Abstract
Purpose
Although African-Americans experience higher cancer morbidity and mortality rates compared to their White counterparts, their participation in biospecimen research is lower than that of their white peers. This study investigated the prevalence and predictors of biospecimen donation in a large, cohort study of Black women.
Methods
The BWHS is a follow-up study of U.S. Black women aged 21–69 years enrolled through postal health questionnaires. Between January 2004 and December 2007, participants were sent a consent form with a postage-paid return envelope, and a mouthwash collection kit. Univariate and age- and educational status-adjusted logistic regression models were used to estimate the association of socio-demographic, lifestyle and medical factors with donation of biospecimens.
Results
Buccal cells with consent forms were obtained from 26,790 women, for a response rate of 51 %. The strongest predictors of biospecimen donation were age: response increased from 48.6 % among those aged <40 to 63.1 % among those aged 60 and older [RR 1.30 (95 % CI 1.27, 1.34)]; multivitamin use [RR (95 % CI) 1.32 (1.30, 1.34)]; physician visit in the previous 2 years [RR (95 % CI) 1.61 (1.58, 1.65)], and a history of breast [RR (95 % CI) 1.59 (1.56, 1.63)], colon [RR (95 % CI) 1.18 (1.16, 1.20)], and cervical [RR (95 % CI) 1.63 (1.60, 1.67)] cancer screening.
Conclusions
We found that 51 % of women in the geographically-dispersed Black Women’s Health Study cohort were willing to provide mouthwash samples to be used for genetic analyses. The response in this study is encouraging given published findings of low overall participation rates of African-Americans in genetic studies.
Keywords: Biospecimens, Blacks, Women, Buccal cells, DNA, Genetics
Introduction
The collection of high quality specimens is essential to the advancement of health research and the mission focused on personalized medicine [1–4]. Epidemiologists are increasingly seeking to supplement observational data with high-quality genomic DNA in order to examine the role of genetic factors in disease occurrence and to evaluate gene–environment interactions. African-Americans’ participation in biospecimen research is lower than Whites [5, 6] although they experience a greater cancer burden, i.e., higher cancer morbidity and mortality rates compared to their White counterparts.
Feasible methods of DNA collection that are acceptable to study participants are needed to overcome the paucity of data about gene–disease associations in African Americans. [7–10] Buccal cell collection by the mouthwash swish method is a viable alternative to blood collection for obtaining DNA in large cohort studies in which study subjects may be geographically dispersed [11, 12]. In contrast to blood collection, buccal cell collection is non-invasive and can be self-administered, and samples can be transported via the postal system [11–14]. This method has been found to provide adequate DNA yield and quality [11, 12, 14–18].
The present study represents the first study to investigate predictors of biospecimen donation in the donation of DNA biospecimens within the Black Women’s Health Study (BWHS), a large, geographically dispersed cohort of U.S. Black women. To the best of our knowledge data on biospecimen donation rate and covariates associated with donation have not been published from other large cohorts.
Materials and methods
BWHS cohort and follow-up
The human subject protocol for this study was reviewed and approved by the Boston University Medical Center, Howard University Cancer Center, and Georgetown University Institutional Review Boards. The BWHS is a follow-up study of U.S. Black women that began in 1995 when 59,000 women aged 21–69 years enrolled through postal health questionnaires, which were sent mainly to subscribers of Essence magazine, members of selected Black women’s professional organizations, and friends and relatives of early respondents. At baseline in 1995, subjects had a median age of 38 years, 97 % had completed high school, and 44 % had completed college. Over 80 % were from California, Georgia, Illinois, Indiana, Louisiana, Maryland, Massachusetts, Michigan, New Jersey, New York, South Carolina, Virginia, and the District of Columbia. Biennial surveys collect data on demographic, reproductive, and lifestyle factors and the occurrence of major illnesses. Questionnaires are mailed up to 8 times within the 2-year period in order to increase participation [19]. Follow-up has been successful for 87 % of potential person-years after eight completed follow-up cycles.
The present analyses used data reported at baseline in 1995 and during follow-up; we used the information that was collected closest in time to 2005: age (2005); education (2003); occupation (1995); working a second job (1995); marital status (2005); childcare/parental responsibilities (1995); smoking status (2005); height (1995); weight (2005); vigorous physical activity (2001); alcohol consumption (2005); multivitamin use (2005); personal history of heart attack, stroke, diabetes, hypertension, high cholesterol, and cancer (1995–2005); family history of breast cancer (1999); family history of heart attack or stroke (1999); family history of diabetes (1999); and physician visits (2005). Data on food intake in 1995 was collected with the NCI-Block short food frequency questionnaire [11, 12], from which total energy intake and total fruit and vegetable intake were derived. We used data collected in 2005 on use of colonoscopy/sigmoidoscopy; mammography; and Pap smear. The women’s addresses in 2005 were linked to U.S. census data on education, employment, and income at the census block group level, from which a neighborhood socioeconomic score was derived [3]. Body mass index was calculated as weight in kg divided by ht2 in meters.
DNA study recruitment and participation
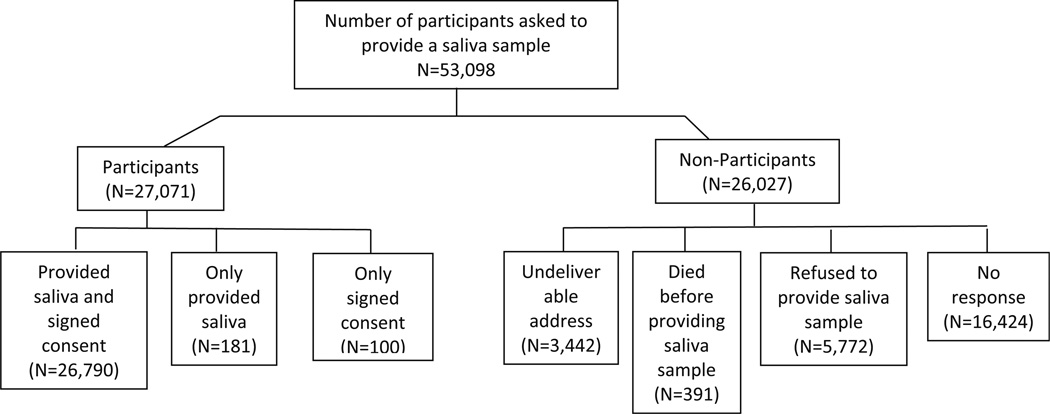
Between January 2004 and December 2007, 53,098 BWHS participants were sent a packet containing an invitational letter, a consent form with a postage-paid return envelope, and a mouthwash collection kit. They were instructed to return signed consent forms to Boston University, and to mail mouthwash samples to the National Human Genome Center at Howard University, Washington, D.C. Once both items were received, participants were mailed a $15 check. Reminder letters were mailed to non-responders 3 weeks after the initial kits were mailed. Women who did not respond after an additional 3 weeks were telephoned, with calls made both during the day, evening, and weekend hours, for a total of up to nine calls per person (Fig. 1).
Fig. 1.
Flowchart of recruitment and participation, Black Women’s Health Study
DNA sample collection
Mouthwash collection kits consisted of a 45 ml (1.5 oz) bottle of Mint Fresh Scope® (Proctor & Gamble), a 15 ml screw-top polypropylene container, printed instructions, a bubble lined envelope, a Ziploc plastic bag, and 2 first class postage-paid envelopes. Subjects were instructed to take a mouthful of Scope, swish vigorously for 45 s, and spit the sample into the polypropylene jar. After the screw-top was secured, the jar was to be placed first into the bubble-lined envelope, which was to be sealed and then placed into the Ziploc bag. Subjects were asked to record the time and date of rinsing on the instruction sheet, and to mail the instruction sheet to the laboratory with the sample. The study initially requested one-swish, but based on data from other studies [12, 17], the protocol was amended in October 2004, to request two consecutive swishes to be spit into two separate containers.
After receipt in the laboratory, mouthwash samples were centrifuged, and pellets were split into two portions for storage at −20 °C. One half of the frozen sample was shipped to the Molecular Genetics Core Laboratory at Boston University School of Medicine, Boston, MA for DNA extraction and long-term storage at −80 °C. DNA extracted from the samples has been used for studies of numerous conditions, including breast cancer [20]. DNA extraction was performed using QIAAMP® DNA Mini Kit (Qiagen USA, Valencia, CA) according to manufacturer’s instructions (available at: www.qiagen.com). DNA concentrations were determined by UV spectrophotometry. As described elsewhere [11], the DNA was found to be of high quality in genotyping of NAT-2.
Statistical analysis
Response rate for buccal samples was calculated by dividing the number of women who returned saliva samples with a signed consent by the number of women who were alive at the time of saliva collection and could be reached by mail/phone. Univariate and age- and educational status-adjusted logistic regression models were used to estimate the association of socio-demographic, lifestyle and medical factors with donation of biospecimens. All analyses were performed using SAS for Windows (SAS Institute, Cary, NC), version 9.3.
Results
Of the 53,098 participants asked to provide a buccal sample 48,986 (92 %) were included in the current analyses: 3,442 (6.5 %) could not be reached due to an undeliverable address, 391 (0.7 %) died before providing a saliva sample, and 281 (0.5 %) provided a saliva sample without signed informed consent or a signed consent without the saliva sample. Buccal cell samples with consent forms were obtained from 26,790 women for a response rate of 51 %. The demographic predictors of mouthwash donation are shown in Table 1. The strongest predictor of donation was age: response increased from 49 % among those aged <40 to 63 % among those aged 60 and older [OR 1.30 (95 % CI 1.27, 1.34) for age 60+ relative to <40]. Response rates were not meaningfully different from the null value of 1.00 and very similar across strata of other demographic factors.
Table 1.
Demographic predictors of mouthwash donation among women in the BWHS (2005)
| Characteristic | Total analytic sample 48,986 |
Returned mouthwash kit 26,790 |
Crude RR | Age and education adjusted RR |
|---|---|---|---|---|
| Age (2005) | ||||
| <40 | 10,634 | 48.6 | 1.00 | 1.00 |
| 40–49 | 16,593 | 52.1 | 1.07 (1.05, 1.10) | 1.07 (1.05, 1.10) |
| 50–59 | 13,921 | 57.7 | 1.19 (1.16, 1.22) | 1.19 (1.16, 1.22) |
| 60+ | 7,838 | 63.1 | 1.30 (1.27, 1.33) | 1.30 (1.27, 1.34) |
| Education (years) (2003) | ||||
| ≤12 | 8,774 | 55.6 | 1.00 | 1.00 |
| 13–15 | 17,345 | 53.8 | 0.98 (0.95, 0.99) | 1.00 (0.98, 1.03) |
| 16 | 12,009 | 54.1 | 0.97 (0.95, 1.00) | 1.02 (1.00, 1.05) |
| 17+ | 10,799 | 56.3 | 1.01 (0.99, 1.04) | 1.03 (1.00, 1.05) |
| Occupation (1995) | ||||
| Professional/technical/manager/administrator | 27,177 | 55.0 | 1.00 | 1.00 |
| Sales/clerical/service worker | 15,142 | 54.3 | 0.99 (0.97, 1.00) | 1.02 (1.00, 1.05) |
| Other | 2,096 | 57.3 | 1.04 (1.00, 1.08) | 1.06 (1.01, 1.10) |
| Works second job (1995) | ||||
| No | 38,876 | 54.3 | 1.00 | 1.00 |
| Yes | 8,924 | 56.5 | 1.04 (1.02, 1.06) | 1.05 (1.02, 1.07) |
| Marital status (2005) | ||||
| Single | 7,139 | 64.9 | 1.00 | 1.00 |
| Married/living as married | 15,012 | 64.1 | 0.99 (0.97, 1.01) | 0.96 (0.94, 0.98) |
| Divorced/separated/widowed | 10,919 | 68.2 | 1.05 (1.03, 1.07) | 0.99 (0.97, 1.01) |
| Childcare/parent care responsibilities (1995) | ||||
| No | 25,030 | 54.4 | 1.00 | 1.00 |
| Yes | 23,956 | 55.1 | 1.01 (1.00, 1.03) | 1.02 (1.00, 1.04) |
| Geographic region (2005) | ||||
| Northeast | 9,933 | 60.8 | 0.97 (0.95, 0.99) | 0.97 (0.95, 0.99) |
| South | 14,502 | 62.7 | 1.00 | 1.00 |
| Midwest | 8,964 | 63.8 | 1.02 (1.00, 1.04) | 1.01 (0.99, 1.03) |
| West | 7,181 | 61.2 | 0.98 (0.96, 1.00) | 0.97 (0.95, 0.99) |
| Neighborhood SES score (quintiles) (2005) | ||||
| 1 (lowest) | 8,786 | 57.1 | 1.00 | 1.00 |
| 2 | 8,981 | 54.9 | 0.96 (0.94, 0.99) | 0.96 (0.94, 0.99) |
| 3 | 9,070 | 53.8 | 0.94 (0.92, 0.97) | 0.95 (0.92, 0.97) |
| 4 | 9,152 | 54.2 | 0.95 (0.92, 0.97) | 0.95 (0.93, 0.98) |
| 5 (highest) | 9,229 | 54.0 | 0.95 (0.92, 0.97) | 0.94 (0.92, 0.97) |
Regular multivitamin intake (3 times/week) was the only lifestyle factor meaningfully associated with sample donation with a RR of 1.32 (95 % CI 1.30, 1.34) (Table 2).
Table 2.
Lifestyle predictors of mouthwash donation among women in the BWHS (2005)
| Characteristic | Total analytic sample 48,986 |
Returned mouthwash kit 26,790 |
Crude RR | Age and education adjusted RR |
|---|---|---|---|---|
| Cigarette smoking (2005) | ||||
| Current | 5,985 | 51.5 | 1.00 | 1.00 |
| Former | 11,354 | 59.3 | 1.15 (1.12, 1.18) | 1.11 (1.08, 1.14) |
| Never | 31,577 | 53.7 | 1.04 (1.01, 1.07) | 1.06 (1.03, 1.09) |
| Body mass index (kg/m2) (2005) | ||||
| <25 | 10,097 | 57.6 | 1.00 | 1.00 |
| 25–29.9 | 14,268 | 58.8 | 1.02 (1.00, 1.04) | 1.00 (0.98, 1.02) |
| ≥30 | 18,832 | 59.9 | 1.04 (1.02, 1.06) | 1.02 (1.00, 1.05) |
| Vigorous physical activity (hours/week) (2001) | ||||
| None | 19,736 | 60.0 | 1.00 | 1.00 |
| ≤1 | 11,854 | 59.7 | 1.00 (0.98, 1.01) | 1.02 (1.00, 1.04) |
| 2 | 3,724 | 58.8 | 0.98 (0.95, 1.01) | 1.01 (0.98, 1.03) |
| ≥3 | 2,995 | 57.5 | 0.96 (0.93, 0.99) | 0.98 (0.95, 1.02) |
| Alcohol intake (2005) | ||||
| Current | 12,153 | 53.5 | 1.00 | 1.00 |
| Former | 16,930 | 58.2 | 1.09 (1.06, 1.11) | 1.08 (1.06, 1.10) |
| Never | 19,683 | 52.5 | 0.98 (0.96, 1.00) | 0.98 (0.96, 1.00) |
| Multivitamin intake (3+ times/week) (2005) | ||||
| No | 25,730 | 47.1 | 1.00 | 1.00 |
| Yes | 23,256 | 63.1 | 1.34 (1.32, 1.36) | 1.32 (1.30, 1.34) |
| Total energy intake (quartiles) (2001) | ||||
| 1 (lowest) | 9,296 | 59.5 | 1.00 | 1.00 |
| 2 | 9,369 | 61.4 | 1.03 (1.01, 1.06) | 1.03 (1.01, 1.06) |
| 3 | 9,382 | 62.0 | 1.04 (1.02, 1.07) | 1.05 (1.03, 1.08) |
| 4 (highest) | 9,285 | 61.7 | 1.04 (1.01, 1.06) | 1.06 (1.03, 1.08) |
| Total fruit and vegetable intake (quartiles) (2001) | ||||
| 1 (lowest) | 10,302 | 59.6 | 1.00 | 1.00 |
| 2 | 8,249 | 61.0 | 1.02 (1.00, 1.05) | 1.01 (0.99, 1.04) |
| 3 | 7,717 | 61.9 | 1.04 (1.01, 1.06) | 1.02 (1.00, 1.04) |
| 4 (highest) | 9,066 | 63.3 | 1.06 (1.04, 1.09) | 1.04 (1.02, 1.06) |
Biospecimen donation was similar across medical history and family history of cancer (Table 3). However, physician visit in the previous 2 years and a history of breast, colon, and cervical cancer screening were associated with a statistically significant increase in biospecimen donation compared to the referent groups (Table 3).
Table 3.
Medical history predictors of mouthwash donation among women in the BWHS (2005)
| Characteristic | Total analytic sample 48,986 |
Returned mouthwash kit 26,790 |
Crude RR | Age and education adjusted RR |
|---|---|---|---|---|
| Reported a chronic condition between 1995 and 2005 | ||||
| Heart attack | 887 | 58.5 | 1.07 (1.01, 1.13) | 1.00 (0.95, 1.06) |
| Stroke | 904 | 61.4 | 1.13 (1.07, 1.19) | 1.05 (1.00, 1.11) |
| Diabetes | 5,864 | 59.4 | 1.10 (1.07, 1.12) | 1.04 (1.02, 1.07) |
| Hypertension | 20,865 | 58.2 | 1.12 (1.10,1.14) | 1.05 (1.04, 1.07) |
| High cholesterol | 16,938 | 58.9 | 1.12 (1.10, 1.14) | 1.07 (1.05, 1.09) |
| Cancer | 2,398 | 61.6 | 1.12 (1.09, 1.15) | 1.06 (1.03, 1.10) |
| Any of above | 20,902 | 58.6 | 1.14 (1.10, 1.18) | 1.07 (1.06, 1.09) |
| None of above | 28,084 | 51.8 | 1.00 | 1.00 |
| Family history of breast cancer (1999) | ||||
| No | 45,749 | 54.6 | 1.00 | 1.00 |
| Yes | 3,237 | 56.5 | 1.04 (1.00, 1.07) | 1.01 (0.98, 1.04) |
| Family history of other cancers (1999) | ||||
| No | 35,110 | 53.2 | 1.00 | 1.00 |
| Yes | 13,876 | 58.4 | 1.10 (1.08, 1.12) | 1.06 (1.04, 1.07) |
| Family history of heart attack/stroke (1999) | ||||
| No | 33,751 | 53.5 | 1.00 | 1.00 |
| Yes | 15,235 | 57.3 | 1.07 (1.05, 1.09) | 1.03 (1.01, 1.05) |
| Family history of diabetes (1999) | ||||
| No | 35,733 | 54.1 | 1.00 | 1.00 |
| Yes | 13,253 | 56.3 | 1.04 (1.02, 1.06) | 1.03 (1.01, 1.04) |
| Physician visit between 2003 and 2005 | ||||
| No | 15,604 | 38.1 | 1.00 | 1.00 |
| Yes | 33,382 | 62.5 | 1.64 (1.60, 1.68) | 1.61 (1.58, 1.65) |
| Colonoscopy or sigmoidoscopy between 2003 and 2005 | ||||
| No | 39,526 | 52.2 | 1.00 | 1.00 |
| Yes | 9,460 | 65.2 | 1.25 (1.23, 1.27) | 1.18 (1.16, 1.20) |
| Mammogram between 2003 and 2005 | ||||
| No | 18,604 | 40.2 | 1.00 | 1.00 |
| Yes | 30,382 | 63.6 | 1.58 (1.55, 1.61) | 1.59 (1.56, 1.63) |
| Pap smear between 2003 and 2005 | ||||
| No | 15,155 | 38.2 | 1.00 | 1.00 |
| Yes | 33,831 | 62.1 | 1.62 (1.59, 1.66) | 1.63 (1.60, 1.67) |
Discussion
We found that 51 % of women in the geographically-diverse Black Women’s Health Study cohort were willing to provide mouthwash samples to be used for genetic analyses. There were few differences in terms of demographic or lifestyle factors between responders and non-responders. Older age was a strong predictor of donation: 63.3 % of women aged ≥60 years provided a cheek cell sample compared with 48.6 % of younger women (<40 years of age). Differences by other factors, such as educational status, marital status, BMI, smoking status, and dietary variables were small. Multivitamin use, recent physician visit and history of breast, colon, and cervical cancer screening were associated with higher proportion of biospecimen donation, indicating that health conscious women were more likely to participate.
This is the first study to investigate predictors of biospecimen donation in a large cohort of African-American women. To the best of our knowledge data on biospecimen donation rate and covariates associated with donation have not been published from other large cohorts. The response in this study is encouraging given published findings of low overall participation rates (<40 %) of African-Americans in genetic studies [7–10], with the exception of research involving the hemoglobin disorders [7]. Previous studies have shown that African-Americans report concerns regarding genetic research [21, 22] and are less likely to participate in genetic registries, even after participating in previous research associated with registry collaborators [23]. In the study by Dash et al. [24], the most common barriers to biospecimen donation reported among African-Americans were not knowing ‘‘how biospecimens will be used in research’’ and ‘‘lack of knowledge about biospecimens,’’ whereas ‘‘distrust of medical community’’ was the least frequently reported. The Tuskegee Syphilis Study [25] or any other unethical biomedical cases [26, 27], were not mentioned as barriers to ‘willingness to donate specimens.’ The BWHS has been ongoing since 1995 and the observed participation rates may reflect the trust developed in this long-term relationship.
Limitations of the current study include generalizability of the study results to all African-Americans. Women in the BWHS have higher educational status compared to the general African-American population and most of the women had at least a high school education. Thus our results may not be generalizable to African-American women with <12 years of education [28]. It is unclear whether participation rates would be similar for biospecimens other than buccal samples. A further limitation is the narrow scope of analyses that can be performed on buccal samples as compared to other biospecimens, namely whole blood. For example, we are unable to collect the biomarker data available in either serum or plasma. Additionally, we are unable to perform DNA methylation on buccal samples given the requirement of relatively large quantities of DNA of high purity [29]. On the other hand, the DNA obtained from our buccal samples has proven to be of sufficiently high quality, and we have successfully carried out genetic analyses of breast cancer [30], uterine fibroids [31], and sarcoidosis [32].
This study demonstrated that self-administered DNA sample collection was successful in a large cohort of Black women. Future studies that incorporate minorities into clinical research involving collection and use of biospecimens are warranted.
Acknowledgments
The study was supported by Grant CA098663 from the National Cancer Institute. The BWHS is supported by CA58420 and UM1CA164974.
Footnotes
Compliance with ethical standards
Conflict of interest There is no conflict of interest reported from the authors of this manuscript.
References
- 1.Vaught J, Rogers J, Myers K, Lim MD, Lockhart N, Moore H, et al. An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr. 2011;(42):1–7. doi: 10.1093/jncimonographs/lgr006. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011;23(1):112–119. doi: 10.1097/CCO.0b013e32834161b8. [DOI] [PubMed] [Google Scholar]
- 3.Moore HM, Compton CC, Lim MD, Vaught J, Christiansen KN, Alper J. Biospecimen research network symposium: advancing cancer research through biospecimen science. Cancer Res. 2009;69(17):6770–6772. doi: 10.1158/0008-5472.CAN-09-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waltz E. Tracking down tissues. Nat Biotechnol. 2007;25(11):1204–1206. doi: 10.1038/nbt1107-1204. [DOI] [PubMed] [Google Scholar]
- 5.Luque JS, Quinn GP, Montel-Ishino FA, Arevalo M, Bynum SA, Noel-Thomas S, et al. Formative research on perceptions of biobanking: what community members think. J Cancer Educ. 2012;27(1):91–99. doi: 10.1007/s13187-011-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiviniemi MT, Saad-Harfouche FG, Ciupak GL, Davis W, Moysich K, Hargrave NC, et al. Pilot intervention outcomes of an educational program for biospecimen research participation. J Cancer Educ. 2013;28(1):52–59. doi: 10.1007/s13187-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal C, Baffoe-Bonnie A, Kittles R, Powell I, Bennett J, Hoke G, et al. Recruitment experience in the first phase of the African American Hereditary Prostate Cancer (AAHPC) study. Ann Epidemiol. 2000;10(8 Suppl):S68–S77. doi: 10.1016/s1047-2797(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 8.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12(4):248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 9.Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2(1–2):31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 10.Gamble VN. A legacy of distrust: African Americans and medical research. Am J Prev Med. 1993;9(6 Suppl):35–38. [PubMed] [Google Scholar]
- 11.Cozier YC, Palmer JR, Rosenberg L. Comparison of methods for collection of DNA samples by mail in the Black Women’s Health Study. Ann Epidemiol. 2004;14(2):117–122. doi: 10.1016/S1047-2797(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 12.Le Marchand L, Lum-Jones A, Saltzman B, Visaya V, Nomura AM, Kolonel LN. Feasibility of collecting buccal cell DNA by mail in a cohort study. Cancer Epidemiol Biomarkers Prev. 2001;10(6):701–703. [PubMed] [Google Scholar]
- 13.Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27(3):251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Closas M, Egan KM, Abruzzo J, Newcomb PA, Titus-Ernstoff L, Franklin T, et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(6):687–696. [PubMed] [Google Scholar]
- 15.Rogers NL, Cole SA, Lan HC, Crossa A, Demerath EW. New saliva DNA collection method compared to buccal cell collection techniques for epidemiological studies. Am J Hum Biol. 2007;19(3):319–326. doi: 10.1002/ajhb.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lum A, Le Marchand L. A simple mouthwash method for obtaining genomic DNA in molecular epidemiological studies. Cancer Epidemiol Biomarkers Prev. 1998;7(8):719–724. [PubMed] [Google Scholar]
- 17.Andrisin TE, Humma LM, Johnson JA. Collection of genomic DNA by the noninvasive mouthwash method for use in pharmacogenetic studies. Pharmacotherapy. 2002;22(8):954–960. doi: 10.1592/phco.22.12.954.33598. [DOI] [PubMed] [Google Scholar]
- 18.Feigelson HS, Rodriguez C, Robertson AS, Jacobs EJ, Calle EE, Reid YA, et al. Determinants of DNA yield and quality from buccal cell samples collected with mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(9):1005–1008. [PubMed] [Google Scholar]
- 19.Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154(9):845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- 20.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, Cupples LA, Cozier YC, Adams-Campbell LL, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013;22(1):127–134. doi: 10.1158/1055-9965.EPI-12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furr LA. Perceptions of genetics research as harmful to society: differences among samples of African-Americans and European-Americans. Genet Test. 2002;6(1):25–30. doi: 10.1089/109065702760093889. [DOI] [PubMed] [Google Scholar]
- 22.Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003;51(3):217–227. doi: 10.1016/s0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 23.Moorman PG, Skinner CS, Evans JP, Newman B, Sorenson JR, Calingaert B, et al. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1349–1354. [PubMed] [Google Scholar]
- 24.Dash C, Wallington SF, Muthra S, Dodson E, Mandelblatt J, Adams-Campbell LL. Disparities in knowledge and willingness to donate research biospecimens: a mixed-methods study in an underserved urban community. J Community Genet. 2014;5(4):329–336. doi: 10.1007/s12687-014-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997;87(11):1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern AM. Sterilized in the name of public health: race, immigration, and reproductive control in modern California. Am J Public Health. 2005;95(7):1128–1138. doi: 10.2105/AJPH.2004.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randall VR. Slavery, segregation and racism: trusting the health care system ain’t always easy! An African American perspective on bioethics. St Louis Univ Public Law Rev. 1996;15(2):191–235. [PubMed] [Google Scholar]
- 28.Day JC, Curry AE. Current Population Reports. US Department of Commerce; 1998. Educational attainment in the United States. March 1998 (update) [Google Scholar]
- 29.Liard PW. Principles and challenges of genome-wide DNA methylation analysis. Nat Rev Genet. 2010;11:191–203. doi: 10.1038/nrg2732. [DOI] [PubMed] [Google Scholar]
- 30.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, Cupples LA, Cozier YC, Adams-Campbell LL, Rosenberg L. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013;22(1):127–134. doi: 10.1158/1055-9965.EPI-12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wise LA, Palmer JR, Ruiz-Narvaez E, Reich DE, Rosenberg L. Is the observed association between dairy intake and fibroids in African Americans explained by genetic ancestry? Am J Epidemiol. 2013;178(7):1114–1119. doi: 10.1093/aje/kwt091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cozier YC, Ruiz-Narvaez E, McKinnon C, Berman J, Rosenberg L, Palmer J. Replication of genetic loci for sarcoidosis in US Black women: data from the Black Women’s Health Study. Hum Genet. 2013;132(7):803–810. doi: 10.1007/s00439-013-1292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]