Abstract
Primordial germ cells are usually made early in the development of an organism. These are the mother of all stem cells that are necessary for propagation of the species, yet use highly diverse mechanisms between organisms. How they are specified, and when and where they form, are central to developmental biology. Using diverse organisms to study this development is illuminating for understanding the mechanics these cells use in this essential function, and for identifying the breadth of evolutionary changes that have occurred between species. This essay emphasizes how echinoderms may contribute to the patch-work quilt of our understanding of germ line formation during embryogenesis.
Introduction
The field of reproductive biology is as old a biological science as they come. Our fascination in the process of reproduction, the application to agriculture, the impact on society and the family unit, and perhaps the enormous variations in how plants, animals, and microbes reproduce has captivated the attention of science for centuries. In 1966, at the birth of the first volume of Current Topics in Developmental Biology, we saw the field of reproduction very differently than we do in 2016. We have indeed experienced great progress in the field during this time, and much of it is captured in the pages of this series. This progress has formed great intersections with all aspects of biology and society; think for example of the Nobel Prize in 2010 to Robert Edwards for his work in development of human in vitro fertilization (Steptoe and Edwards, 1978).
It is of great fun to page through the early volumes of the Current Topics series and to see how investigators thought, what the pressing questions of the time were, and how they were approached. It is not surprising that these topics were priorities in the early volumes of CTDB. Not only were they important topics in the field, but they were key for how the gametes functioned, and eventually for how the germ line was established. One of the original editors of CTDB was Alberto Monroy, a leader in molecular biology at the time and in dissecting translational mechanisms in transitions of eggs to embryos, largely in the sea urchin. His interest and role in this field of germ line and gamete function was highly impactful.
In context
The germ line is that lineage of cells that eventually forms the eggs and sperm in the adult. The precursor germ line cells, the primordial germ cells, are formed in the early embryo and will eventually migrate to the developing gonad, form the germ line stem cells, and in the adult, make the gametes, the eggs or sperm. What is clear in 2016 is that animals have tinkered enormously with the mechanisms to make the primordial germ cells in the embryo. Perhaps the strongest, and certainly the earliest impacts of germ line mechanics was revealed in Drosophila with the wonderful genetic screens that resulted in the identification of many germ line mechanisms, factors, and determinants – molecules which are sufficient to initiate a germ line fate in the early embryo (Cohen, 1995; Nusslein-Volhard and Wieschaus, 1980; Roush, 1995); for current reviews on this biology see (Slaidina and Lehmann, 2014; Weil, 2014). Drosophila determines its germ line in the posterior region of the syncytial embryo by accumulation of factors scaffolded by oskar – a highly divergent germ line determinant in Diptera that is essential for germ line formation (Lehmann and Nusslein-Volhard, 1986). This type of germ line formation is largely dependent on the localization of oskar in the posterior pole, followed by aggregation of many diverse functional elements that lead to a germ line fate (Lehmann and Nusslein-Volhard, 1986; Little et al., 2015; Lynch et al., 2011; Ryu and Macdonald, 2015; Yang et al., 2015). Cytoplasmic localization of factors is the main strategy for germ line formation in Drosophila, C. elegans, zebrafish, and Xenopus, among many other animals (Extavour and Akam, 2003).
But wait! This strategy of germ line determination based on localized material in the egg and early embryo appears to be a highly derived character in development (Extavour and Akam, 2003). Studies in the mouse embryo show an extreme opposite approach to germ line formation; intercellular signaling between different sites of the embryo initiate new gene expression that leads to chromatin modifications, the results of which lead to PGCs. This type of determination in the germ line is now referred to as an inductive mechanism, to contrast it from an acquired, or inherited mechanism. The most is known for these mechanics in mouse, in which the general region of the embryo wherein the germ line forms will otherwise develop into mesodermal fates (McLaren, 1998; Tam and Zhou, 1996). Yet, a small cohort of eventually as many as 40 cells, receive a combination of BMP and Wnt signals from adjacent regions of the embryo that “rescues” them from a somatic cell fate, and redirects them to a germ line fate. This inductive mechanism to make primordial germ cells is distinct from the localized cytoplasmic materials seen in e.g. Drosophila, and appears to be a basally derived strategy for germ line formation. An inductive mechanism is used by a wide variety of organisms, not just mammals, and includes representatives from all major phyla, including salamanders, some arthropods, and cnidarian (Extavour and Akam, 2003). Although the mechanisms of this inductive process are not as well described for most animals as in the mouse, the fact that many animals only make a germ line in adults strongly suggests that those animals also use inductive mechanisms, if only for the argument that it is hard to imagine how a cytoplasmic deposit in the egg and early embryo could have been passed on through enormous numbers of cell divisions and end up in a cell to become the germ line. The parsimonious explanation for such animals is that at some point in the adult, a stem-like cell is stimulated by intercellular signaling to acquire a germ line fate that may lead directly to gamete development, or to a precursor cell that eventually develops directly into a gamete.
The formation of a mammalian germ line precursor cell (primordial germ cell) can now even be recapitulated in vitro beginning with iPS cells (Hayashi et al., 2007). Beginning with a pluripotent cell, investigators can sequentially stimulate these cells to divert their fate from a somatic cell fate to one of a germ line precursor cell. Although complete development to functional gamete is now been recapitulated, the later steps of gamete formation must occur via transplant back into a host mouse. It is likely only a matter of time though before the complete process of pluripotent stem cell - to - functional gamete is possible in a dish – especially with the recent successful engineering of 3-D organoids of a variety of tissue types in vitro, and of biofunctional matrices made synthetically. For more thorough reviews of the mechanisms, and the variations, in germ line determination between animals, the reader is referred instead to several significant references (Ewen-Campen et al., 2010; Paksa and Raz, 2015; Rangan et al., 2008; Seydoux and Braun, 2006; Strome and Updike, 2015; Surani, 2015; Williamson and Lehmann, 1996).
The emphasis of this essay is to look forward. Here I will address several important areas for future research in germ line determination mechanisms, with an emphasis of how especially echinoderms may serve as an important model system for answering these questions.
Echinoderms have recently become an exciting model organism for understanding mechanisms in germ line determination (Wessel et al., 2014a; Wessel et al., 2014b). The animals yield millions of oocytes and eggs, develop readily in vitro with excellent access to observation, enormous genome resources are available (echinobase.org), and a rich literature of gene regulatory networks each add to our understanding of the embryonic context for the reproductive cells and their precursors. At the birth of CTDB 50 years ago, sea urchins were primary sources of cells for many landmark observations. These include revealing mRNA biosynthesis and polyadenylation, regulation of mRNA translation, complexity of the genome and transcribed mRNAs, mechanisms of cell and tissue morphogenesis, egg activation and signal transduction mechanisms, and cell-type specific transcriptional mechanics. This rich history adds to the utility of sea urchins currently for studying the germ line determination mechanisms.
Four important directions in germ line determination mechanisms are posed here with how echinoderms might be particularly relevant to these studies.
1. Is nuage, or germinal granules, essential for animals using acquired factors for germ line determination?
In animals utilizing cytoplasmically localized materials to determine their germ line, distinct aggregates of proteins and mRNAs are seen, and appear important, if not essential for germ line determination. A popular example of this structure/function relationship is in Drosophila in which the polar granules are formed in the posterior. Transplanting this cytoplasm containing the polar granules to the anterior pole of an embryo is sufficient to form pole cells (the primordial germ cells in this animal) in the anterior pole (Illmensee and Mahowald, 1974; Mahowald et al., 1976). Indeed, even introduction of the oskar protein in the opposite end of the embryo, the anterior, is sufficient to initiate new pole cell formation ectopically in this organism (Ephrussi and Lehmann, 1992; Wharton and Struhl, 1991). Further, germinal granules likely form following oskar introduction, suggesting these structures as a necessary prerequisite for the germ line determination mechanism. Many animals have similar such aggregates important for acquired germ line formation.
Sea urchins also appear to utilize localized determination mechanisms for germ line formation (Voronina et al., 2008; Yajima and Wessel, 2012). The major difference in sea urchins though is that no germinal granules have ever been reported. Sea urchin eggs and early embryos were prime subjects of examination for electron microscopy studies in the era of the birth of the CTDB series. Innumerable reports of egg structures that included cytoplasmic organelles, nuclear structures, cell surface changes in fertilization etc, were wonderfully documented in sea urchins, yet no germinal granule-aggregate looking structures have ever been reported.
However, peri-nuclear granules are seem with Vasa antibodies, and to be induced by Vasa over-expression. This later approach of Vasa-GFP expression may be one way to identify if aggregates are actually present. Peri-nuclear granules are apparent following introduction of Vasa-GFP into the embryo (and when appropriately mutated so it is better able to accumulate outside of the germ line), it is clear that all cells are capable of accumulating peri-nuclear granular structures as seen in other organisms whose germ lines are determined by acquired cytoplasmic factors. The possible explanation for these observations is that either a) all cells of the early sea urchin embryo already have peri-nuclear granules not otherwise apparent by electron microscopy, or b) they do not have such granules, and by over expressing Vasa, induces formation of new peri-nuclear granules. This may be analogous to the example of oskar mis-expression in the anterior region of the Drosophila embryo in which a nucleating stimulus is essential for aggregate formation, although this would be a surprising function for Vasa. The sea urchin embryos over-expressing Vasa have not been grown to sexual maturity, so it is not clear what impact, if any, may result in germ line functions with potentially induced peri-nuclear granule phenotype.
2. The broad utility of germ line factors in somatic cells and cancer
Some of the first factors identified to be involved in germ line determination were identified genetically (Ephrussi et al., 1991; Ephrussi and Lehmann, 1992; Gavis and Lehmann, 1992; Irish et al., 1989; Lasko and Ashburner, 1988). A mutation in the RNA – binding protein nanos, in Drosophila resulted in both posterior patterning defects (by virtue of its early regulation in hunchback translation) and in germ line viability. Nanos was subsequently found to be limited in its expression to the germ line. The RNA helicase Vasa was similarly identified and found in flies to be selectively expressed in the germ line. At a time (early-mid 1980s) when some transcription factors were discovered to be true developmental determinants – that is, transfect a cell with the factor, and the cell became the tissue in which the factors is normally expressed. This concept was highly popularized by the discovery of e.g. myoD, myogenin and their role in skeletal muscle formation (Edmondson and Olson, 1989; Tapscott et al., 1988; Wright et al., 1989). It was tempting then to look for the same type of “determinant function” in the germ line mechanism.
What we now know however, is that in most animals the germ line factors are more general factors used broadly in development, and even into adulthood. An early report of this conclusion was in the mud snail, Ilyanassa, in which Vasa and Nanos were found in the 4d lineage, a cell that gives rise to many different mesoderm derivatives, as well as to the germ line (Juliano et al., 2010; Swartz et al., 2008). In the Ilyanassa embryo though, it was clearly functioning much more broadly than in making a germ line stem cell, and instead was used in somatic progenitor cells for many different cells types (Rabinowitz et al., 2008; Swartz et al., 2008). Subsequent to that finding, other animal embryos were examined with similar results. The sea urchin embryo was of particular interest. Vasa expression was present throughout the early embryo, in cells for example, that gave rise only to somatic ectoderm, or in larval tissues of the ectoderm (Yajima and Wessel, 2015), and recently reported even in tissues of the adult tube feet (Reinardy et al., 2015). Moreover, when Vasa expression was knocked-down in the embryos, the cell cycle of those embryos was highly disorganized (Yajima and Wessel, 2011). Upon closer inspection Vasa was found to accumulate on the spindle during mitosis, and potentially be involved in localized translation of messages important for cell cycle completion. Indeed, in this embryo, Vasa appeared to bind to most, if not all, mRNAs and knocking down Vasa caused broad translational cessation.
At about the same time, reports began to arrive that documented Vasa and other germ line factors are involved in tumor formation and progression. The most startling example was in flies in which one of the polycomb repressor groups was knocked out, causing a malignant brain tumor-like phenotype (Janic et al., 2010). Within these tumorigenic cells was high levels of expression of Vasa, nanos, piwi, and other factors normally restricted to the germ line. On its own, this is interesting, but not exciting, since after all tumor cells are messed up and they may aberrantly express many different genes without posing a functional output. The key though was that when the investigators knocked out one or another of the “germ line factors”, the tumors regressed, demonstrating not just aberrant expression, but functional contribution to the tumor phenotype. A significant percentage (estimated at ~10–15%) of some human tumor types also express one or another “germ line factor”, the functionality of which is not yet known (Lasko, 2013).
Our rich dataset of “germ line factors” remains enigmatic. Vasa for example, when it is present in a cell, seems essential to that cell’s general functionality. Clearly though not all cells have Vasa expression, but when they do, it seems essential for viability of that cell. It is not clear what Vasa may be doing in the balance of cell’s translation machinery, but the biochemical role of this factor, as well as the other major germline factors, needs to be teased apart. The approach for this understanding in these cases will likely not be completely served by knocking genes out of the genome, even conditionally. Instead, in vitro biochemical assays for functionality may lend an important perspective to the role these factors play both in the germ line for those animals in which it is highly restricted, and for a host of other organisms in which these same factors appear to be more broadly functional. Moreover, the role of germ line factors in tumorigenesis is both paradigm shifting as well as clinically important. While the sea urchin and other echinoderms may not be direct models for tumorigenesis, the tractability of the many embryos to such experimentation is feasible is highly attractive.
3. Consequences of early germ line determination
It is strongly concluded from many studies that the ancestral mechanism for germ line determination is by inductive cell interactions, and that an acquired mechanism is a derived state. This conclusion is based on broad phylogenetic analysis integrated into the developmental morphogenesis mechanism of the animal (Extavour and Akam, 2003). The derived character, acquisition, seems to have been evolutionarily tested and adopted throughout each major taxon, suggesting that it occurs broadly, is not constrained by any of the developmental mechanisms currently in place in those embryos, and that there must be some selection to transition from the inductive mechanism into the acquisition mode. It also argues that the transition might be more readily accomplished than perhaps thought just a decade ago.
As for mechanism(s) of the transition, it is likely that the acquired and inductive mechanisms are a continuum of characters (Juliano and Wessel, 2010). It is comfortable to speak of one versus the other, black versus white, but in actuality these features are likely many shades of gray. An important point to this issue is what is the selection, if any, for having an acquired mechanism of germ line determination? Since the ancestral state is an inductive event, an acquired mechanism must in some way be favorable to fitness to have occurred so frequently and so broadly.
Recent research suggests that one selection mechanism may be that embryos using acquired mechanics are able to diversify their developmental program more rapidly, and thereby capitalize on new developmental niches to outcompete other organisms (Johnson and Alberio, 2015). Since their germ line is segregated early, sometimes very early (C.elegans), and even just prior to cellularization (Drosophila), that any changes in the genome for a new developmental mechanism or feature would still be passed on to the next generation because the germ line is already established early in development. An inductive mechanism necessarily occurs later in development, may be constrained by the positioning of the various signaling and receiving cells, and may therefore be limited in what developmental changes might be possible, while still retaining an ability to specify their germ line.
The sea urchin appears to set aside its germ line extremely early in development, at the 5th cell division (Wessel et al., 2013). Even though these cells do not seem to have germinal granules, or nuage, or perinuclear granules early in development, the vast amount of data suggesting an acquired germ line mechanism in these organisms is overwhelming. Importantly, the other taxa of echinoderms, which include sea stars, crinoids, brittle stars, and sea cucumbers, each appear instead to make their germ line following gastrulation, after the assemblage of thousands of cells and following significant amounts of major tissue morphogenesis. Although the detailed mechanism of germ line determination in these other, non-echinoid echinoderms is not yet clear, they instead appear to use inductive mechanisms.
A further consequence of early germ line formation is that in many cases, the primordial germ cells are made so early, that the embryo has yet to begin formation of a gonadal precursor. In fact in Drosophila, C.elegans, and in sea urchins, no cells of the embryo are even migrating at the time of primordial germ cell formation. As a consequence, the primordial germ cells “wait” for the rest of the embryo to catch up. For this mechanism, at least some germ cells in various organisms demonstrate that they become quiescent. In sea urchins, for example, the PGCs are quiescent in terms of cell division, transcription, translation, motility, and even mitochondrial activity. Indeed, these cells appear to be a wonderful model for the quiescent stem cell phenotype that is also seen in neuronal stem cells, and hematopoietic stem cells of the mammal (Cheung and Rando, 2013; Nakamura-Ishizu et al., 2014). Thus, with the readily accessible PGCs of the sea urchin, their ability to replicate the in vivo condition in vitro, and the strong manipulations possible in these cells, they may serve as an excellent model for general quiescent stem cell characteristics.
4. Diversity in mechanisms of germ line formation
Echinodermata has 5 well defined clades, Crinoidea (sea lilies and feather stars), Ophiuroidea (basket stars and brittle stars), Asteroidea (starfishes), Echinoidea (sea urchins, sand dollars, and sea biscuits), and Holothuroidea (sea cucumbers). Sea urchins appear to be the only clade that uses acquired germ line determination mechanisms. All other echinoderms appear instead to specify their germ line late in development, necessitating inductive mechanisms. Sea stars have been the most intensively studied clade outside of echinoids, and the site of germ line formation appears to be within the posterior enterocoele (PE). This structure buds off the endoderm following gastrulation and becomes progressively well defined by mRNAs of germ line factors (e.g. Vasa, Nanos, Piwi) and the absence of somatic markers (e.g. Blimp). Thus, although less is known about this group of animals in general than in the sea urchin, this phylum lends itself well to understanding both the acquired mechanism as seen in flies, roundworms, and fish, as well as the inductive mechanism seen in for example, mammals. With the many manipulations feasibly in sea stars, one may be able to more readily dissect inductive mechanisms for germ line determination, and contribute to those studies which instead rely largely on genetic manipulations.
Final comments
No one animal is ideal, or sufficient, to test for mechanisms of germ line determination mechanisms. Some animals are beautifully situated for genetic manipulations, or for transplantations, or visualizations, or biochemical approaches, but no animal offers all the important features necessary for efficient and rapid progress. This essay does point to several important goals in the field and how echinoderms might be particularly useful as a model organism for reaching these goals. An important part of study in this area, as could be argued for much of developmental biology, is a reliance of comparative analysis; how a patchwork of observations may reveal results not generally accessible with only one or another experimental approach. Genetic applications over the past several decades have made enormous progress in this and many fields of the discipline, and now adding perspectives from different approaches, and different organisms less intensively documented to this point, should be of great benefit to the advancement of the field as a whole. Change is inevitable in biology, especially in the area of reproduction, and embracing these changes and differences is essential for real progress.
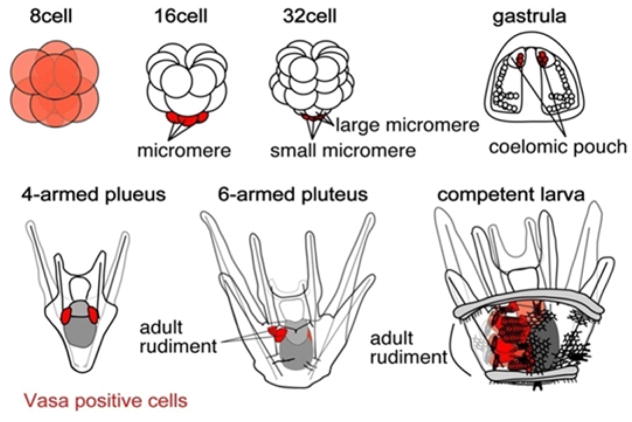
Figure 1.
Diagram of the development of a sea urchin. Early development yields Vasa-positive cells (shown in red, beginning with a uniform Vasa positive egg and early embryo). At the 32-cell stage, the sMics are uniquely Vasa-positive. These cells move into the coelom during gastrulation, segregate into the left and right coelomic pouches, and expand to contribute to the germ cells of the adult, and likely also to some somatic cells of the rudiment. (from Wessel at al., 2014)
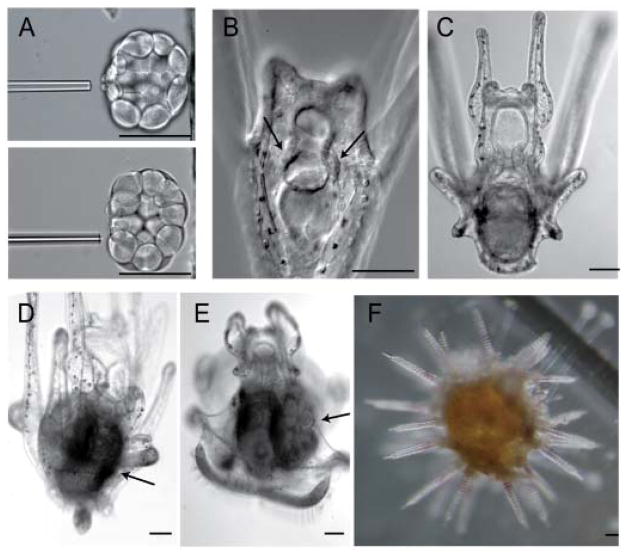
Figure 2.
The sMics are dispersible for development but required for gamete development. A: Method of sMic removal with a glass pipette. The resulting embryos develop into a larva (B–E), form an adult rudiment (D, E, arrow in E), and metamorphos into an adult (F) that does not produce gametes. Scale bars, 80 μm. (from Yajima and Wessel, 2011)
Figure 3. Vasa in perinuclear granules throughout the embryo.
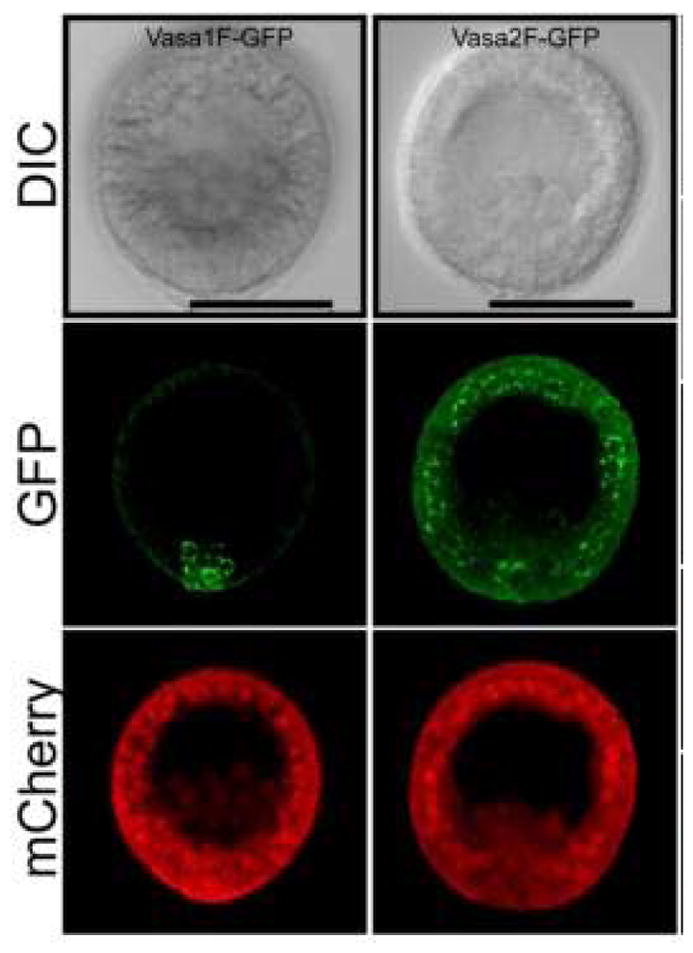
The S. purpuratus Vasa open reading frame is sufficient for specific small micromere enrichment of Vasa protein (left column), whereas deletion of 141 N-terminal amino acids causes broad Vasa expression throughout the embryo in perinuclear granules. Scale bar = 50 μm. (from Gustafson et al., 2011)
Acknowledgments
Fifty years of Current Topics in Developmental Biology! - Happy Golden Anniversary! You just keep looking better, and healthier too! Your volumes keep reproducing and like a germ line stem cell – you retain your potential for immortality! Congratulations to you! I look forward to another 50 of the same!
The author thanks Paul Wassarman for the opportunity to be involved in this project, and to members of PRIMO for rich discussions and discoveries. The author acknowledges the NIH (HD028152 and HD075561) for research support.
This work is dedicate to the memory of Betty (March 15, 1929 – August 21, 2015) and Bob (December 30, 1923 – September 6, 2015) Wessel. They were great people, special parents, and are missed dearly.
References
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nature reviews Molecular cell biology. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. Nobel committee rewards pioneers of development studies in fruitflies. Nature. 1995;377:465. doi: 10.1038/377465a0. [DOI] [PubMed] [Google Scholar]
- Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes & development. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- Ewen-Campen B, Schwager EE, Extavour CG. The molecular machinery of germ line specification. Mol Reprod Dev. 2010;77:3–18. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130:5869–5884. doi: 10.1242/dev.00804. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Mahowald AP. Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:1016–1020. doi: 10.1073/pnas.71.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish V, Lehmann R, Akam M. The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature. 1989;338:646–648. doi: 10.1038/338646a0. [DOI] [PubMed] [Google Scholar]
- Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–1827. doi: 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Alberio R. Primordial germ cells: the first cell lineage or the last cells standing? Development. 2015;142:2730–2739. doi: 10.1242/dev.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C, Wessel G. Versatile Germline Genes. Science. 2010;329:640–641. doi: 10.1126/science.1194037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano CE, Swartz SZ, Wessel GM. A conserved germline multipotency program. Development. 2010;137:4113–4126. doi: 10.1242/dev.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P. The DEAD-box helicase Vasa: evidence for a multiplicity of functions in RNA processes and developmental biology. Biochimica et biophysica acta. 2013;1829:810–816. doi: 10.1016/j.bbagrm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Nusslein-Volhard C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 1986;47:141–152. doi: 10.1016/0092-8674(86)90375-2. [DOI] [PubMed] [Google Scholar]
- Little SC, Sinsimer KS, Lee JJ, Wieschaus EF, Gavis ER. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nature cell biology. 2015;17:558–568. doi: 10.1038/ncb3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JA, Ozuak O, Khila A, Abouheif E, Desplan C, Roth S. The phylogenetic origin of oskar coincided with the origin of maternally provisioned germ plasm and pole cells at the base of the Holometabola. PLoS genetics. 2011;7:e1002029. doi: 10.1371/journal.pgen.1002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP, Illmensee K, Turner FR. Interspecific transplantation of polar plasm between Drosophila embryos. The Journal of cell biology. 1976;70:358–373. doi: 10.1083/jcb.70.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Germ cells and germ cell transplantation. The International journal of developmental biology. 1998;42:855–860. [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141:4656–4666. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Paksa A, Raz E. Zebrafish germ cells: motility and guided migration. Curr Opin Cell Biol. 2015;36:80–85. doi: 10.1016/j.ceb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JS, Chan XY, Kingsley EP, Duan Y, Lambert JD. Nanos is required in somatic blast cell lineages in the posterior of a mollusk embryo. Current biology : CB. 2008;18:331–336. doi: 10.1016/j.cub.2008.01.055. [DOI] [PubMed] [Google Scholar]
- Rangan P, DeGennaro M, Lehmann R. Regulating gene expression in the Drosophila germ line. Cold Spring Harbor symposia on quantitative biology. 2008;73:1–8. doi: 10.1101/sqb.2008.73.057. [DOI] [PubMed] [Google Scholar]
- Reinardy HC, Emerson CE, Manley JM, Bodnar AG. Tissue Regeneration and Biomineralization in Sea Urchins: Role of Notch Signaling and Presence of Stem Cell Markers. PloS one. 2015;10:e0133860. doi: 10.1371/journal.pone.0133860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush W. Nobel prizes: fly development work bears prize-winning fruit. Science. 1995;270:380–381. doi: 10.1126/science.270.5235.380. [DOI] [PubMed] [Google Scholar]
- Ryu YH, Macdonald PM. RNA Sequences required for the noncoding function of oskar RNA also mediate regulation of oskar protein expression by Bicoid stability factor. Developmental biology. 2015 doi: 10.1016/j.ydbio.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127:891–904. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Slaidina M, Lehmann R. Translational control in germline stem cell development. The Journal of cell biology. 2014;207:13–21. doi: 10.1083/jcb.201407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Strome S, Updike D. Specifying and protecting germ cell fate. Nature reviews Molecular cell biology. 2015;16:406–416. doi: 10.1038/nrm4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani MA. Human Germline: A New Research Frontier. Stem cell reports. 2015;4:955–960. doi: 10.1016/j.stemcr.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz SZ, Chan XY, Lambert JD. Localization of Vasa mRNA during early cleavage of the snail Ilyanassa. Dev Genes Evol. 2008;218:107–113. doi: 10.1007/s00427-008-0203-6. [DOI] [PubMed] [Google Scholar]
- Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Developmental biology. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Voronina E, Lopez M, Juliano CE, Gustafson E, Song JL, Extavour C, George S, Oliveri P, McClay D, Wessel G. Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Developmental biology. 2008;314:276–286. doi: 10.1016/j.ydbio.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil TT. mRNA localization in the Drosophila germline. RNA biology. 2014;11:1010–1018. doi: 10.4161/rna.36097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Brayboy L, Fresques T, Gustafson EA, Oulhen N, Ramos I, Reich A, Swartz SZ, Yajima M, Zazueta V. The biology of the germ line in echinoderms. Mol Reprod Dev. 2013 doi: 10.1002/mrd.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Brayboy L, Fresques T, Gustafson EA, Oulhen N, Ramos I, Reich A, Swartz SZ, Yajima M, Zazueta V. The biology of the germ line in echinoderms. Mol Reprod Dev. 2014a;81:679–711. doi: 10.1002/mrd.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Fresques T, Kiyomoto M, Yajima M, Zazueta V. Origin and development of the germ line in sea stars. Genesis. 2014b;52:367–377. doi: 10.1002/dvg.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991;67:955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Williamson A, Lehmann R. Germ cell development in Drosophila. Annual review of cell and developmental biology. 1996;12:365–391. doi: 10.1146/annurev.cellbio.12.1.365. [DOI] [PubMed] [Google Scholar]
- Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. The DEAD-box RNA helicase Vasa functions in embryonic mitotic progression in the sea urchin. Development. 2011;138:2217–2222. doi: 10.1242/dev.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Autonomy in specification of primordial germ cells and their passive translocation in the sea urchin. Development. 2012;139:3786–3794. doi: 10.1242/dev.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Essential elements for translation: the germline factor Vasa functions broadly in somatic cells. Development. 2015;142:1960–1970. doi: 10.1242/dev.118448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Yu Z, Hu M, Wang M, Lehmann R, Xu RM. Structure of Drosophila Oskar reveals a novel RNA binding protein. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:11541–11546. doi: 10.1073/pnas.1515568112. [DOI] [PMC free article] [PubMed] [Google Scholar]