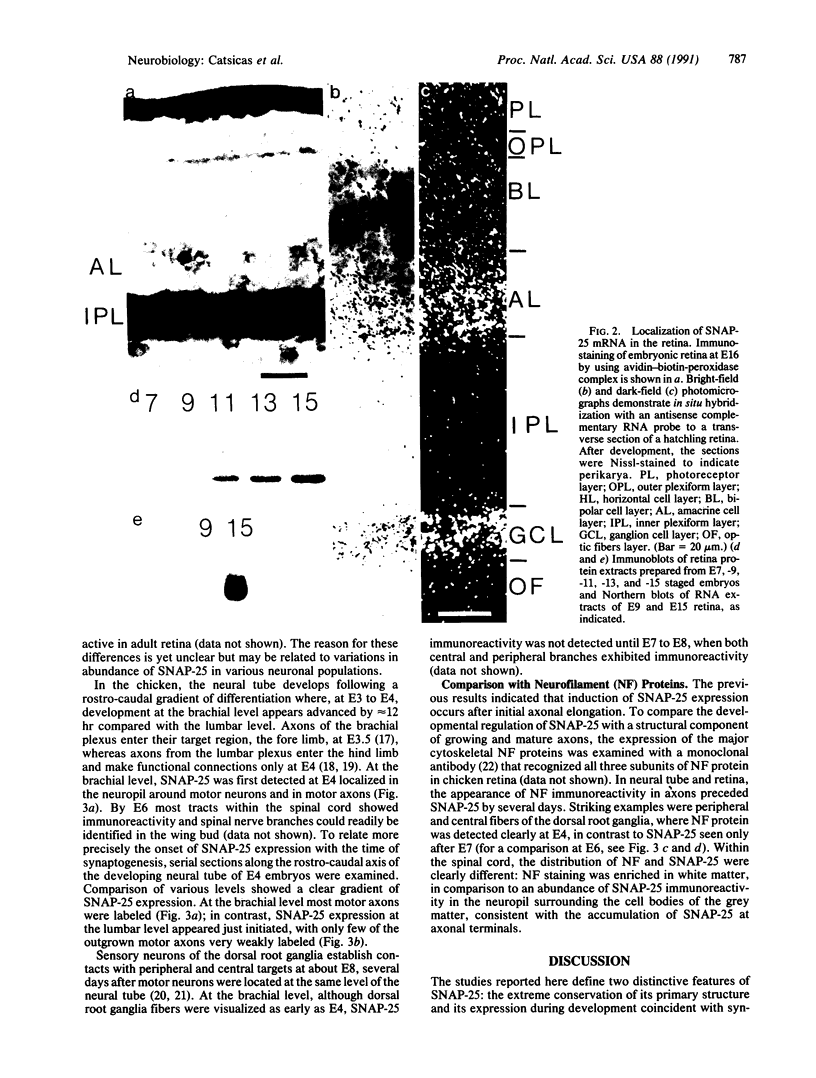
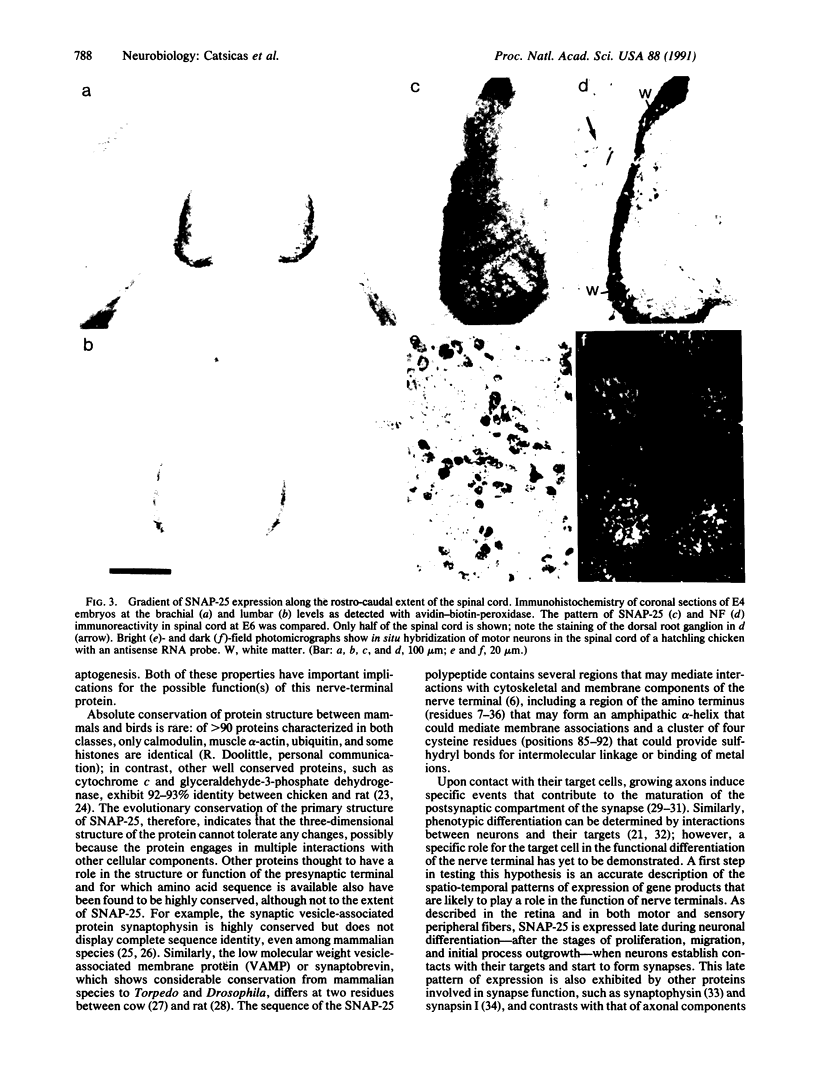
Abstract
Contact of axons with target territories results in the formation of synapses, specific junctional complexes that may represent a final stage of neuronal maturation. Synaptosomal-associated protein 25 (SNAP-25) is a component of particular nerve terminals recently identified in rodent brain. To evaluate the structure and regulation of molecular components of the synapse, we investigated the expression of SNAP-25 in the developing chicken nervous system. Analysis of SNAP-25 cDNA clones demonstrated that the chicken homologue is identical in amino acid sequence to the mouse protein. In chicken retina and neural tube, the onset of SNAP-25 mRNA and protein expression was found to correspond to the time of synaptogenesis. These results suggest that SNAP-25 plays a role in the physiology of mature nerve terminals and that its expression may be regulated by specific cell-cell interactions occurring during synapse formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branks P. L., Wilson M. C. Patterns of gene expression in the murine brain revealed by in situ hybridization of brain-specific mRNAs. Brain Res. 1986 Jul;387(1):1–16. doi: 10.1016/0169-328x(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Clarke P. G. Spatiotemporal gradients of kainate-sensitivity in the developing chicken retina. J Comp Neurol. 1987 Aug 22;262(4):512–522. doi: 10.1002/cne.902620405. [DOI] [PubMed] [Google Scholar]
- Daniels M. P., Vogel Z. Localization of alpha-bungarotoxin binding sites in synapses of the developing chick retina. Brain Res. 1980 Nov 10;201(1):45–56. doi: 10.1016/0006-8993(80)90774-x. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Trimble W. S., Scheller R. H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989 Jul 5;264(19):11061–11064. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J. W., Hess E. J., Hart R. A., Kesslak J. P., Cotman C. W., Wilson M. C. Lesions of hippocampal circuitry define synaptosomal-associated protein-25 (SNAP-25) as a novel presynaptic marker. Neuroscience. 1990;38(2):515–525. doi: 10.1016/0306-4522(90)90047-8. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haas C. A., DeGennaro L. J. Multiple synapsin I messenger RNAs are differentially regulated during neuronal development. J Cell Biol. 1988 Jan;106(1):195–203. doi: 10.1083/jcb.106.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Brunso-Bechtold J. K., Yip J. W. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981 Jan;1(1):60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. D., Virág I., Skene J. H. A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in rat CNS. J Neurosci. 1986 Jun;6(6):1843–1855. doi: 10.1523/JNEUROSCI.06-06-01843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Jahn R., Südhof T. C. Transmembrane topography and evolutionary conservation of synaptophysin. J Biol Chem. 1989 Jan 15;264(2):1268–1273. [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus P., Betz H., Rehm H. Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem. 1986 Oct;47(4):1302–1304. doi: 10.1111/j.1471-4159.1986.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The development of motor projection patterns in the chick hind limb. J Physiol. 1978 Nov;284:391–414. doi: 10.1113/jphysiol.1978.sp012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large T. H., Rauh J. J., De Mello F. G., Klein W. L. Two molecular weight forms of muscarinic acetylcholine receptors in the avian central nervous system: switch in predominant form during differentiation of synapses. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8785–8789. doi: 10.1073/pnas.82.24.8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell J. W., Townes-Anderson E., Czernik A. J., Cameron R., Greengard P., De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990 Jul;5(1):19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Heaton M. B. The retrograde transport of horseradish peroxidase from the developing limb of the chick embryo. Brain Res. 1975 Nov 14;98(2):291–302. doi: 10.1016/0006-8993(75)90007-4. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe E., Garosi M., Droz B. Influence of peripheral and central targets on subpopulations of sensory neurons expressing calbindin immunoreactivity in the dorsal root ganglion of the chick embryo. Neuroscience. 1988 Jul;26(1):225–232. doi: 10.1016/0306-4522(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Rager G. Morphogenesis and physiogenesis of the retino-tectal connection in the chicken. I. The retinal ganglion cells and their axons. Proc R Soc Lond B Biol Sci. 1976 Feb 17;192(1108):331–352. doi: 10.1098/rspb.1976.0017. [DOI] [PubMed] [Google Scholar]
- Role L. W. Neural regulation of acetylcholine sensitivity in embryonic sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2825–2829. doi: 10.1073/pnas.85.8.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotzinger R. J., Landis S. C. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature. 1988 Oct 13;335(6191):637–639. doi: 10.1038/335637a0. [DOI] [PubMed] [Google Scholar]
- Swanson G. J., Lewis J. The timetable of innervation and its control in the chick wing bud. J Embryol Exp Morphol. 1982 Oct;71:121–137. [PubMed] [Google Scholar]
- Südhof T. C., Baumert M., Perin M. S., Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989 May;2(5):1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Lottspeich F., Greengard P., Mehl E., Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987 Nov 20;238(4830):1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Gray T. S., Elferink L. A., Wilson M. C., Scheller R. H. Distinct patterns of expression of two VAMP genes within the rat brain. J Neurosci. 1990 Apr;10(4):1380–1387. doi: 10.1523/JNEUROSCI.10-04-01380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Scheller R. H. Molecular biology of synaptic vesicle-associated proteins. Trends Neurosci. 1988 Jun;11(6):241–242. doi: 10.1016/0166-2236(88)90098-7. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Binder L. I., Matus A. I. Neuronal microtubule-associated proteins in the embryonic avian spinal cord. J Comp Neurol. 1988 May 1;271(1):44–55. doi: 10.1002/cne.902710106. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]