sole site of colonization. In 33% of dogs (3/9), no metastases were identified in pulmonary tissues at all, but rather successful colonization developed in unexpected anatomic compartments including the lymphatic, nervous, and cutaneous tissues. Subjectively, a disproportionate fraction of dogs (4/9) had extensive metastatic colonization of abdominal visceral organs including the spleen, liver, kidney, and adrenal glands.
Discussion
Complex and interactive biologic processes likely contribute to the conserved patterns of metastases associated with specific solid tumor malignancies, such as canine OS. Scientific and clinical evidence support the proactive involvement of tumor cells in the process of distant tissue colonization, which includes subversion of chemokine receptor signaling cascades.10 Understanding the role of chemokine receptors in metastatic cell migration and the capacity of conventional or experimental therapeutics in modifying chemokine‐induced intracellular responses, serve as fundamental and necessary steps toward developing treatment strategies that might alter, and ideally inhibit, metastatic progression. Considering the critical role of CXCR4 signaling in various diseases including solid tumor metastases, several blocking strategies including peptides, peptide analogues, and antibodies have been developed and proven to be effective in delaying metastatic progression in preclinical experimental systems, including murine models of OS.11, 12, 23, 24 However, some blocking strategies are limited in the prevention of successful metastatic seeding, and do not exert activity against established micrometastatic disease.24 Additionally, the capacity of zoledronate to inhibit OS metastatic progression in different rodent models has not generated uniform results, including the exertion of antimetastatic, neutral, or prometastatic activities.11, 12, 23, 24, 25, 26, 27, 28 Nonetheless, collectively based upon this therapeutic promise, clinical trials in people evaluating CXCR4 antagonism for the management of advanced stage cancers have been recently reported.13
Complementing the development of specific CXCR4 inhibitors as potential anti‐metastatic agents, prior reports have suggested that zoledronate not only reduces CXCR4 expression in breast cancer cells, but also decreases tumor recurrence rates in postmenopausal women diagnosed with breast carcinoma.17, 18 Given the safety and confirmed biological activity of zoledronate in cancer‐bearing dogs,20, 29 in conjunction with the potential role of CXCR4 in canine OS biology,20 the major purpose of the current study was to investigate if zoledronate could modulate metastatic behaviors in canine OS. Exposure of canine OS cells to biologically achievable concentrations of zoledronate,21 resulted in decreased CXCR4 expression in 1 of 3 cell lines investigated; however, the observed differential response could not be solely explained by FPPS expression levels, the enzymatic target of aminobisphosphonates.30 Based upon these findings, differences in cytosolic concentrations of FPPS might only partially contribute toward relative resistance to the CXCR4‐modulating activities of zoledronate in canine OS cells. Interestingly, the differential responses to zoledronate observed in the current study parallel previous studies describing the acquisition of metabolic resistance in OS cells grown in long‐term culture with low concentrations of aminobisphosphonates.31
Chemokine receptors undergo constant recycling which regulates their cellular longevity.32 In the K003 cell line, reduced expression of CXCR4 following zoledronate exposure was likely mediated through augmented protein ubiquitination, as co‐incubation of K003 cells with zoledronate and MG132, a potent proteasome inhibitor, abolished zoledronate's capacity to downregulate CXCR4. Concordant with our findings, downregulation of CXCR4 in breast cancer cell lines following exposure to a novel biphenyl urea derivative has been similarly reported to be dependent upon proteasome degradation.33 In our current study, although proteasome degradation was responsible for reducing CXCR4 expressions in K003 cells, the precipitating mechanism for zoledronate‐induced CXCR4 ubiquitination was not definitively elucidated, but could be mediated through the induction of the unfolded protein response, previously reported to be a cellular consequence of mevalonate pathway inhibition.34
In K003 cells, the magnitude in which zoledronate inhibited cAMP generation and directional cell migration was discordant with the moderate reductions in CXCR4 protein achieved, and supported the existence of additional molecular mechanisms altered by zoledronate exposure. Given the potential cytotoxic properties of aminobisphosphonates against various cell lines, including canine OS,35, 36 zoledronate's capacity to induce global cellular dysfunction as a consequence of early apoptosis, plausibly could have contributed to the impaired CXCR4 functionality and directional migration observed in K003 cells. However, given the low concentrations evaluated in the current study, nonspecific cytotoxic effects of zoledronate as a mechanism for reduced CXCR4 functionality were excluded as no difference in apoptosis or cleaved caspase‐3 activities were identified between untreated and zoledronate‐exposed K003 cells. Alternatively, the disruption of heterotrimeric G‐protein activities were further considered candidate targets of zoledronate based upon the requisite prenylation of βγ subunits necessary for appropriate subcellular localization and consequent cell signaling.7 As a surrogate measure for appropriate heterotrimeric G‐protein assembly, we explored zoledronate's effect on γ5, a subunit which requires prenylation and heterodimerizes with various β proteins prior to localizing within the plasma membrane.8 Following exposure to zoledronate, K003 cells demonstrated reductions in γ5 expression which could be completely rescued with the co‐incubation of GGOH, a metabolite that can be converted into isoprenoid pyrophosphates in the absence of FPPS activity; findings which support zoledronate's capacity to inhibit γ5 prenylation. Derived from these data, the loss of CXCR4 functionality following zoledronate exposure could be partially attributed to dysregulated subcellular localization of βγ subunits with consequent impaired heterotrimeric G‐protein assembly and signaling.
To evaluate the translational relevance of the in vitro findings identified in cell lines, correlative in vivo studies were conducted and provided additional indirect evidence for the capacity of zoledronate to alter CXCR4 expressions in a limited cohort of dogs with OS. Dogs (n = 9) receiving serial intravenous infusions of zoledronate on a monthly basis prior to amputation demonstrated reductions in CXCR4 expression by OS cells comprising the primary tumor in comparison with dogs not receiving zoledronate (n = 10). Concordant with the observed downregulation of CXCR4 expression by primary tumor OS cells, in a separate cohort of dogs with OS (n = 20) paired plasma samples collected before and after first‐time zoledronate administration revealed reductions in circulating CXCR4 concentrations in the majority of dogs (18/20). Collectively, these findings suggest that zoledronate exposure in dogs with OS has the capacity to alter CXCR4 expressions at the level of the primary tumor and within systemic circulation.
The potential for reduced CXCR4 expressions to alter patterns of metastases were qualitatively described in the same cohort of dogs (n = 9) receiving serial zoledronate therapy prior to death. Based upon the limited number of animals evaluable, observed patterns of metastases at time of death were qualitatively different in comparison with historical reports,1, 2 and provide anecdotal evidence for zoledronate's capacity to perturb the migratory behavior of metastatic OS cells. Only a minority of dogs (2/9) had evidence of only pulmonary parenchymal colonization, while an unexpectedly high percentage (66%) of dogs developed metastatic lesions within atypical locations including lymphatic, cutaneous, nervous, and abdominal visceral organs. Collectively, these in vivo findings lend additional, albeit descriptive, support for the possibility of zoledronate to alter chemokine receptor expressions and consequent patterns of metastases.
Although the current study provides novel information pertaining to canine OS metastatic biology, several limitations should be recognized. First, the number of cell lines utilized in the current investigation was limited, and therefore strong conclusions regarding the biologic consequences exerted by zoledronate might not be conserved globally across all OS histologies. In particular, any biologic response to zoledronate might be dependent upon multiple factors including FPPS activities. Although protein expression of FPPS was qualitatively evaluated, direct enzymatic activity was not and therefore the experimental design of the current study was incomplete in scope and could provide a plausible explanation for the imperfect association identified between cell lineage susceptibilities to zoledronate and cytosolic FPPS expressions. Second, the study design of the current investigation did not provide any definitive evidence to prove the exact mechanisms responsible for CXCR4 downregulation following zoledronate exposure, only that ultimate proteasome degradation was involved. Mevalonate pathway blockade results in endoplasmic reticulum stress with consequent induction of the unfolded protein response.34 It is probable that zoledronate elicits similar cellular responses in canine OS cells; however, additional studies characterizing the upregulation of various chaperone proteins, such as glucose‐regulated protein 78, following zoledronate exposure would be required to associate the unfolded protein response and CXCR4 downregulation observed in the current study. Third, the observed reductions in circulating CXCR4 concentrations following zoledronate exposure in dogs with OS is a novel finding; however, the biologic significance of this discovery is uncertain. Soluble cytokine receptors are most often extracellular membrane products of enzymatic cleavage derived from single pass transmembrane receptors, not serpentine receptors like CXCR4.37 However, elevated soluble CXCR4 receptor concentrations have been reported in people with inflammatory and cancerous processes, and has been proposed to serve as a biomarker of disease burden.38 As such, additional research will be necessary to elucidate the biologic mechanisms and clinical significance of soluble CXCR4 concentrations in dogs with OS. Last, although dogs treated with zoledronate manifested with qualitatively atypical metastases, it is not possible to ascribe the anatomic changes in metastatic colonization as a direct effect of reduced CXCR4 expression secondary to zoledronate exposure, especially in light of the small cohort of dogs characterized in the current investigation. Despite the well‐annotated patterns of metastases in dogs with OS treated with surgery alone or with adjuvant chemotherapies, the natural disease progression and sites of preferential metastases in dogs not treated with surgery have not been thoroughly characterized. As such, the perceived atypical metastatic pattern observed in dogs receiving long‐term zoledronate therapy might be attributed to other factors unrelated and separate from reduced CXCR4 expression and zoledronate exposure.
In conclusion, our results showed that CXCR4 expression by canine OS cells can be modulated by zoledronate, and functional signaling and directional migration can be substantively attenuated through impaired heterotrimeric G‐protein activities. In dogs with OS, treatment with zoledronate reduces CXCR4 expressions at the level of the primary tumor and systemic circulation, and potentially alters natural patterns of metastases. Collectively, these findings broaden our understanding of chemokine mediated metastases in canine OS, and provide new information and future opportunities to investigate the use of zoledronate as an adjuvant therapy for changing, and ideally delaying, the onset of metastatic progression in dogs with OS.
Supporting information
Figure S1 (A) Canine cross‐reactivity of anti‐human CXCR4 antibody for western blot application with the identification of a protein band size ~ 45 kD. (B) Identification of FPPS in 3 canine OS cell lines demonstrating variances in basal expression.
Figure S2 Exposure of K003 cells to aqueous vehicle control (sterile water) does not reduce (A) CXCR4 or (B) γ5 subunit as quantified by confocal fluorescent microscopy. Data expressed as mean ± SEM and significance defined as ***P < .001.
Table S1 Canine OS study population signalment.
Data S1 Materials and Methods.
Acknowledgments
The authors thank the technicians and veterinary oncology residents of the University of Illinois Cancer Care Clinic for their contributions to this study.
Conflict of Interest Declaration: Dr. Timothy Fan serves as Associated Editor for Journal of Veterinary Internal Medicine. He was not involved in the review of this manuscript.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was conducted in the Comparative Oncology Research Laboratory and Veterinary Teaching Hospital at the University of Illinois, Urbana, IL 61802.
Footnotes
Abcam, Cambridge, MA
Santa Cruz Biotechnology, Dallas, TX
EMD Millipore, Billerica, MA
Sigma Aldrich, Saint Louis, MO
SelleckChem, Houston, TX
Sino Biological Incorporation, North Wales, PA
Thermo Fisher Scientific, Life Technologies, Schaumburg, IL
Bio‐Rad, Hercules, CA
National Institutes of Health, Bethesda, MD
cAMP XP kit; Cell Signaling Technology, Danvers, MA
QCM Chemotaxis Cell Migration Assay; EMD Millipore, Billerica, MA
FITC Annexin V Apoptosis Detection kit; BD Biosciences, San Jose, CA
Caspase‐3 colorimetric assay kit; R&D Systems, Minneapolis, MN
BioGenex, Fremont, CA
ABC Vector Laboratories, Burlingame, CA
Canine chemokine C‐X‐C‐Motif Receptor 4 ELISA kit, MBS094822, San Diego, CA
GraphPad InStat, Version 3.10; GraphPad Software, Inc. La Jolla, CA
References
- 1. Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978‐1988). J Am Vet Med Assoc 1992;200:995–999. [PubMed] [Google Scholar]
- 2. Selmic LE, Burton JH, Thamm DH, et al. Comparison of carboplatin and doxorubicin‐based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med 2014;28:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brodey RS, Riser WH. Canine osteosarcoma. A clinicopathologic study of 194 cases. Clin Orthop Relat Res 1969;62:54–64. [PubMed] [Google Scholar]
- 4. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98–101. [PubMed] [Google Scholar]
- 5. Dong F, Budhu AS, Wang XW. Translating the metastasis paradigm from scientific theory to clinical oncology. Clin Cancer Res 2009;15:2588–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med 2001;345:833–835. [DOI] [PubMed] [Google Scholar]
- 7. Higgins JB, Casey PJ. The role of prenylation in G‐protein assembly and function. Cell Signal 1996;8:433–437. [DOI] [PubMed] [Google Scholar]
- 8. Marrari Y, Crouthamel M, Irannejad R, et al. Assembly and trafficking of heterotrimeric G proteins. Biochemistry 2007;46:7665–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oldham WM, Hamm HE. Heterotrimeric G protein activation by G‐protein‐coupled receptors. Nat Rev Mol Cell Biol 2008;9:60–71. [DOI] [PubMed] [Google Scholar]
- 10. Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol 2008;215:211–213. [DOI] [PubMed] [Google Scholar]
- 11. Richert MM, Vaidya KS, Mills CN, et al. Inhibition of CXCR4 by CTCE‐9908 inhibits breast cancer metastasis to lung and bone. Oncol Rep 2009;21:761–767. [PubMed] [Google Scholar]
- 12. Wong D, Kandagatla P, Korz W, et al. Targeting CXCR4 with CTCE‐9908 inhibits prostate tumor metastasis. BMC Urol 2014;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galsky MD, Vogelzang NJ, Conkling P, et al. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin Cancer Res 2014;20:3581–3588. [DOI] [PubMed] [Google Scholar]
- 14. Berenson JR. Zoledronic acid in cancer patients with bone metastases: results of Phase I and II trials. Semin Oncol 2001;28:25–34. [DOI] [PubMed] [Google Scholar]
- 15. Fournier PG, Stresing V, Ebetino FH, et al. How do bisphosphonates inhibit bone metastasis in vivo? Neoplasia 2010;12:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen‐containing bisphosphonates inhibit the mevalonate pathway and prevent post‐translational prenylation of GTP‐binding proteins, including Ras. J Bone Miner Res 1998;13:581–589. [DOI] [PubMed] [Google Scholar]
- 17. Denoyelle C, Hong L, Vannier JP, et al. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA‐dependent and ‐independent effects. Br J Cancer 2003;88:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open‐label phase 3 trial. Lancet Oncol 2014;15:997–1006. [DOI] [PubMed] [Google Scholar]
- 19. Im KS, Graef AJ, Breen M, et al. Interactions between CXCR4 and CXCL12 promote cell migration and invasion of canine hemangiosarcoma. Vet Comp Oncol 2015; Sep 3. doi:10.1111/vco.12165. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan TM, Barger AM, Fredrickson RL, et al. Investigating CXCR4 expression in canine appendicular osteosarcoma. J Vet Intern Med 2008;22:602–608. [DOI] [PubMed] [Google Scholar]
- 21. Weiss HM, Pfaar U, Schweitzer A, et al. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 2008;36:2043–2049. [DOI] [PubMed] [Google Scholar]
- 22. Fernandis AZ, Cherla RP, Chernock RD, et al. CXCR4/CCR5 down‐modulation and chemotaxis are regulated by the proteasome pathway. J Biol Chem 2002;277:18111–18117. [DOI] [PubMed] [Google Scholar]
- 23. Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res 2014;124:31–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SY, Lee CH, Midura BV, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis 2008;25:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Labrinidis A, Hay S, Liapis V, et al. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clin Cancer Res 2009;15:3451–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Endo‐Munoz L, Cumming A, Rickwood D, et al. Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Res 2010;70:7063–7072. [DOI] [PubMed] [Google Scholar]
- 27. Wolfe TD, Pillai SP, Hildreth BE 3rd, et al. Effect of zoledronic acid and amputation on bone invasion and lung metastasis of canine osteosarcoma in nude mice. Clin Exp Metastasis 2011;28:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labrinidis A, Hay S, Liapis V, et al. Zoledronic acid protects against osteosarcoma‐induced bone destruction but lacks efficacy against pulmonary metastases in a syngeneic rat model. Int J Cancer 2010;127:345–354. [DOI] [PubMed] [Google Scholar]
- 29. Spugnini EP, Vincenzi B, Caruso G, et al. Zoledronic acid for the treatment of appendicular osteosarcoma in a dog. J Small Anim Pract 2009;50:44–46. [DOI] [PubMed] [Google Scholar]
- 30. Gibbs JB, Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol 1997;37:143–166. [DOI] [PubMed] [Google Scholar]
- 31. Ory B, Moriceau G, Trichet V, et al. Farnesyl diphosphate synthase is involved in the resistance to zoledronic acid of osteosarcoma cells. J Cell Mol Med 2008;12:928–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 2007;1768:952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhan Y, Zhang H, Li J, et al. A novel biphenyl urea derivate inhibits the invasion of breast cancer through the modulation of CXCR4. J Cell Mol Med 2015;19:1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res 2012;18:3524–3531. [DOI] [PubMed] [Google Scholar]
- 35. Farese JP, Ashton J, Milner R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim 2004;40:113–117. [DOI] [PubMed] [Google Scholar]
- 36. Poirier VJ, Huelsmeyer MK, Kurzman ID, et al. The bisphosphonates alendronate and zoledronate are inhibitors of canine and human osteosarcoma cell growth in vitro. Vet Comp Oncol 2003;1:207–215. [DOI] [PubMed] [Google Scholar]
- 37. Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol 1998;64:135–146. [DOI] [PubMed] [Google Scholar]
- 38. Malvoisin E, Livrozet JM, Makloufi D, et al. Soluble chemokine receptor CXCR4 is present in human sera. Anal Biochem 2011;414:202–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) Canine cross‐reactivity of anti‐human CXCR4 antibody for western blot application with the identification of a protein band size ~ 45 kD. (B) Identification of FPPS in 3 canine OS cell lines demonstrating variances in basal expression.
Figure S2 Exposure of K003 cells to aqueous vehicle control (sterile water) does not reduce (A) CXCR4 or (B) γ5 subunit as quantified by confocal fluorescent microscopy. Data expressed as mean ± SEM and significance defined as ***P < .001.
Table S1 Canine OS study population signalment.
Data S1 Materials and Methods.


