Abstract
1. Scope
The composition of the gastrointestinal (GIT) microbiota, particularly in early life, influences the development of metabolic diseases later in life. The maternal microbiota is the main source of bacteria colonising the infant GIT and can be modified by dietary prebiotics. Our objective was to determine the effects of prenatal consumption of prebiotic caprine milk oligosaccharides (CMO) on the large intestine of female mice, milk composition, and offspring's development.
2. Methods and results
C57BL/6 mice were fed either a control diet, CMO diet, or galacto‐oligosaccharide diet from mating to weaning. From weaning, some pups nursed by CMO, GOS, and control‐dams were fed the control diet for 30 days. CMO or GOS‐fed dams had increased colon length and milk protein concentration compared to control‐fed dams. At weaning, pups from CMO‐fed dams had increased body weight and colon length and increased proportions of colonic Bifidobacterium spp compared to the pups from control‐fed dams. Thirty days after weaning, pups from CMO‐fed dams had increased visceral fat weight compared to pups from control‐fed dams.
3. Conclusion
Consumption of CMO by the dams during gestation and lactation improved the development of the pups, and the relative abundance of bifidobacteria and butyric acid in the colon, at weaning.
Keywords: Caprine milk oligosaccharides, Development, Prebiotic, Pregnancy, Prenatal diet
Abbreviations
- CMO
caprine milk oligosaccharides
- CMOF
caprine milk oligosaccharide enriched fraction
- GIT
gastrointestinal tract
- GOS
galacto‐oligosaccharide
1. Introduction
The composition of the microbiota, particularly in early life, profoundly influences the development and maturation of the infant mucosal immune system 1 and metabolism 2. A higher risk of metabolic diseases in adults, for example, has been associated with changes in the GIT microbiota in early life 2. The maternal microbiota is the main source of bacteria colonizing the infant GIT during labor 3 and breast feeding 4. Emerging evidence suggests that these bacteria may also colonise the infant GIT before birth 5, 6. The manipulation of the maternal microbiota (in humans and rodents) through antibiotics 7, diet 8, and prebiotic intake 9, 10 has been shown to affect the microbiota transmitted to the offspring both quantitatively and qualitatively and offspring development 11. Therefore, the period from pregnancy to weaning is likely to be a critical window of opportunity for modifying the maternal GIT microbiota for improved infant development and long‐term health benefits.
Caprine milk has oligosaccharides structurally similar to human milk oligosaccharides 12, known to stimulate the development, maturation, and colonization of the neonate's GIT 13. Important differences in the profile of goat and milk oligosaccharides were, however, also described 14, 15. The ratio of fucosyl‐oligosaccharides, for example, is high in human milk oligosaccharides, while this is extremely small in caprine milk. Human milk sialyl‐oligosaccharides contain only the monosaccharide Neu5Ac, while the ratio of Neu5Ac/Neu5Gc in caprine milk is 36/64. In human milk the type 1 oligosaccharides, which contain Gal(β1‐3)GlcNAc, predominate over the type 2, which contain Gal(β1‐4)Glc, while caprine milk contains only type 2 oligosaccharides 14, 15. The impact of these different milk oligosaccharides profiles on the GIT microbiota and host physiology have been poorly explored.
We have previously shown that a caprine oligosaccharide‐enriched fraction (CMOF) supported the growth of selected bifidobacterial strains isolated from breast‐fed infants, and stimulated in vitro production of acetate and lactate 16. Oliveira et al. (2012) 17 has also demonstrated the prebiotic activity of CMOF in a batch culture system inoculated with adult human feces. The aim of this study was to determine the effects of prenatal consumption of CMOF on the colon microbiota and milk composition of the dams, and the offspring's development. In this study, we also examined whether the effects of maternal diet on pups’ development persisted after the pups’ diet was switched to the control diet for 30 days after weaning.
2. Materials and methods
2.1. Animals and study design
Animal experimentation was approved by AgResearch Grasslands Animal Ethics Committee (AE12579), in accordance with the New Zealand Animal Welfare Act 1999, New Zealand. Sixty three C57BL/6 mice (42 female and 21 male) were obtained from the AgResearch Ruakura Small Animal Facility and housed in a temperature and humidity controlled facility with a 12‐h light/dark cycle. After acclimatization, 9‐week‐old mice were randomly assigned to groups of two females and one male and fed one of the following diets; AIN‐76A (control diet), AIN‐76A supplemented with 1% GOS (GOS diet), or AIN‐76A supplemented with CMOF containing 1% caprine milk oligosaccharides (CMO diet). Diets were formulated by Research Diets, Inc. (NJ, USA). The CMO diet also contained 0.2% of GOS and other sugars as a result of the method used to obtain the CMOF (Table 1). CMOF was obtained by the method previous described in 18. In short, caprine milk whey was processed by a combination of ultrafiltration, enzymatic hydrolysis of the lactose, solid‐phase extraction, and rotary vacuum evaporation. Oligosaccharide characterization and quantification was performed on a Thermo Scientific LTQ XL‐Linear Ion Trap Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with electrospray ionization in negative mode using appropriated standards. Other sugars (lactose, galactose and glucose) were characterized and quantified using high performance liquid chromatography connected to a Thermo Hypercarb column. More information on diet composition and analysis is provided in Supporting Information Table 1.
Table 1.
Composition of diets and caprine milk oligosaccharide enriched fraction
| Ingredientsa | Control diet (AIN76A) | GOS diet | CMO diet |
|---|---|---|---|
| Grams | Grams | Grams | |
| Casein | 200 | 200 | 200 |
| DL‐Methionine | 3 | 3 | 3 |
| Corn Starch | 150 | 500 | 500 |
| Maltodextrin | 0 | 150 | 150 |
| Sucroseb | 500 | 0 | 0 |
| Cellulose, BW200 | 50 | 50 | 50 |
| Corn oil | 50 | 50 | 50 |
| Mineral Mix S10001 | 35 | 35 | 35 |
| Vitamin Mix V10001 | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 |
| Composition of CMOF | Components | grams | grams | grams |
|---|---|---|---|---|
| Protein | 0.014 | |||
| GOS | 10 | 2 | ||
| Lactose | 3.34 | 3.34 | ||
| Glucose | 10 | 10 | ||
| Galactose | 6.68 | 6.68 | ||
| Oligosaccharidec | 11 | |||
| Calcium | 0.028 | |||
| Magnesium | 0.014 | |||
| Potassium | 0.377 | |||
| Sodium | 0.21 | |||
| Iodine | 0.0001 | |||
| Selenium | 0.000001 | |||
| Total | 1000.00 | 1022.02 | 1033.6 |
All ingredients of the AIN‐76A, GOS and CMO diet (except CMOF, sourced from New Zealand, and GOS, sourced from Yakult, Japan), were supplied by Research Diets.
The sucrose concentration was adjusted in the CMO and GOS diets to balance the energy and nutritional content of the AIN‐76A diet.
Caprine milk oligosaccharides and their abundance: (13%) 3’‐ and/or 6’‐galactosyl‐lactose, (27%) 3’‐ and/or 6’‐sialyl‐lactose, (32%) 6’‐glycolyl‐neuraminyl‐lactose, (9%) lacto‐N‐hexaose, (11%) disialyl‐N‐lactose, (8%) 6’‐N‐acetyL‐glucosaminyl‐lactose.
Animals were mated for 11 days. After delivery, pups remained with their dams up to weaning (21 days) where dams and some of the pups were euthanized and sampled. To determine the long‐term effects of maternal diet, the remaining pups were fed the control diet for 30 days, then euthanized and samples collected.
2.2. Sampling and analysis
2.2.1. Milk sample collection and nutrient composition
At days 5, 12, and 19 of lactation, mice were anesthetized with 0.2 mL of ketamine/xylazine/acepromazine mix (50 mg/mL ketamine, 5 mg/mL xylazine, and 0.5 mg/mL acepromazine), then injected with oxytocin (0.15 iu/mouse). Milking was done manually into a sterile tube and samples obtained for each mouse on each of the three sampling occasions were pooled prior to compositional analysis.
The dry matter and crude fat were determined using a method described by Gor et al. 19. In short, dry matter was measured in triplicate on 10 μL of milk in precombusted and preweighed tin capsules. After weighing, the samples were dried at 55°C for 5 h, cooled to room temperature, and reweighed, and the dry matter calculated. Total fat was measured using 100 μL of milk which was transferred to a 20 mL glass tube and diluted ten times with Milli‐Q water. Two hundred microliters of NH3 solution (25%), 1 mL ethanol (100%), 3 mL diethyl ether, 3 mL petroleum ether (40–60°C), and 800 μL Milli‐Q water were added, with 30‐s vortexing after each step. The sample was then centrifuged at 3000 × g for 10 min. Four milliliters of the upper layer was transferred to a preweighed and precombusted glass vial and dried by nitrogen evaporation. The samples were dried for 2 h at 105°C, cooled in a dissecator, and weighed to determine the fat percentage. Milk sugar (lactose, glucose, and galactose) composition was determined by diluting 20 μL of milk with 80 μL of Milli‐Q water followed by centrifugation at 21 000 × g for 20 min at 4°C to separate fat and whey. Whey (60 μL) was transferred to a clean tube and diluted ten times with Milli‐Q water and further centrifuged at 16 100 × g for 10 min at 4°C. Diluted whey (50 μL) was separated isocratically by HPLC as previously described 18. The total protein concentration of milk was determined using a Bradford assay 20 with α‐casein (Sigma, Aldrich, Auckland; 70% α‐casein, 30% other proteins) as the external standard.
2.2.2. SCFA analysis
Cecum digesta was collected post‐mortem, snap frozen, and kept at −80°C prior to analyses. Cecum digesta (approximately 100 mg) was mixed with 1 mL of PBS and 30 μL of internal standard (200 mM/L 2‐ethyl butyric acid). Samples were homogenized using a hand‐held homogenizer (OMNI international, Kennesaw, USA) at full speed for 10 s and then centrifuged (12 000 × g for 5 min at 4°C) to remove particulate material, following which 1 mL of supernatant was transferred to a glass tube. Extraction of SCFA into an organic solvent and derivatisation were performed using a previously described method 21.
2.2.3. Leptin analysis
Blood was collected by cardiac puncture and serum leptin concentrations were measured using a Mouse Leptin Elisa kit (Millipore, Thermofisher NZ) according to the manufacturer's instructions.
2.2.4. Femur mineral composition
Both femurs were cleaned of all adherent tissues, weighed, then defatted and dried using two 16‐h exposures to acetone and then ethyl ether following a previously published method 22. Femurs were air dried and any visible remaining cartilage material was removed. Samples were placed in acid‐leached, preweighed 20 mL borosilicate scintillation vials with acid‐leached polypropylene caps and frozen at −80°C for 1 h. Femurs were then freeze dried overnight (FTS Systems, SP Scientific, Philadelphia, USA) and the dry weight recorded 23.
Bone samples were digested using a previously described method 24 with the following modifications. All samples received 2.5 mL HNO3 (67% Aristar BDH, London, UK) and 0.5 mL HCl (34–37%, Trace Metal Grade Fisher) and heated in a foil covered aluminium block with acid leached funnels at 85°C for 1 h. Funnels were removed, and samples were heated up to 110°C until dry. Five milliliters of analytical matrix (i.e. 1 mL HNO3 (67% Aristar BDH, London, UK): 1 mL HCl (34‐37% Trace Metal Grade Fisher): 5 mL H2O) were added and weighed, and samples decanted to acid‐leached polypropylene 10 mL tubes for analysis.
Blanks were used to control for background levels in all steps (de‐fatting, lyophilisation, and digestion). Samples were digested alongside blanks and Certified Reference Material (CRM) (IAEA‐H‐5) to test for contamination and to calculate recovery rates (Ca, 96–98%; Mg 93–100% and Zn 100%). Samples were measured on an Inductively Coupled Plasma MS instrument (ICP‐MS, Thermo Electron Corporation, England) by Hill Laboratories, Hamilton, New Zealand, for calcium, magnesium and zinc concentrations (limits of detection: Ca 1.0 g/m3, Mg 0.4 g/m3, Zn 0.02 g/m3).
2.2.5. Colon histology and microbial analysis
Transverse sections of the proximal colon were collected and stored in 10% formalin solution at room temperature for subsequent assessment of colonic crypt length and goblet cell number.
In preparation for microbial analysis, approximately 50 mg of colon digesta DNA was extracted using a NucleoSpin Soil kit accordingly to the manufacturer's instructions (Macherey Nagel, Düren, Germany). For pyrotag sequencing, the V4‐V6 region of the bacterial 16S rRNA gene was amplified. The primers and PCR conditions used have been previously published by our group 25. Purified products were pooled in equivalent quantities and sent to Macrogen (Seoul, Korea) for unidirectional sequencing from the forward primer using the Roche GS‐FLX sequencer with Titanium chemistry. Sequences were analyzed accordingly with a method previously described 25.
2.2.6. PICRUSt analysis of 16S rRNA amplicon sequencing data
PICRUSt is a tool designed to infer metagenomics information from 16S rRNA amplicon sequencing data 26. In the present study, analysis of 16S rRNA amplicon sequencing data was performed using the default settings of PICRUSt (version 0.9.1). Predicted enzymes/gene functions were grouped at level 2 of the KEGG Orthology and clusters of orthologous groups classification schemes for statistical analyses by single factor permutation ANOVA (500 permutations) as implemented by the RVAideMemoire package in R 27.
2.2.7. Statistical analysis
All data are presented as means with their standard errors. Statistical analysis was performed using ANOVA in GenStat version 12 (VSN International Ltd). Differences between means were considered significant when probability was less than 0.05 (p<0.05). Trends were defined as p > 0.05 but <0.10.
Analysis of the colon microbiota sequencing data was performed using the nonparametric Kruskal–Wallis test in R 3.0.2 28, the results of which were corrected for multiple testing using the Benjamini and Hochberg false discovery rate (FDR) adjustment.
3. Results
3.1. Body parameters
There was no evidence that diet affected dams’ food intake (data not shown), body weight, GIT length, SI length or stomach, colon (no digesta), spleen, kidneys, brain, femur, and visceral fat weight (Supporting Information Table 2) during the experimental period. However, dams fed the CMO diet had greater colon length (control, 1.41 ± 0.04; GOS, 1.65 ± 0.14; CMO, 1.94 ± 0.14; mean [mm/g] ± SE; p‐value = 0.05) and lower liver weight (control, 85.8 ± 2.6; GOS, 86.0 ± 2.9; CMO, 73.1 ± 3.8; mean [mg/g] ± SE; p‐value = 0.05) than control fed dams.
At weaning, pups from dams fed the CMO diet had increased body weight (control, 4.69 ± 0.1; GOS, 4.98 ± 0.1; CMO, 5.20 ± 0.09; mean [mg/g] ± SE; p‐value = 0.02) and colon length (control, 3.06 ± 0.06; GOS, 3.33 ± 0.22; CMO, 4.52 ± 0.30; mean [mm/g] ± SE; p‐value < 0.001) compared to pups from dams fed the control diet (Supporting Information Table 2). Body length was higher for weaned pups from dams fed the CMO and GOS diets (control, 5.17 ± 0.06; GOS, 5.35 ± 0.06; CMO, 5.38 ± 0.04; mean [mm/g] ± SE; p‐value = 0.04) than for pups from dams fed the control diet. After 30 days of receiving the control diet, pups from dams fed the CMO diet had increased visceral fat weight (control, 46.2 ± 1.7; GOS, 52.2 ± 2.5; CMO, 55.9 ± 2.8; mean [mg/g] ± SE; p‐value = 0.05) compared to pups from dams fed the control diet (Supporting Information Table 2).
3.2. Leptin serum concentration
Treatment did not affect dams’ serum leptin concentration (control, 14.9 ± 1.1; GOS, 12.8 ± 2.1; CMO, 11.9 ± 1.8; ng/mL, mean ± SE), however, 30 days after weaning, pups from dams fed the CMO diet had higher concentrations of serum leptin than pups from control‐fed dams (control, 7.1 ± 0.8; GOS, 9.5 ± 0.9; CMO, 10.4 ± 1.2; ng/mL, mean ± SE; p = 0.05).
3.3. Milk composition
Each milk sample utilized for compositional analysis is a pool of three milk samples collected from one animal at lactation days 5, 12, and 19. Due to variation in milk volume, three control; four GOS; and five CMO samples were analyzed for dry matter, total protein, and sugars and 1 control; three GOS; and two CMO samples were analyzed for fat content. Different sample numbers for total fat analysis (n = Control, 1; GOS, 3; CMO, 2) a, b, Values with similar letters in rows do not differ significantly (p<0.05). Feeding the CMO and GOS diets to the dams resulted in an increase of the total protein levels in milk (control, 74.8 ± 11.2; GOS, 115.9 ± 9.1; CMO, 115.0 ± 3.9; mg/mL, mean ± SE, p = 0.02) (Supporting Information Table 3). Diet did not significantly affect dry matter, fat, lactose, glucose, or galactose concentrations in milk.
3.4. Femur mineral composition
There was no effect on femur mineral composition in dams consuming the CMO diet or their pups 30 days after weaning were observed (Supporting Information Table 4). At weaning, pups from dams fed the CMO and GOS diets had increased calcium concentration (control, 96.4 ± 2.7; GOS, 108.4 ± 2.9; CMO, 105.7 ± 2.0; mg/g, mean ± SE. p = 0.004) in the femur compared to pups from dams fed the control diet. At weaning, pups from dams fed the GOS diet also had higher magnesium concentration (control, 2.58 ± 0.04; GOS, 2.75 ± 0.06; CMO, 2.62 ± 0.04; mg/g, mean ± SE, p = 0.02) than pups from dams fed the CMO and control diets (Supporting Information Table 4).
3.5. Cecum SCFA concentrations
CMO‐fed dams showed higher concentrations of cecal formic acid than control diet‐fed dams (control, 0.06 ± 0.02; GOS, 0.16 ± 0.05; CMO, 0.48 ± 0.17; μmol/g, mean ± SE. P‐value = 0.04) (Supporting Information Table 5). CMO and GOS‐fed dams showed lower concentrations of cecal isobutyric acid than control‐fed dams (control, 0.43 ± 0.02; GOS, 0.36 ± 0.03; CMO, 0.33 ± 0.02; μmol/g, mean ± SE. p = 0.04). Dams fed the GOS diet showed higher concentrations of cecal propionic (control, 3.1 ± 0.1; GOS, 5.1 ± 0. 3; CMO, 3.8 ± 0.3; μmol/g, mean ± SE. p = 0.01) and butyric acids (control, 6.1 ± 1.0; GOS, 8.6 ± 0.5; CMO, 5.5 ± 0.7; μmol/g, mean ± SE. p = 0.04) than dams fed the control or CMO diet.
Maternal dietary intervention was also shown to modify the pups SCFA production (Supporting Information Table 5). At weaning, pups from CMO‐fed dams had a higher concentration of cecal butyric acid than pups from dams fed control or GOS diets (control, 0.7 ± 0.3; GOS, 1.3 ± 0.2; CMO, 2.9 ± 0.3; μmol/g, mean ± SE. p = 0.001). Thirty days after weaning, pups from the dams fed the GOS diet showed higher concentrations of cecal propionic acid than pups from the dams fed the control diet (control, 1.3 ± 0.2; GOS, 2.9 ± 0.7; CMO, 2.4 ± 0.3; μmol/g, mean ± SE. p = 0.02) (Supporting Information Table 5).
3.6. Colon histology and microbial composition
There was no evidence that diet influenced colon crypt length or goblet cell number of the dams or the pups (Supporting Information Table 6).
The microbiota composition of the colon contents of dams and pups after the experimental period was examined by high throughput 454 pyrosequencing of the bacterial 16S rRNA gene. Principal coordinate analysis (PCoA) of Unifrac phylogenetic distances showed that broad differences in community structure could be discerned between dams, pups at weaning and pups 30 days after weaning (Supporting Information Fig. 1A). However, differences between dietary treatments were less clear (Supporting Information Fig. 1B). Significant differences in community diversity were observed between pups at weaning, pups 30 days after weaning, and dams (p<0.001) (Fig. 1A). Posthoc Wilcoxon rank sum analysis showed that the diversity of dams’ microbiota was significantly higher than that of pups at weaning and 30 days postweaning, while the pups at 30 days postweaning had higher diversity than pups at weaning (Fig. 1A). Community diversity was significantly higher in pups at weaning fed the CMO diet compared to those fed the control diet (p = 0.01) (Fig. 1B). No other significant differences in diversity were observed between dietary treatments.
Figure 1.
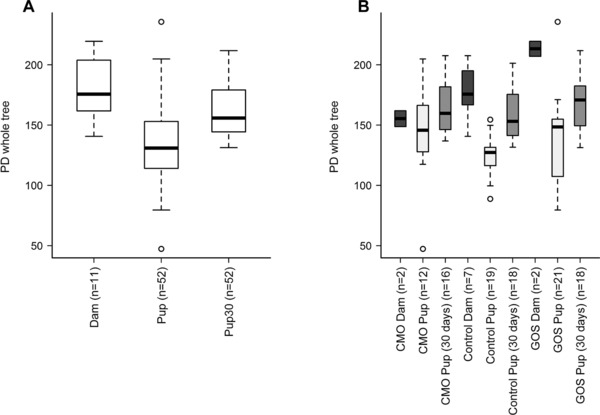
Boxplots of Faith's Phylogenetic Distances (2660 sampling depth) showing community diversity by (A) age and (B) age by diet. Significant differences in diversity were observed between dams, pups at weaning and pups 30 days postweaning (*p<0.001). Communities from pups at weaning fed the CMO diet were also significantly more diverse than communities from pups at weaning fed the Control diet (# p = 0.01).
Although dietary intervention did not cause broad alterations in the microbiota of the dam colon digesta, the proportion of members of Odoribacter spp. (control, 0.4%; GOS, 0.6%; CMO, 1.8%) and Oscillibacter spp. (control, 0.7%; GOS, 0.1%; CMO, 1.5%) was increased in the colon digesta of CMO‐fed dams compared to that of the GOS and control‐fed dams (Supporting Information Table 7). Other members of the Firmicutes family, Lactococcus spp, (control, 0.02%; GOS, 0.14%; CMO, 0.05%), and the Allobaculum spp. (control, 0.3%; GOS, 3.9%; CMO, 1.9%) were found in higher proportions in the colon digesta of GOS‐fed dams compared to that of the control and CMO‐fed dams (Supporting Information Table 7).
At weaning, pups from dams fed the CMO diet showed genus alterations in the colon digesta community compared to pups from control and GOS‐fed dams, with significantly higher proportions of Bifidobacterium spp. (control, 0.05%; GOS, 0.07%; CMO, 1.37%) and Parabacteroides spp. (control, 1.5%; GOS, 0.5%; CMO, 4.5%) (Supporting Information Table 7).
Thirty days after weaning, increased proportions of Allobaculum spp. (control, 5.5%; GOS, 3.0%; CMO, 10.7%) and decreased proportions of Alistipes spp (control, 8.3%; GOS, 7.1%; CMO, 5.1%) were observed in pups from dams fed CMO diet compared to control. The greatest alterations in the colon digesta community were observed in pups from dams fed the GOS diet. Changes in the proportions of Sporacetigenium spp. (control, 0.2%; GOS, 1.9%; CMO, 0.5%), Clostridium spp (control, 0.1%; GOS, 0.3%; CMO, 0.1%), Turicibacter spp (control, 0.09%; GOS, 1.7%; CMO, 0.1%), unclassified Clostridiaceae (control, 0.1%; GOS, 0.3%; CMO, 0.08%) and unclassified Peptostreptococcaceae (control, 0.3%; GOS, 2.7%; CMO, 0.6%) were increased in pups from dams fed GOS diet compared to pups from the dams fed the control and CMO diets, (Supporting Information Table 7).
3.7. Predicted metabolism‐related gene functions in the colon microbiota associated with maternal diet
Overall the PICRUSt analysis showed a constant representation of metabolic functions across age and diet (Supporting Information Fig. 2). However, specific differences in the mean relative predicted abundance of gene categories (COGs and KOs) were found within age groups accordingly to the dam's diet (Fig. 2). Pups at weaning from CMO and GOS‐fed dams had increased KEGG metabolic pathways of amino acid, energy, and vitamin metabolism compared to pups from control‐fed dams. Pups at weaning from GOS‐fed dams also had increased KEGG metabolic pathways of carbohydrate and lipids metabolism. Pups from CMO and GOS‐fed dams had only an increase in carbohydrate KEEG metabolic pathways 30 days postweaning.
Figure 2.
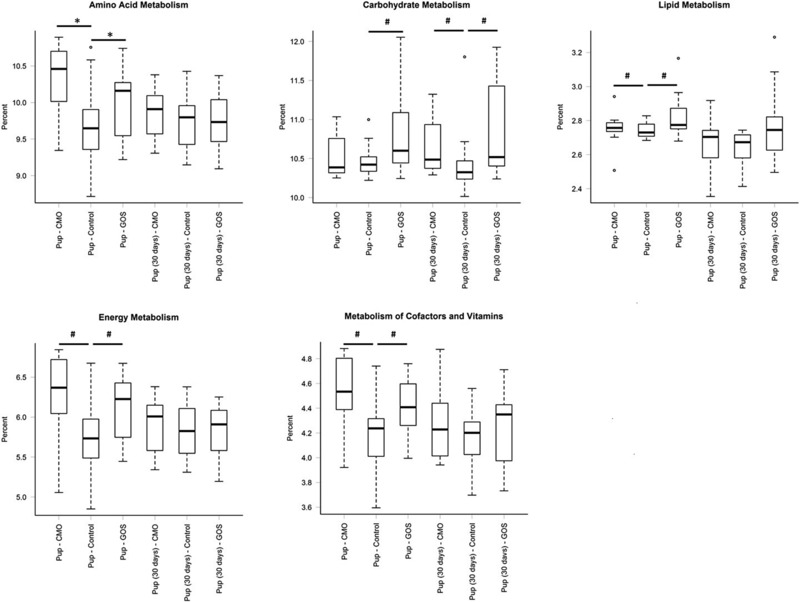
Relative abundances of predicted level 2 KEGG pathways involved in amino acid, carbohydrate, lipid, energy, and vitamin metabolism in the pups at weaning and 30 days postweaning compared within the groups (*p < 0.005, # p <0.01).
4. Discussion
The present study demonstrates that the consumption of CMO by dams during gestation and lactation is associated with changes in the colon microbiota, and in milk composition, which affected pups development. Our major findings, related directly or indirectly to CMO and GOS consumption compared to control diet are summarised in Table 2. The specific findings related to CMO consumption by the dams include (i) an increase in colon length in dams and pups at weaning; (ii) increased body weight and proportions of bifidobacteria in the colon of pups at weaning and (iii) increased body fat and serum leptin concentration in pups 30 days after weaning. The consumption of CMO and GOS by the dams also (iv) increased milk protein concentration; (v) altered cecal SCFA production in dams and pups at weaning; and finally (vi) increased body length and calcium concentration in the femur of pups at weaning. These findings highlight the potential for indirect exposures to nutrients via maternal diet to influence offspring development, GIT composition, and metabolism.
Table 2.
Summary of main findings on the effects of CMO and GOS diet compared to control diet on dams, pups at weaning, and pups 30 days postweaning. Similar letters in rows do not differ significantly (p<0.05)
| Animal | Parameters analyzed | Control | GOS | CMO | |
|---|---|---|---|---|---|
| Dam | Body parameters | Colon length | a | ab | ↑b |
| Liver weight | a | a | ↓b | ||
| Milk composition | Protein | a | ↑b | ↑b | |
| Femur mineral concentration | Zinc | ab | ↑a | b | |
| Cecum SCFA | Formic acid | a | ab | ↑b | |
| Propionic acid | a | ↑b | a | ||
| Butyric acid | a | ↑b | a | ||
| Isobutyric acid | a | ↑b | ↑b | ||
| Main colonic taxa modulated by diet | Odoribacter spp | a | a | ↑b | |
| Oscillibacter spp | a | a | ↑b | ||
| Lactococcus spp | a | ↑b | a | ||
| Pups weaning | Body parameters | Body weight | a | ab | ↑b |
| Body length | a | ↑b | ↑b | ||
| Colon length | a | a | ↑b | ||
| Femur mineral concentration | Calcium | a | ↑b | ↑b | |
| Magnesium | a | ↑b | a | ||
| Zinc | ab | ↑a | b | ||
| Cecum SCFA | Butyric acid | a | a | ↑b | |
| Main colonic taxa modulated by diet | Bifidobacterim spp | a | a | ↑b | |
| Parabacteroides spp | a | a | ↑b | ||
| Barnesiella spp | a | ↓b | ab | ||
| Pups 30 days postweaning | Body parameters | Visceral fat | a | ab | ↑b |
| Serum leptin | a | ab | ↑b | ||
| Cecum SCFA | Propionic acid | a | ↑b | ab | |
| Main colonic taxa modulated by diet | Turicibacter spp | a | ↑b | a | |
| Sporacetigenium spp | a | ↑b | ab | ||
| Clostridium spp | a | ↑b | a | ||
| Alistipes spp | a | ab | ↓b | ||
| Allobaculum spp | a | a | ↑b | ||
↑ or ↓ indicate specific parameter increased or decreased compared to treatments with different letters. Number of animals used for the analysis of body parameters: dams (control, 8; GOS, 9; CMO, 7); pup at weaning (control, 27; GOS, 27; CMO, 23); pup 30 days postweaning (control, 18; GOS, 19; CMO, 16). Variations in the number of animals may have occurred for specific analysis, please see supplement material for exact number.
The CMO diet modified dams’ colon microbiota by increasing the concentrations of the genera Oscillibacter spp. and Odoribacter spp. compared to dams fed GOS and control diets. Recent data suggest that the abundance of Oscillibacter spp 29, 30 and Odoribacter spp 31 are increased in obese individuals and negatively correlate with a functional intestinal barrier. Although, no difference was found in dams’ body weight or visceral fat, a decrease of liver weight may indicate a decreased fat accumulation in this organ 32 and an altered lipid metabolism 33. This is at odds with studies which have shown that the consumption of prebiotics by adults (human and rodents) and also pregnant mice improve the GIT microbiota composition, especially increasing the faecal concentrations of bifidobacteria and/or lactobacillus 8, 34.
The CMO diet also impacted cecal microbial fermentation through an increase in the concentration of formic acid and a decrease in the concentration of isobutyric acid, compared to the control diet. Formic acid is a volatile SCFA rarely detected in nutritional studies 35, being an intermediate product, not an end‐product, of bacterial fermentation which is converted readily to CO2 and water. Another common end‐product of bacterial fermentation, isobutyric acid, is produced by fermentation of proteins 36 when carbohydrate availability becomes a limiting factor for microbial fermentation 37. Collectively, this could explain the decline in isobutyric acid in the cecum of CMO and GOS‐fed dams, as the preferential metabolism of CMOF and GOS by carbohydrate‐fermenting bacteria may have altered the microbiota and associated fermentation toward a type that was less proteolytic 38.
Increased milk protein concentration observed in dams fed the CMO and GOS diet may have contributed to increased body weight and length and increased muscle mass 11 observed in the pups at weaning. Accordingly, PICRUST analysis showed an increased representation of KEGG metabolic pathways of amino acid, carbohydrate, energy, and vitamin metabolism in pups at weaning from CMO and GOS‐fed dams. However, apart from changes in carbohydrate metabolism, these differences in predicted functions were largely absent by 30 days postweaning. Early nutrition affects expression of genetic growth potential; this can have short‐term and long‐term effects on growth, development, metabolic programming, and disease risk 39. In neonatal rats, higher protein intake via the enteral route was reported to enhance short‐term weight gain, insulin resistance, and modification of the expression of glucose transporters. However, these differences were not sustained into adult life 40. In human neonates, high protein milk formula (2 versus 1.5 g/100 mL) fed to babies considered small for gestational weight 41 resulted in an increase in total adiposity between 2 and 6 years of age 42. The concentration of protein reported in murine milk, however, is highly variable (32 and 125 mg/mL) 19, 43, thus caution is needed to infer any physiological effect in the pups due to this variation.
As is typically observed as a consequence of direct high fiber intake 44, pups at weaning from CMO‐fed dams had increased proportions of Bifidobacterium spp. and Parabacteroides spp, in the colon, and increased concentrations of butyric acid, in the cecum, as an indirect consequence of CMO intake by the dams. Increased in butyric acid in the dams cecum however, was only observed in dams which consumed GOS diet. The production of butyric acid is often positively correlated with the presence of bifidobacteria, most likely because bifidobacteria produce acetate and lactate, which are then converted by other bacteria (e.g., clostridial cluster 4, and 14a) into butyrate. Of the major SCFAs, butyrate is of special interest because it is the preferred energy source for colonocytes 45 and promotes a normal phenotype in these cells and hence may protect against cancer and other serious colonic diseases 46.
Dams’ CMO intake also indirectly increased the microbial diversity in pups at weaning compared to pups from GOS and control‐fed dams. Microbial diversity in the large intestine in early life has been shown to be negatively correlated with the development of metabolic diseases and inflammatory bowel diseases later in life. It is certain, however, that limited direct exposure to the diets may have occurred when pups began to eat their dam's diet as they approached weaning 47. Increased consumption of dietary fibres and production of butyric acid 48 are associated with increased intestinal length 49 and increased mineral absorption 50, 51, 52, 53 as observed in this study by increased calcium concentration in the pups femur at weaning. The effects on colon length are usually accompanied by an increase in weight and crypt size 54, 55, 56 which was not observed in the present study. Changes in the milk oligosaccharide profile of the dam by diet (not determined in this study) may also favour the increase in the proportions of these commensal bacteria and increase colon length in the pups 57.
Thirty days after weaning, increases in the proportions of Allobaculum spp. and decreases of the genus Alistipes spp. were observed in pups from CMO‐fed dams. Allobaculum spp. were reported to be affected by a high‐fat diet 58 and dominant in the intestine of rodents fed a high fiber diet 59, 60 or prebiotic 58. Conversely, 30 days after weaning, pups from dams fed the CMO diet had an increase in leptin concentration and fat mass without change in body weight. Interestingly, Desbuards et al., 61 reported a trend for increased leptin concentration 28 days after weaning in pups which had consumed the same prebiotic diet as the dams.
In conclusion, consumption of CMO or GOS by the dams during gestation and lactation differently altered dam's colon microbiota and cecal SCFA, although, both beneficially effected pup's development at weaning. Consumption of CMO diet only, however, increased the microbial diversity and the relative the abundance of bifidobacteria and butyric acid in the colon of pups at weaning. Specific changes of maternal GIT microbiota and liver weight may indicate that maternal lipid metabolism was modified by CMO dietary intake. Those effects could also be observed by an increase in body fat and leptin concentration in pups 30 days after weaning. Further studies, however, are needed to more comprehensively understand the physiological effects of CMO in the maternal/infant pair.
C.T., A.C., N.R., and W.M. designed the study. C.T. supported by W.Y. performed the experiment, analyzed the data, and wrote the paper. All authors proof‐read the paper.
The authors have declared no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Material
Acknowledgments
Caroline Thum acknowledges the Ministry of Business, Innovation and Employment, New Zealand (C10×0907), the Riddet Institute Centre of Research Excellence (CoRE) and AgResearch for the funding and the PhD Scholarship.
5 References
- 1. El Aidy, S. , van Baarlen, P. , Derrien, M. , Lindenbergh‐Kortleve, D. J. et al., Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012, 5, 567–579. [DOI] [PubMed] [Google Scholar]
- 2. Cox, L. M. , Yamanishi, S. , Sohn, J. , Alekseyenko, A. V. et al., Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bettelheim, K. , Breadon, A. , Faiers, M. C. , O'Farrell, S. M. et al., The origin of O serotypes of Escherichia coli in babies after normal delivery. J. Hyg. (Lond). 1974, 72, 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jost, T. , Lacroix, C. , Braegger, C. P. , Rochat, F. et al., Vertical mother‐neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 2014, 16, 2891–2904. [DOI] [PubMed] [Google Scholar]
- 5. Jimenez, E. , Fernandez, L. , Marin, M. L. , Martin, R. et al., Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [DOI] [PubMed] [Google Scholar]
- 6. Jimenez, E. , Marin, M. L. , Martin, R. , Odriozola, J. M. et al., Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [DOI] [PubMed] [Google Scholar]
- 7. Fak, F. , Ahrne, S. , Molin, G. , Jeppsson, B. et al., Microbial manipulation of the rat dam changes bacterial colonization and alters properties of the gut in her offspring. Am. J. Physiol. 2008, 294, G148–G154. [DOI] [PubMed] [Google Scholar]
- 8. Shadid, R. , Haarman, M. , Knol, J. , Theis, W. et al., Effects of galactooligosaccharide and long‐chain fructooligosaccharide supplementation during pregnancy on maternal and neonatal microbiota and immunity–a randomized, double‐blind, placebo‐controlled study. Am. J. Clin. Nutr. 2007, 86, 1426–1437. [DOI] [PubMed] [Google Scholar]
- 9. Fujiwara, R. , Takemura, N. , Watanabe, J. , Sonoyama, K. , Maternal consumption of fructo‐oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br. J. Nutr. 2010, 103, 530–538. [DOI] [PubMed] [Google Scholar]
- 10. Fujiwara, R. , Watanabe, J. , Sonoyama, K. , Assessing changes in composition of intestinal microbiota in neonatal BALB/c mice through cluster analysis of molecular markers. Br. J. Nutr. 2008, 99, 1174–1177. [DOI] [PubMed] [Google Scholar]
- 11. Desbuards, N. , Gourbeyre, P. , Haure‐Mirande, V. , Darmaun, D. et al., Impact of perinatal prebiotic consumption on gestating mice and their offspring: a preliminary report. Br. J. Nutr. 2012, 107, 1245–1248. [DOI] [PubMed] [Google Scholar]
- 12. Martinez‐Ferez, A. , Rudloff, C. , Guadix, A. , Henkel, C. A. et al., Goats’ milk as a natural source of lactose‐derived oligosaccharides: Isolation by membrane technology. Internat. Dairy J. 2006, 16, 173–181. [Google Scholar]
- 13. Kobata, A. , Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci 2010, 86, 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albrecht, S. , Lane, J. A. , Marino, K. , Al Busadah, K. A. et al., A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [DOI] [PubMed] [Google Scholar]
- 15. Urashima, T. , Taufik, E. , Fukuda, K. , Asakuma, S. , Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [DOI] [PubMed] [Google Scholar]
- 16. Thum, C. , Cookson, A. , Otter, D. , McNabb, W. et al., In Vitro fermentation of Caprine milk oligosaccharides by bifidobacteria isolated from breast‐fed infants. Gut Microbes 2015, 6, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliveira, D. L. , Costabile, A. , Wilbey, R. A. , Grandison, A. S. et al., In vitro evaluation of the fermentation properties and potential prebiotic activity of caprine cheese whey oligosaccharides in batch culture systems. Biofactors 2012, 6, 440–449. [DOI] [PubMed] [Google Scholar]
- 18. Thum, C. , Cookson, A. , McNabb, W. , Roy, C. N. et al., Composition and enrichment of caprine milk oligosaccharides from New Zealand Saneen goat cheese whey. J. Food Compos. Anal. 2015, 42, 30–37. [Google Scholar]
- 19. Gors, S. , Kucia, M. , Langhammer, M. , Junghans, P. et al., Technical note: milk composition in mice–methodological aspects and effects of mouse strain and lactation day. J. Dairy Sci. 2009, 92, 632–637. [DOI] [PubMed] [Google Scholar]
- 20. Bradford, M. M. , A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 21. Jensen, M. , Cox, R. , Jensen, B. B. , Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. 1995, 61, 293–304. [Google Scholar]
- 22. Warner, S. , Shea, J. , Miller, S. , Shaw, J. , Adaptations in cortical and trabecular bone in response to mechanical loading with and without weight bearing. Calcif. Tissue Int. 2006, 79, 395–403. [DOI] [PubMed] [Google Scholar]
- 23. Grotti, M. , Abelmoschi, M. L. , Dalla Riva, S. , Soggia, F. et al., Determination of lead in bone tissues by axially viewed inductively coupled plasma multichannel‐based emission spectrometry. Analyt. Bioanalyt. Chem. 2005, 381, 1395–1400. [DOI] [PubMed] [Google Scholar]
- 24. Hirayama, M. , Iijima, S. , Iwashita, M. , Akiyama, S. et al., Aging effects of major and trace elements in rat bones and their mutual correlations. J. Trace Elem. Med. Biol. 2011, 25, 73–84. [DOI] [PubMed] [Google Scholar]
- 25. Young, W. , Egert, M. , Bassett, S. A. , Bibiloni, R. , Detection of sialic acid‐utilising bacteria in a caecal community batch culture using RNA‐based stable isotope probing. Nutrients 2015, 7, 2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langille, M. G. , Zaneveld, J. , Caporaso, J. G. , McDonald, D. et al., Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hervé, M. , R Package 2015.
- 28. Team, R. C. , R: A language and environment for statistical computing, R. Foundation for Statistical Computing 2009.
- 29. Serino, M. , Luche, E. , Gres, S. , Baylac, A. et al., Metabolic adaptation to a high‐fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam, Y. Y. , Ha, C. W. , Campbell, C. R. , Mitchell, A. J. et al., Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet‐induced obese mice. PLoS One 2012, 7, e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geurts, L. , Lazarevic, V. , Derrien, M. , Everard, A. et al., Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin‐resistant mice: impact on apelin regulation in adipose tissue. Front. Microbiol. 2011, 2, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parnell, J. A. , Reimer, R. A. , Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose–response study in JCR: LA‐cp rats. Br. J. Nutr. 2010, 103, 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen, P. , Leray, V. , Diez, M. , Serisier, S. et al., Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [DOI] [PubMed] [Google Scholar]
- 34. Davis, L. M. , Martinez, I. , Walter, J. , Hutkins, R. , A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int. J. Food Microbiol. 2010, 144, 285–292. [DOI] [PubMed] [Google Scholar]
- 35. Huda‐Faujan, N. , Abdulamir, A. S. , Fatimah, A. , Anas, O. M. et al., The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macfarlane, G. , Gibson, G. , Beatty, E. , Cummings, J. , Estimation of short‐chain fatty acid production from protein by human intestinal bacteria based on branched‐chain fatty acid measurements. FEMS Microbiol. Lett. 1992, 101, 81–88. [Google Scholar]
- 37. Jensen, B. B. , 8th Symposium on Digestive Physiology in Pigs. Workshop on: Gut Environment. Influence of luminal factors, Aarhus University, Uppsala, Sweden 2000, pp. 12–14.
- 38. Hansen, C. F. , Hernández, A. , Mansfield, J. , Hidalgo, Á. et al., A high dietary concentration of inulin is necessary to reduce the incidence of swine dysentery in pigs experimentally challenged with Brachyspira hyodysenteriae. Br. J. Nutr. 2011, 106, 1506–1513. [DOI] [PubMed] [Google Scholar]
- 39. Guilloteau, P. , Zabielski, R. , Hammon, H. , Metges, C. , Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J. Physiol. Pharmacol. 2009, 60, 17–35. [PubMed] [Google Scholar]
- 40. Robert, C. d. , Li, N. , Caicedo, R. , Frost, S. et al., Metabolic effects of different protein intakes after short term undernutrition in artificially reared infant rats. Early Hum. Dev. 2009, 85, 41–49. [DOI] [PubMed] [Google Scholar]
- 41. Lucas, A. , Morley, R. , Cole, T. J. , Randomised trial of early diet in preterm babies and later intelligence quotient. Br. J. Nutr. 1998, 317, 1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rolland‐Cachera, M. , Thibault, H. , Souberbielle, J. , Soulie, D. et al., Massive obesity in adolescents: dietary interventions and behaviours associated with weight regain at 2 y follow‐up. Int. J. Obes. 2004, 28, 514–519. [DOI] [PubMed] [Google Scholar]
- 43. Boumahrou, N. , Andrei, S. , Miranda, G. , Henry, C. et al., The major protein fraction of mouse milk revisited using proven proteomic tools. J. Physiol. Pharmacol. 2009, 60 Suppl 3, 113–118. [PubMed] [Google Scholar]
- 44. Graf, D. , Di Cagno, R. , Fak, F. , Flint, H. J. et al., Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 2015, 26, 26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roediger, W. , Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [PubMed] [Google Scholar]
- 46. Mortensen, P. B. , Clausen, M. R. , Short‐chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. 1996, 31, 132–148. [DOI] [PubMed] [Google Scholar]
- 47. Blass, E. M. , Teicher, M. H. , Suckling. Science 1980, 210, 15–22. [DOI] [PubMed] [Google Scholar]
- 48. Jacobs, L. R. , Schneeman, B. O. , Effects of dietary wheat bran on rat colonic structure and mucosal cell growth. J. Nutr. 1981, 111, 798–803. [DOI] [PubMed] [Google Scholar]
- 49. Aghazadeh, A. , TahaYazdi, M. , Effect of butyric acid supplementation and whole wheat inclusion on the performance and carcass traits of broilers. South African J. Anim. Sci. 2012, 42, 241–248. [Google Scholar]
- 50. Chonan, O. , Watanuki, M. , Effect of galactooligosaccharides on calcium absorption in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 1995, 41, 95–104. [DOI] [PubMed] [Google Scholar]
- 51. Pérez‐Conesa, D. , López, G. , Abellán, P. , Ros, G. , Bioavailability of calcium, magnesium and phosphorus in rats fed probiotic, prebiotic and synbiotic powder follow‐up infant formulas and their effect on physiological and nutritional parameters. J. Sci. Food Agric. 2006, 86, 2327–2336. [Google Scholar]
- 52. Weaver, C. M. , Martin, B. R. , Nakatsu, C. H. , Armstrong, A. P. et al., Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [DOI] [PubMed] [Google Scholar]
- 53. van den Heuvel, E. G. , Schoterman, M. H. , Muijs, T. , Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J. Nutr. 2000, 130, 2938–2942. [DOI] [PubMed] [Google Scholar]
- 54. Sakata, T. , Adachi, M. , Hashida, M. , Sato, N. et al., Effect of n‐butyric acid on epithelial cell proliferation of pig colonic mucosa in short‐term culture. DTW. Dtsch. Tierarztl. Wochenschr. 1995, 102, 163–164. [PubMed] [Google Scholar]
- 55. Freitas, K. d. C., Amancio, O. M. S. , de Morais, M. B. , High‐performance inulin and oligofructose prebiotics increase the intestinal absorption of iron in rats with iron deficiency anaemia during the growth phase. Br. J. Nutr. 2012, 108, 1008–1016. [DOI] [PubMed] [Google Scholar]
- 56. McCullough, J. , Ratcliffe, B. , Mandir, N. , Carr, K. et al., Dietary fibre and intestinal microflora: effects on intestinal morphometry and crypt branching. Gut 1998, 42, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hallam, M. C. , Barile, D. , Meyrand, M. , German, J. B. et al., Maternal high protein or prebiotic fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity 2014, 22, 2344–2351. [DOI] [PubMed] [Google Scholar]
- 58. Everard, A. , Lazarevic, V. , Gaia, N. , Johansson, M. et al., Microbiome of prebiotic‐treated mice reveals novel targets involved in host response during obesity. ISME J 2014, 8, 2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. An, C. , Kuda, T. , Yazaki, T. , Takahashi, H. et al., FLX pyrosequencing analysis of the effects of the brown‐algal fermentable polysaccharides alginate and laminaran on rat cecal microbiotas. Appl. Environ. Microbiol. 2013, 79, 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miriam, B. , Buenviaje, M. , Quantitative sputum culture and gram stain: pulmonary infection vs. colonization. Philipp. J. Microbiol. Infect. Dis 1989, 18, 28–35. [Google Scholar]
- 61. Karlsson, C. L. , Molin, G. , Fak, F. , Johansson Hagslatt, M. L. et al., Effects on weight gain and gut microbiota in rats given bacterial supplements and a high‐energy‐dense diet from fetal life through to 6 months of age. Br. J. Nutr. 2011, 106, 887–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Material


