Abstract
Introduction
Perinatal management and prognostic value of clinical evaluation and diagnostic tools have changed with the generalization of therapeutic hypothermia (TH) in infants with hypoxic-ischemic encephalopathy (HIE)
Aim
to ascertain the prognostic value of amplitude integrated electroencephalogram (aEEG) in neonates with HIE considering hours of life and treatment with TH.
Methods
A systematic review was performed. Inclusion criteria were studies including data of neonates with HIE, treated or not with TH, monitored with aEEG and with neurodevelopmental follow-up of at least 12 months. The period of bibliographic search was until February 2016. No language restrictions were initially applied. Consulted databases were MEDLINE, Scopus, CINHAL and the Spanish language databases GuiaSalud and Bravo. Article selection was performed by two independent reviewers. Quality for each individual paper selected was evaluated using QUADAS-2. Review Manager (RevMan) version 5.3 software was used. Forest plots were constructed to graphically show sensitivity and specificity for all included studies, separating patients treated or not with hypothermia. Summary statistics were estimated using bivariate models and random effects approaches with the R package MADA from summary ROC curves. Meta-regression was used to estimate heterogeneity and trends.
Results
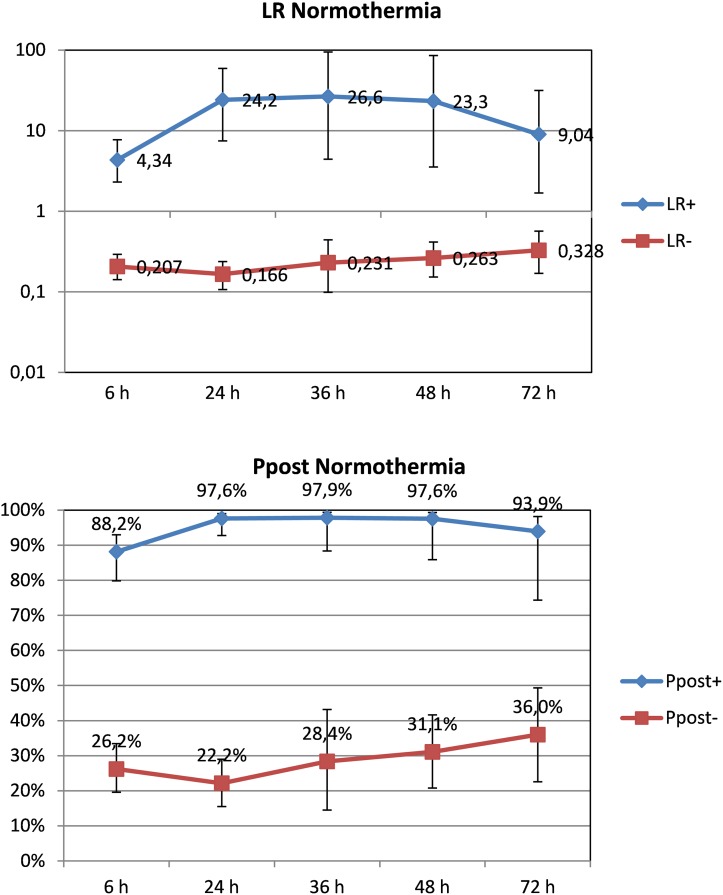
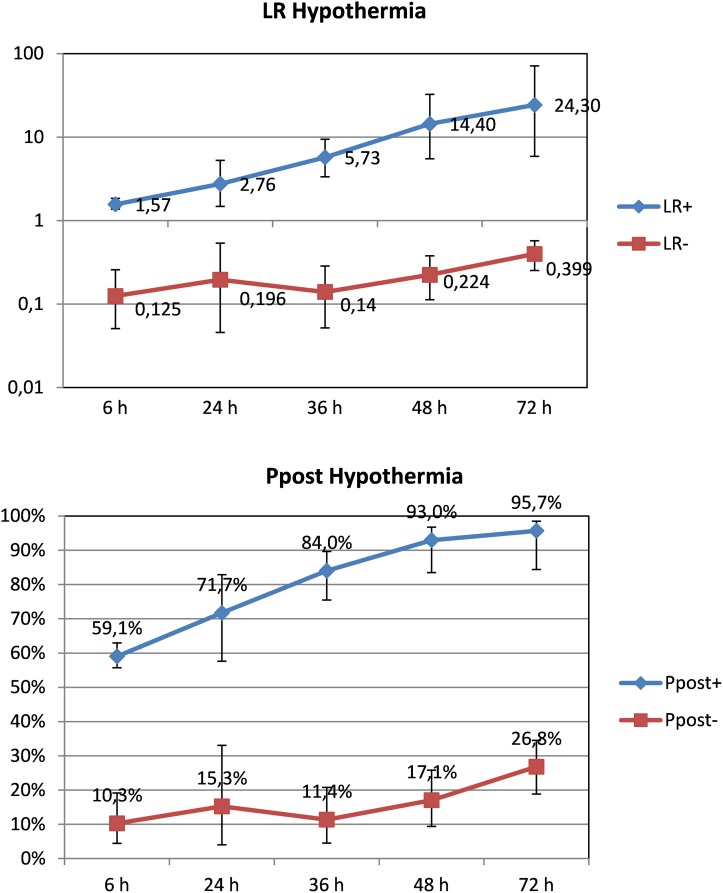
from the 403 articles initially identified, 17 were finally included and critically reviewed. In infants not treated with hypothermia the maximum reliability of an abnormal aEEG background to predict death or moderate/severe disability was at 36 hours of life, when a positive post-test probability of 97.90% was achieved (95%CI 88.40 to 99.40%). Positive likelihood ratio (+LR) at these hours of life was 26.60 (95%CI 4.40 to 94.90) and negative likelihood ratio (-LR) was 0.23 (95%CI 0.10 to 0.44). A high predictive value was already present at 6 hours of life in this group of patients, with a positive post-test probability of 88.20% (95%CI 79.80 to 93%) and a +LR of 4.34 (95%CI 2.31 to 7.73). In patients treated with TH the maximum predictive reliability was achieved at 72 hours of life (post-test probability of 95.70%, 95%CI 84.40 to 98.50%). +LR at this age was 24.30 (95%CI 5.89 to 71.30) and–LR was 0.40 (95%CI 0.25 to 0.57). Predictive value of aEEG at 6 hours of life was low in these patients (59.10%, 95%CI 55.70 to 63%).
Conclusion
This study confirms that aEEG´s background activity, as recorded during the first 72 hours after birth, has a strong predictive value in infants with HIE treated or not with TH. Predictive values of traces throughout the following 72 hours are a helpful guide when considering and counselling parents about the foreseeable long-term neurological outcome
Introduction
Perinatal hypoxic ischemic encephalopathy (HIE) is an important cause of early mortality and permanent major disability in full term infants [1,2]. Therapeutic hypothermia (TH) remains the only neuroprotective treatment available for these patients today [3,4]. Perinatal management and prognostic value of clinical evaluation and diagnostic tools have changed with the generalization of TH [5–7].
Amplitude-integrated electroencephalography (aEEG) is a simplified bedside neurophysiology tool that is nowadays widely used in neonatal units today [8] In term newborns with HIE, different authors have described a good correlation of aEEG with conventional EEG findings [9–11]. aEEG traces are assesed using two methods: voltage and background pattern [12,13] Both methods seem to be comparable for rapid clasification of electrocortical activity in newborns [14].
Prior to the use of TH for neonates with HIE, abnormal aEEG background pattern during the first 3–6 postnatal hours was reported to be highly predictive of adverse outcome, with the combination of aEEG pattern and clinical examination being more predictive than either of these parameters independently [12,15–16]. With the use of TH some authors described that normal aEEG background pattern continued to relate to good neurological outcome whilst persistance of an abnormal aEEG tracing beyond 48 hours of life related to later altered neurodevelopmental outcome [17]. However, the temporal profile of the predictive ability of the aEEG in infants treated with TH during the first 72 hours of life has not been established [18].
A systematic review of the medical literature was performed to ascertain the prognostic value of aEEG in newborns with HIE considering hours of life and treatment with hypothermia. This review was part of the Spanish National Health System Clinical Practice Guideline (CPG) on HIE [19].
Methods
We conducted a systematic review and performed a meta-analysis (MA). Inclusion criteria were studies including newborns with HIE, treated or not with hypothermia, monitored with aEEG and with neurodevelopmental follow-up of at least 12 months.
Data sources and search strategy
Librarians and the CPG´s panel group performed the electronic literature research. Consulted databases were MEDLINE, Scopus, CINHAL and the Spanish language databases GuiaSalud and Bravo. Clinical trials registers were checked for on-going studies. The period of bibliographic search was until February 2016. No language restrictions were initially applied. Bibliographic references of selected papers were tracked if pertinent. A combination of the following subject headings and keywords was adapted for each electronic database: “Hypoxic ischemic encephalopathy”, “hypoxia-ischemia”, “brain”, “anoxia”, “brain injuries”, “hypoxia brain”, “brain ischemia”, “asphyxia”, “newborn”, “developmental disabilities”, “neurodevelopment”, “cerebral palsy”, “infant mortality”, “hypothermia”, “hypothermia induced”, “neurological morbidity”, “cerebral damage”, “severe hypoxia”, “asphyxia neonatorum”, “oxygen inhalation therapy”, “resuscitation”, “fatal outcome”, “treatment outcome”, “seizures” and “electroencephalography”. Initial search strategy used in Pubmed is shown in S1 Fig. PRISMA checklist is shown in S2 Fig.
Study selection and data extraction
Article selection was performed by two independent reviewers (DB and RR) and in case of discordance a third author intervened (AGA). Only studies including: 1) human infants with a gestational age of 35 weeks or more, 2) neonatal encephalopathy caused by perinatal asphyxia and 3) neurodevelopmental outcome data available at a postnatal age of at least 12 months were eligible. Review articles, opinion articles, and editorials were excluded as well as abstracts and conference proceedings. A standardized extraction form was used to record the characteristics of each study. For each paper aEEG background pattern at 6, 24, 26, 48 and 72 hours of life was analysed and neurodevelopmental outcome after follow up was recorded. Rates of true positive, true negative, false positive and false negative were recorded. aEEG background trace was considered abnormal according to the pattern classification if the trace was flat, low voltage or burst suppression [13]. Using the voltage classification, aEEG was considered abnormal if the trace’s lower margin was below 5uV and its upper margin was below 10uV [12]. Adverse neurological outcome was defined as the combination of death or moderate/severe disability.
Quality assessment
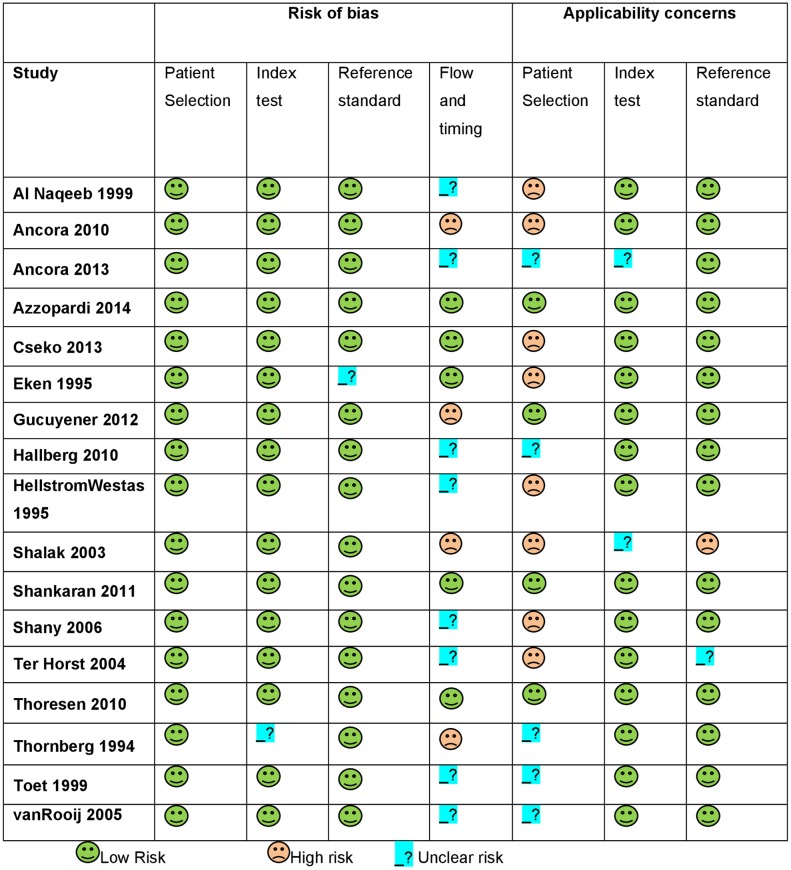
Quality for each individual paper selected was evaluated using QUADAS-2 [20]. Quality was considered high if included population and outcomes were well defined. Quality was lowered if population was not well defined, follow-up was irregular or outcomes were not well specified. Influence of aEEG´s result on withdrawal of life support was not specifically described by any of the included articles except for the paper by Eken et al [21].
Statistical methods
Using Review Manager (RevMan) version 5.3 software, forest plots were constructed to graphically show sensitivity and specificity for all included studies, separating patients receving TH from patients not receiving this therapy. Summary statistics (sensitivity, specificity, likelihood ratios, diagnostic odds ratio) were estimated using bivariate models and random effects approaches with the R package MADA, from the summary receiver operative operating characteristic curve (SROC). MADA uses a linear mixed model with known variances of the random effects, similar to the computational approach by Reitsma et al [22]. Heterogeneity and trends were explored using meta-regression with the same package (S1 Table). Significant heterogeneity at 6 and 24 hours of life, depending on hypothermia treatment led us to separate summary estimates for patients receiving and not receiving hypothermia treatment. Post-test probabilities were computed for different hours of life, using our likelihood ratio estimations. For pre-test probabilities, the point estimations of incidences of death and moderate/severe disability reported by Tagin et al [3] were used.
Results
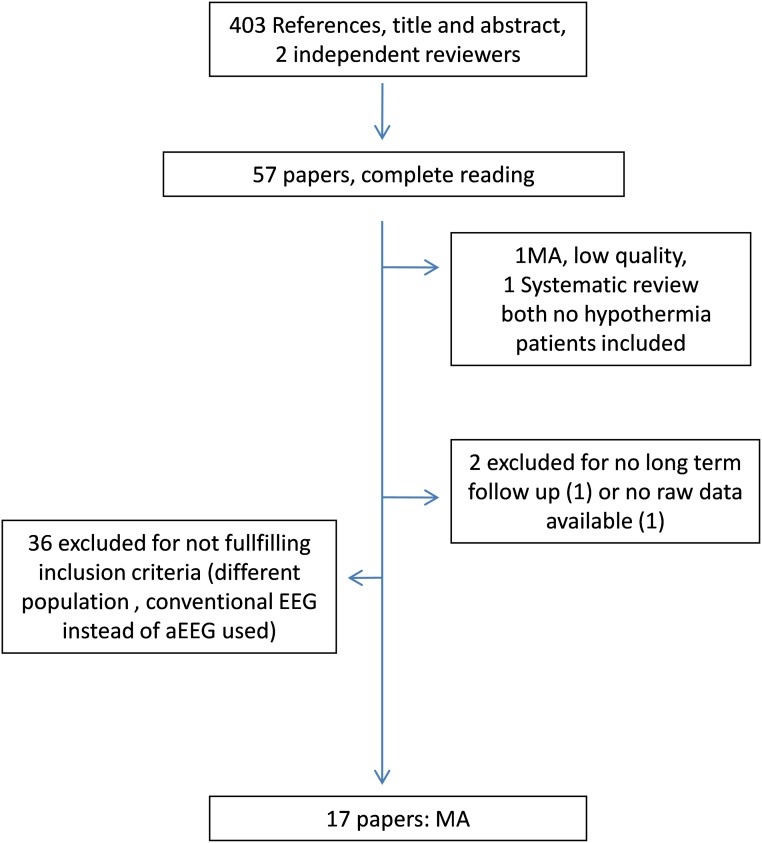
Four hundred and three articles were identified. Fifty-seven publications were initially preselected on the basis of the article´s title and abstract. Finally, only 17 articles met the inclusion and exclusion criteria (Fig 1) and were critically reviewed. Tables 1 and 2 present the characteristics of all included articles [12,15–17,21,23–34]. HIE stage was graded according to criteria by Sarnat [35]. QUADAS-2 evaluation for these papers is shown in Fig 2. Excluded full text papers and reasons for exclusion are shown in S2 Table.
Fig 1. Search and section process flowchart.
Table 1. Characteristics of included articles with no hypothermia treatment.
| Study and year of publication | HIE stage 1/2/3 (n) | NT | Follow-up | Neurodevelopmenta assessment. Definition of adverse outcome | Quality; Reason for reduction |
|---|---|---|---|---|---|
| Thornberg1994 [24] | NS, total n = 38 | 38 | 18 mo | Denver; Death or CP ± mental retardation | Moderate; Small sample size, stage of encephalopathy NS, variable follow-up |
| Hellström-Westas 1995 [23] | NS, total n = 47, stage 2–3 n = 24, unspecified number of non-encephalopathic infants | 47 | 12–18 mo | NS; Death, CP or psychomotor retardation requiring special training | Moderate; Small sample size, patients with no encephalopathy or stage 1 included, unprecise definition of neurological impairment |
| Eken 1995 [21] | 11/7/16 | 34 | 24 mo | Griffiths and neurologic examination according to Amiel-Tison, Grenier and Touwen.; Death,,DQ <85 or CP | Moderate; Small sample size, patients with HIE stage 1 included |
| al Naqeeb 1999 [12] | 14/17/7, stage NA in 2 | 40 | 18–24 mo | Griffiths or Optimality Score and neurologic examination; Death, neuromotor abnormalities, Optimality Score <20 or DQ <85 | Moderate; Small sample size, patients with HIE stage 1 included |
| Toet 1999 [15] | NS, total n = 68 | 68 | ≥12 mo | Griffiths and neurologic examination according to Amiel-Tison, Grenier and Touwen. Alberta Infant Motor Scale in children younger than 18 mo.; Death, CP or DQ <80 | Moderate; Stage of encephalopathy NS, variable follow-up |
| Shalak 2003 [16] | 3/17/2, normal n = 15, stage 1–2 n = 13 | 50 | 18 mo | Bayley, neurological examination, visual and hearing evaluation.; Death, CP, non-specified low Bayley score, altered vision or hearing | Low; Small sample size, patients with no encephalopathy or stage 1 included, disability not well defined |
| Ter Horst 2004 [25] | 4/18/5, stage NA in 3 | 30 | 24 mo | Neurologic examination based on Touwen.; Death, severe mental and motor delay, infantile spasms or CP | Low; Retrospective, small sample size, patients with HIE stage 1 included |
| Van Rooij 2005 [26] | NS, total n = 160 | 160 | 24 mo | Griffiths; Death, CP, DQ <85 | Moderate; Retrospective, stage of encephalopathy NS, variable follow-up |
| Shany 2006 [27] | 16/14/9 | 39 | 30 mo | Bayley II for children ≤42 mo and Leiter-R test for children > 42 mo. Neurologic evaluation according to Amiel-Tison; Death, developmental score <80 or CP | Low; Retrospective, developmental results needing adjustment for maternal education, patients with HIE stage 1 included |
| Ancora 2010 [28] | 17/13/2 | 32 | 24 mo | Griffiths, neurologic examination and psychomotor assessment via Milani-Comparetti’s neuroevolutive assessment, Brazelton’s behavioural assessment and Prechtl’s general movement assessment. Brainstem auditory evoked responses and assessment of visual function.; Death, CP, DQ <85, hearing loss or cortical visual impairment | Moderate; Small sample size, patients with HIE stage 1 included |
CP, cerebral palsy; DQ, developmental quotient; NA, not assessable; NS, not specified; NT, cases treated in normothermia; mo = months
Table 2. Characteristics of included articles with hypothermia treatment.
| Study and year of publication | HIE stage 1/2/3a (n) | NT/HT (n) | Follow-up | Neurodevelopmental assessment; Definition of adverse outcome | Quality; Reason for reduction |
|---|---|---|---|---|---|
| Hallberg 2010 [29] | 3/16/4 | 0/23 | 12 mo | Serial neurological examinations and AIMS at 4 mo; Death or spasticity and AIMS score <5th percentile | Moderate; Small sample size, retrospective, patients with HIE stage 1 included, short and limited follow-up |
| Thoresen 2010 [17] | NS, total n = 74, all stage 2 or 3 | 31/43 | 18 mo | Bayley II, neurological examination, GMFCS and visual assessment; Death, MDI <70, GMFCS level 3–5 or no useful vision | High |
| Ancora 2013 [30] | 0/8/4 | 0/12 | ≥12 mo | Griffiths; Death, CP or DQ <88.7 | Low; Small sample size, variable follow-up |
| Shankaran 2011 [31] | 0/71/37 | 51/57 | 18 mo | Bayley II, neurological examination, GMFCS, hearing and visual assessment; Death; MDI 70–84 and either a GMFCS level of 2, hearing impairment with no amplification or persistent seizure disorder; MDI <70; GMFCS level 3–5; hearing impairment requiring hearing aids or blindness | High |
| Gucuyener 2012 [32] | 0/6/4 | 0/10 | 8–24 mo | Bayley II; Death, CP, MDI or PDI <70 | Low; Retrospective, inconsistent follow-up |
| Csekö 2013 [33] | NS, total n = 70, all stage 2 or 3 | 0/70 | 18–24 mo | Bayley II; Death, MDI or PDI <70 | Moderate; Retrospective |
| Azzopardi 2014 [34] | NS, total n = 314, all stage 2 or 3 | 158/156 | 18 mo | Bayley II, neurological examination, GMFCS and visual assessmentDeath, CP with GMFCS level 3–5, MDI <70 or bilateral cortical visual impairment | High |
AIMS, Alberta Infant Motor Scale; CP, cerebral palsy; DQ, developmental quotient; GMFCS, Gross Motor Function Classification System; MDI, mental developmental index; NT/HT, ratio of cases treated in normothermia/receiving therapeutic hypothermia; PDI, psychomotor developmental index; mo, months
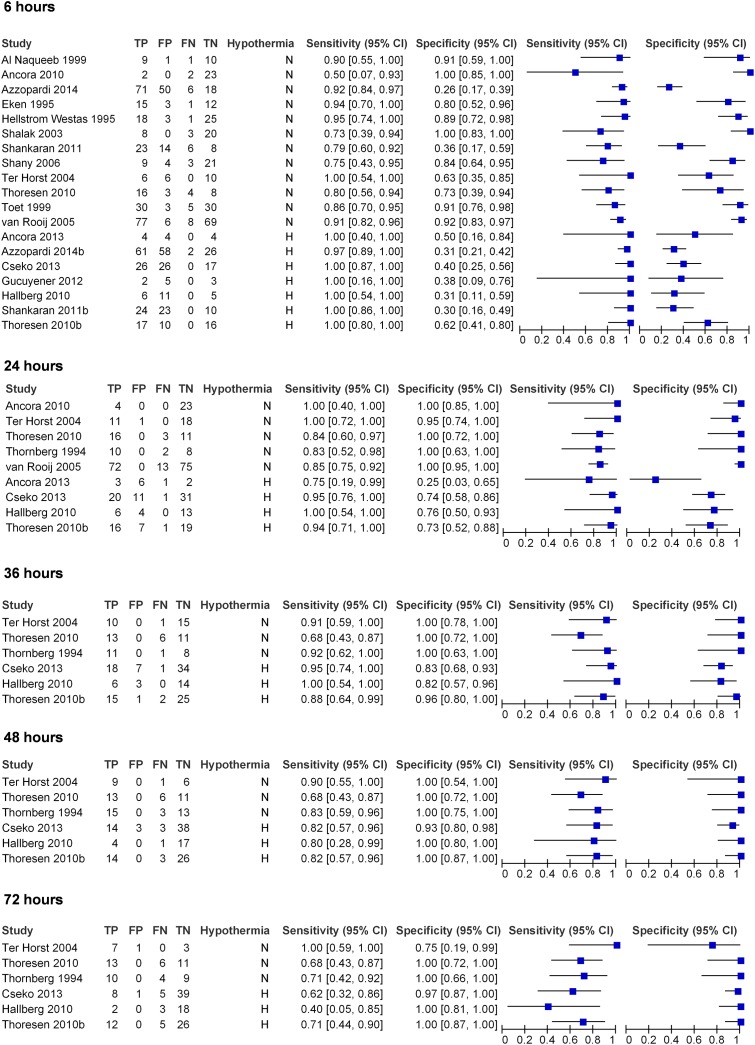
Fig 2. Forest plot for all included studies, considering hours of life and treatment or not with hypothermia.
Forest plot of sensitivity and specificity with 95% confidence intervals is shown in Fig 3. To evaluate the predictive value of an abnormal aEEG in infants not treated with hypothermia thirteen studies including 671 patients were included [12,15–17,21,23–28,31,34]. Seven studies including 360 patients assessed the predictive value of an abnormal aEEG in patients treated with hypothermia [17,29–34].
Fig 3. QUADAS 2 assessment of included papers.
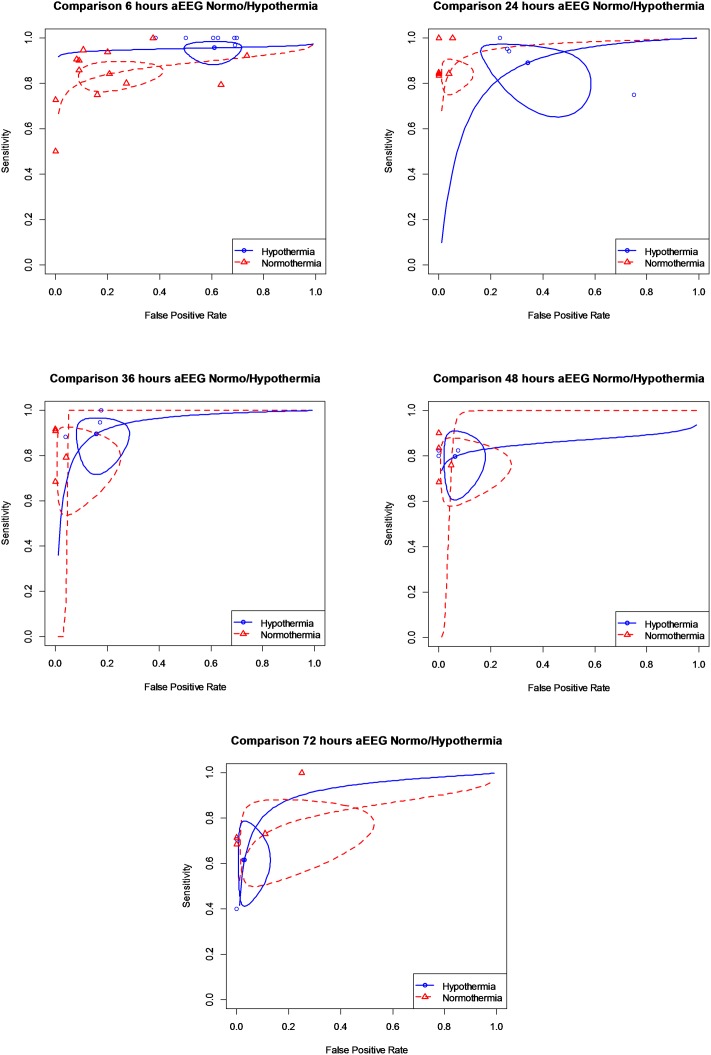
ROC curves for different times of life for patients receiving or not TH are shown in Fig 4. Curves for patients receiving and not receiving TH are presented separately, as the prognostic value of aEEG differed for these two groups of patients at 6 and 24 hours of life. A statistical significant trend exists for sensitivity and specificity with increasing hours of life in patients receiving TH. This trend exists only for sensitivity in normothermia patients. After 36 hours of life, ROC curves for patients receiving and not receiving TH treatment overlapped. Pooled sensitivity and specificity, diagnostic odds ratios and an overall summary of this review are given in Table 3. In infants not treated with hypothermia the maximum reliability of an abnormal aEEG background to predict death or moderate/severe disability was at 36 hours of life, when a positive post-test probability of 97.90% (95%CI 88.40 to 99.40%) was achieved (Fig 5). Positive likelihood ratio at these hours of life was 26.60 (95%CI 4.40 to 94.90) and negative likelihood ratio 0.23 (95%CI 0.10 to 0.44). Positive post-test probability was already very high at 24 hours of life (97.60%, 95%CI 92.80% to 99%). In the following time epochs, aEEG´s predictive reliability remained high at around 95%. Notably, a high predictive value was already present at 6 hours of life in this group of patients, with a positive post-test probability of 88.20% (95%CI 79.80 to 93%) and a positive likelihood ratio of 4.34 (95%CI 2.31 to 7.73).
Fig 4. ROC curves for different hours of life.
Table 3. Pooled sensitivity and specificity, diagnostic odds ratio and likelihood ratios with confidence intervals for abnormal aEEG and death or moderate/severe disability, patients receiving and not receiving TH.
| aEEG-Death/Seriousdisability | Normothermia | Hypothermia | |
|---|---|---|---|
| 6h. | Studies (n) | 12 (671) | 7 (360) |
| AUC | 0.87 | 0.94 | |
| Sensitivity (95%CI) | 0.84 (0.78 to 0.88) | 0.95 (0.9 to 0.98) | |
| Specificity (95%CI) | 0.79 (0.63 to 0.89) | 0.61 (0.51 to 0.69) | |
| Positive LR (95%CI) | 4.34 (2.31 to 7.73) | 1.57 (1.37 to 1.85) | |
| Negative LR (95%CI) | 0.2 (0.14 to 0.29) | 0.12 (0.05 to 0.25) | |
| DOR (95%CI) | 22.2 (8.85 to 46.2) | 15.3 (5.5 to 32.4) | |
| 24h. | Studies (n) | 5 (267) | 4 (141) |
| AUC | 0.95 | 0.86 | |
| Sensitivity (95%CI) | 0.84 (0.77 to 0.89) | 0.89 (0.71 to 0.96) | |
| Specificity (95%CI) | 0.96 (0.89 to 0.98) | 0.65 (0.46 to 0.81) | |
| Positive LR (95%CI) | 24.2 (7.48 to 59.3) | 2.76 (1.48 to 5.27) | |
| Negative LR (95%CI) | 0.16 (0.1 to 0.23) | 0.19 (0.04 to 0.53) | |
| DOR (95%CI) | 155 (41.6 to 451) | 23.9 (2.65 to 95.7) | |
| 36h. | Studies (n) | 3 (76) | 3 (126) |
| AUC | 0.95 | 0.93 | |
| Sensitivity (95%CI) | 0.79 (0.59 to 0.9) | 0.89 (0.76 to 0.95) | |
| Specificity (95%CI) | 0.95 (0.81 to 0.99) | 0.84 (0.74 to 0.9) | |
| Positive LR (95%CI) | 26.6 (4.43 to 94.9) | 5.73 (3.35 to 9.43) | |
| Negative LR (95%CI) | 0.23 (0.09 to 0.44) | 0.14 (0.05 to 0.28) | |
| DOR (95%CI) | 141 (13.6 to 589) | 51.2 (13.2 to 130) | |
| 48h. | Studies (n) | 3 (77) | 3 (123) |
| AUC | 0.95 | 0.85 | |
| Sensitivity (95%CI) | 0.76 (0.61 to 0.86) | 0.79 (0.64 to 0.89) | |
| Specificity (95%CI) | 0.95 (0.79 to 0.99) | 0.93 (0.85 to 0.97) | |
| Positive LR (95%CI) | 23.3 (3.55 to 85.7) | 14.4 (5.51 to 32.5) | |
| Negative LR (95%CI) | 0.26 (0.15 to 0.41) | 0.22 (0.11 to 0.37) | |
| DOR (95%CI) | 101 (11.3 to 385) | 73.7 (17.2 to 200) | |
| 72h. | Studies (n) | 3 (64) | 3 (119) |
| AUC | 0.82 | 0.91 | |
| Sensitivity (95%CI) | 0.73 (0.54 to 0.85) | 0.61 (0.45 to 0.75) | |
| Specificity (95%CI) | 0.89 (0.58 to 0.97) | 0.97 (0.9 to 0.99) | |
| Positive LR (95%CI) | 9.04 (1.69 to 31.6) | 24.3 (5.89 to 71.3) | |
| Negative LR (95%CI) | 0.32 (0.17 to 0.56) | 0.39 (0.25 to 0.57) | |
| DOR (95%CI) | 31.8 (3.36 to 119) | 65.6 (12.7 to 203) | |
AUC: area under the curve; DOR diagnostic odds ratio; LR likelihood ratio
Fig 5. Likelihood ratios and post-test probability of abnormal aEEG and adverse neurological outcome, patients not treated with hypothermia according to hours of life.
The maximum predictive reliability for death or moderate/severe disability of an abnormal aEEG in patients treated with hypothermia was achieved at 72 hours of life (post-test probability of 95.70%, 95%CI 84.40 to 98.50%). Positive likelihood ratio at this age was 24.30 (95%CI 5.89 to 71.30) and negative likelihood ratio 0.40 (95%CI 0.25 to 0.57). Compared with newborns not treated with TH, the predictive value of aEEG at 6 hours of life (Fig 6) was low in these patients (59.10%, 95%CI 55.70 to 63%).
Fig 6. Likelihood ratios and post-test probability of abnormal aEEG and adverse neurological outcome, patients treated with hypothermia according to hours of life.
Discussion
This systematic review provides a comprehensive summary of the prognostic value of aEEG in newborns with HIE. It is the first review that includes infants treated with hypothermia and, more importantly, stratifies the results according to postnatal age. This study confirms that aEEG background activity, as recorded during the first 72 hours after birth, has a strong predictive value in infants with HIE treated or not with TH.
In 2007 Spitzmiller et al [18] published a MA of the prognostic value of aEEG in newborns with HIE. Papers included were published between 1996 and 2005. In this MA, an abnormal aEEG had high positive and negative likelihood ratios for the prediction of death or moderate to severe disability (+LR 10.1, 95%CI 5.5 to 18; -LR 0.09, 95%CI 0.06 to 15). Limitations of this MA were heterogeneity between studies and different times of aEEG pattern assessment. Also no studies with hypothermia were included, as the analysis was performed in the pre-hypothermia era. Van Laerhoven et al recently reviewed the different prognostic tools for newborns with HIE [36]. These authors presented their results for aEEG as pooled sensitivities and specificities at 6, 24 and 72 hours of life. During the first 6 hours of life, pooled sensitivity for the prediction of adverse outcome was of 0.95 (95%CI 0.87 to 0.98) and pooled specificity was of 0.92 (95%CI 0.61 to 0.99). At 72 hours of life, pooled sensitivity was 0.92 (95%CI 0.66 to 0.99) and pooled specificity was 0.83 (95%CI 0.64 to 0.93). Though the authors conclude that their results could be used in infants treated with hypothermia, no hypothermia studies were included in their review.
Determining which infants with HIE will have significant brain damage is crucial. Among the diverse tools that can assist with prognosis are clinical examination, electrophysiological investigations including aEEG or imaging of the brain. Treatment with hypothermia has indeed changed the prognostic value of the initial aEEG trace and the time cutoffs for outcome prediction based on aEEG in newborn infants with HIE. The seminal work by Thoresen et al [17] showed that the positive predictive value of aEEG before 6 hours of life was significant in normothermia-treated neonates following perinatal HIE (84%), but lower among hypothermia-treated infants (59%). Our analysis showed similar predictive values, with clear differences existing between neonates treated or not with TH. As Infants with moderate or severe HIE are cooled within the first 6 hours of life, differences in aEEG´s predictive values at this early age deserves an explanation. Although profound hypothermia depresses EEG voltages, it has been shown that moderate cooling has no effect on aEEG background pattern both in animal models [37] and clinical studies [38]. Reduction in the predictive reliability of early aEEG (6 and 24 hours of life) for adverse outcome in hypothermia-treated infants compared with normothermic infants could be explained by the beneficial effect of TH on the ongoing brain damage [3]. Additional neuroprotection due to sedative drugs frequently administered in patients receiving hypothermia cannot be ruled out [39]. Another important finding in in the work by Thoresen [17] was that whilst all infants cared for at normothermia who normalized their background pattern beyond 24 hours after birth had a poor outcome, some of the hypothermia-treated infants still developed normally after an abnormal aEEG at 24 hours as long as the aEEG recovered before 48 hours. Our study confirmed a similarly high predictive reliability for death or moderate/severe disability of an abnormal aEEG at 48 hours of life in patients treated with hypothermia (post-test probability of 93%, 95%CI 83.5 to 96.8%).
The effect of TH on tools for neurological assessment of the newborn with HIE other than aEEG has been evaluated in individual studies. In a similar way to aEEG, the predictive value of the severity of clinical encephalopathy at less than 6 hours of age is lower for hypothermia-treated than for normothermia-treated infants [40, 41]. The time course of clinical encephalopathy throughout TH is valuable in outcome prediction. While TH reduces brain injury on magnetic resonance imaging (MRI) in newborn infants with HIE, the predictive value of MRI for subsequent outcome is not affected by TH [42]. Combined with clinical and neuroimaging information, aEEG is of valuable help to the clinician when considering the need for neuroprotection and the likely outcome of infants with HIE.
The strength of the present work is the differentiation of prognostic values depending on postnatal age and use or not of TH. Traces at less than 6 hours and in normothermia conditions are useful for the selection of infants with significant HIE needing neuroprotection in order to improve outcome. Predictive values of traces throughout the following 72 hours are a helpful guide when considering and counselling parents about the foreseeable long-term neurological outcome. In order to provide strong evidence of the predictability of aEEG and its 72-hour time course in infants with perinatal HIE a thorough revision of available literature was needed.
We are conscious of several limitations of our work. The aim to include evaluation at different epochs of postnatal life lowered the number of patients included for each period. Also, heterogeneity was high when considering timing and duration of aEEG monitoring, duration of follow-up and neurodevelopmental tests used by different authors. In studies without TH the percentage of patients with mild HIE is higher than in studies using TH, a factor that might influence aEEG´s prognostic reliability. In addition, the ability of different recording equipment used from initial studies to the latest may be different. Our analysis did not include sleep-wake cycling, seizures or administration of anticonvulsants or narcotics. One important limitation is the general lack of information in the original papers on the numbers of deaths that occurred after withdrawal of intensive care as a consequence of abnormal results of prognostic tools, including aEEG. Of the studies included, only one from the era preceding TH by Eken gave account of the causes of death [21]. In this study, 9 of 17 deaths followed withdrawal of intensive care occurring as a result of a flat aEEG trace beyond 24 hours of life. This limitation could introduce a bias in the prognostic reliability of aEEG.
Conclusion
This new MA of the prognostic value of aEEG in newborns with HIE incorporates for the first time hours of life and treatment with hypothermia. With TH the maximum capacity reliability of aEEG to predict adverse neurological outcome is delayed from 24–36 to 48–72 hours of life.
Supporting Information
(DOCX)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
Yolanda Castilla and Ariadna Alberola collaborated in the summaries of the study findings included in Tables 1 and 2 of this review.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This review was part of the Spanish National Health System Clinical Practice Guideline (CPG) on HIE. Funding was received for the development of the whole CPG from the Agencia de Qualitat i Avaluació Sanitàries de Catalunya (public quality agency linked to the Health Department of the Catalan Government).
References
- 1.Smith J, Wells l, Dodd K.The continuing fall in incidence of hypoxic-ischaemic encephalopathy in term infants. BJOG 2000; 107(4): 461–6. [DOI] [PubMed] [Google Scholar]
- 2.García-Alix A, Martinez Biarge M. Neonatal hypoxic-ischemic encephalopathy: Incidence and prevalence in the first decade of the 21st century. An Pediatr (Barc) 2009; 71: 319–26. [DOI] [PubMed] [Google Scholar]
- 3.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med 2012; 166(6): 558–66. 10.1001/archpediatrics.2011.1772 [DOI] [PubMed] [Google Scholar]
- 4.Blanco D, García-Alix A, Valverde E, Tenorio V, Vento M, Cabañas F. Neuroprotection with hypothermia in the newborn with hypoxic-ischaemic encephalopathy. Standard guidelines for its clinical application. An Pediatr (Barc) 2011; 75: 341.e1–20. [DOI] [PubMed] [Google Scholar]
- 5.Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury. National Institute for Health and Clinical Excellence. www.nice.org.uk/guidance/IPG347/publicinfo.
- 6.Chalak L, Kaiser J. Neonatal Guideline Hypoxic-Ischemic Encephalopathy (HIE). Available in: http://www.uams.edu/angels/guidelines.asp. [PubMed]
- 7.Hypothermia for newborns with hypoxic ischemic encephalopathy. Fetus and Newborn Committee Canadian Paediatric Society. Paediatr Child Health 2012; 17: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellström-Westas L, de Vries L, Rosen I. An atlas of amplitude integrated EEGs in the newborn. 1st edition London: Parthenon Publishing; 2003. [Google Scholar]
- 9.Hellstrom-Westas L. Comparison between tape-recorded and amplitude-integrated EEG monitoring in sick newborn infants. Acta Paediatr 1992; 81(10): 812–9. [DOI] [PubMed] [Google Scholar]
- 10.Toet MC, van der Meij W, de Vries LS, Uiterwaal CS, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 2002; 109(5): 772–9. [DOI] [PubMed] [Google Scholar]
- 11.Shah DK, Mackay MT, Lavery S, Watson S, Harvey AS, Zempel J, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics 2008; 121(6): 1146–54. 10.1542/peds.2007-1839 [DOI] [PubMed] [Google Scholar]
- 12.Al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude integrated electroencephalography. Pediatrics 1999; 103: 1263–71. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom Westas L, Rosen I, de Vries LS, Greisen G. Amplitude integrated EEG: Classification and interpretation in preterm and term infants. Neoreviews 2006; 7: e76–87. [Google Scholar]
- 14.Hellström-Westas. Monitoring brain function with aEEG in term asphyxiated infants before and during cooling. Acta Paediatr 2013; 102(7):678–9. 10.1111/apa.12287 [DOI] [PubMed] [Google Scholar]
- 15.Toet MC, Hellström-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999; 81(1): F19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics 2003; 111(2): 351–7. [DOI] [PubMed] [Google Scholar]
- 17.Thoresen M, Hellström-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010; 126(1): e131–9. 10.1542/peds.2009-2938 [DOI] [PubMed] [Google Scholar]
- 18.Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath SB. Amplitude-integrated EEG is useful in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J ChildNeurol 2007; 22(9): 1069–78. [DOI] [PubMed] [Google Scholar]
- 19.Grupo de trabajo de la Guía de Práctica Clínica sobre Encefalopatía Hipóxico-Isquémica Perinatal en el Recién Nacido. Guía de Práctica Clínica sobre Encefalopatía Hipóxico-Isquémica Perinatal en el Recién Nacido. Ministerio de Sanidad, Servicios Sociales e Igualdad. Agència de Qualitat i AvaluacióSanitàries de Catalunya (AQuAS); 2015. Guías de PrácticaClínica en el SNS. http://aquas.gencat.cat/ca/projectes/mes_projectes/guies_eines_decisions/gpc_encefalopatia_nounat/
- 20.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155 (8): 529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 21.Eken P, Toet MC, Groenendaal F, de Vries LS. Predictive value of early neuroimaging, pulsed Doppler and neurophysiology in full term infants with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1995; 73(2): F75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitsma J, Glas A, Rutjes A, Scholten R, Bossuyt P, Zwinderman A. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005; 58: 982–990. 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 23.Hellström-Westas L, Rosén I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed 1995; 72(1): F34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornberg E, Ekström-Jodal B. Cerebral function monitoring: a method of predicting outcome in term neonates after severe perinatal asphyxia. Acta Paediatr 1994; 83(6): 596–601. [DOI] [PubMed] [Google Scholar]
- 25.Ter Horst HJ, Sommer C, Bergman KA, Fock JM, van Weerden T W, Bos AF. Prognostic significance of amplitude-integrated EEG during the first 72 hours after birth in severely asphyxiated neonates. Pediatr Res 2004; 55(6): 1026–33. 10.1203/01.pdr.0000127019.52562.8c [DOI] [PubMed] [Google Scholar]
- 26.van Rooij LG, Toet MC, Osredkar D, van Huffelen AC, Groenendaal F, de Vries LS. Recovery of amplitude integrated electroencephalographic background patterns within 24 hours of perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed.2005; 90(3): F245–51. 10.1136/adc.2004.064964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shany E, Goldstein E, Khvatskin S, Friger MD, Heiman N, Goldstein M, et al. Predictive value of amplitude-integrated electroencephalography pattern and voltage in asphyxiated term infants. Pediatric Neurol 2006; 35(5): 335–42. [DOI] [PubMed] [Google Scholar]
- 28.Ancora G, Soffritti S, Lodi R, Tonon C, Grandi S, Locatelli C, et al. A combined a-EEG and MR spectroscopy study in term newborns with hypoxic-ischemic encephalopathy. Brain Dev 2010; 32(10): 835–42. 10.1016/j.braindev.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 29.Hallberg B, Grossmann K, Bartocci M, Blennow M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr 2010; 99(4): 531–6. 10.1111/j.1651-2227.2009.01653.x [DOI] [PubMed] [Google Scholar]
- 30.Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, Savini S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev 2013; 35(1):26–31. 10.1016/j.braindev.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 31.Shankaran S, Pappas A, McDonald SA, Laptook AR, Bara R, Ehrenkranz RA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics 2011; 128(1): e112–20. 10.1542/peds.2010-2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gucuyener K, Beken S, Ergenekon E, Soysal S, Hirfanoglu I, Turan O, et al. Use of amplitude-integrated electroencephalography (aEEG) and near infrared spectroscopy findings in neonates with asphyxia during selective head cooling. Brain Dev 2012; 34(4): 280–6. 10.1016/j.braindev.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 33.Csekő AJ, Bangó M, Lakatos P, Kárdási J, Pusztai L, Szabó M. Accuracy of amplitude-integrated electroencephalography in the prediction of neurodevelopmental outcome in asphyxiated infants receiving hypothermia treatment. Acta Paediatr 2013; 102(7): 707–11. 10.1111/apa.12226 [DOI] [PubMed] [Google Scholar]
- 34.Azzopardi D; TOBY study group. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. ArchDisChild Fetal Neonatal Ed. 2014; 99(1): F80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976; 33(10): 696–705. [DOI] [PubMed] [Google Scholar]
- 36.van Laerhoven H, de Haan TR, Offringa M, Post B, van der Lee JH. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics 2013; 131: 88–98. 10.1542/peds.2012-1297 [DOI] [PubMed] [Google Scholar]
- 37.Thoresen M. Supportive care during neuroprotective hypothermia in the term newborn: adverse effects and their prevention. Clin Perinatol 2008; 35(4): 749–63. 10.1016/j.clp.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 38.Horan M, Azzopardi D, Edwards AD, Firmin RK, Field D. Lack of influence of mild hypothermia on amplitude integrated-electroencephalography in neonates receiving extracorporeal membrane oxygenation. Early Hum Dev 2007; 83(2): 69–75. 10.1016/j.earlhumdev.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 39.Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics 2008; 12(4) 844–9. [DOI] [PubMed] [Google Scholar]
- 40.Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatalencephalopathy. J Pediatr 2008; 152(1):55–8, 10.1016/j.jpeds.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 41.Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CM, Das A, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2012; 160(4): 567–572. 10.1016/j.jpeds.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magnetic resonance imaging in hypoxic-ischaemic encephalopathy. Early Hum Dev 2010; 86(6): 351–60. 10.1016/j.earlhumdev.2010.05.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.