Abstract
High throughput screening of insulin secretion is intractable with current methods. We developed a secreted insulin-luciferase system (Ins-GLuc) in β cells that is rapid, inexpensive, and amenable to 96- and 384-well formats. We treated stable Ins-GLuc-expressing MIN6 cells overnight with 6298 marine natural product fractions. The cells were then washed to remove media and chemicals, followed by stimulation with glucose in the diazoxide paradigm. These conditions allowed the discovery of many insulin secretion suppressors and potentiators. The mechanisms of action of these natural products must be long-lasting given the continuance of secretory phenotypes in the absence of chemical treatment. We anticipate that these natural products and their target pathways will lead to a greater understanding of glucose-stimulated insulin secretion. Keywords: insulin, Gaussia, high-throughput screening, pancreatic islet beta cell, marine natural products.
Graphical Abstract
Quantitating the secretion of insulin from pancreatic islet β cells is an essential measure of islet function. Insulin secretion is commonly measured using ELISA or RIA although newer methods have been developed. For example, a method to measure insulin and C-peptide levels by FRET using non-overlapping epitope-based antibodies and fluorochrome-labeled oligonucleotides was proposed to replace the ELISA and RIA.1 While these methods directly measure insulin, they can be costly and time-consuming and are not amenable to high-throughput screening. The secreted luciferase from Gaussia princeps2 has recently been used to monitor secretion from beta cells,3 demonstrating the feasibility of this approach in high-throughput. Here, we present a detailed protocol that can be used to measure β cell insulin secretion responses rapidly and inexpensively in the lab using lab-made stock solutions. We expressed the insulin-Gaussia luciferase reporter (Ins-GLuc) using the rat insulin promoter to restrict expression to β cells. Using this system, we have screened a unique natural product library containing 6298 fractions derived from marine bacteria and invertebrates. Our screen yielded potential inhibitors and activators of the amplifying pathway of glucose-stimulated insulin secretion which may lead to the discovery of novel β-cell function regulatory pathways.
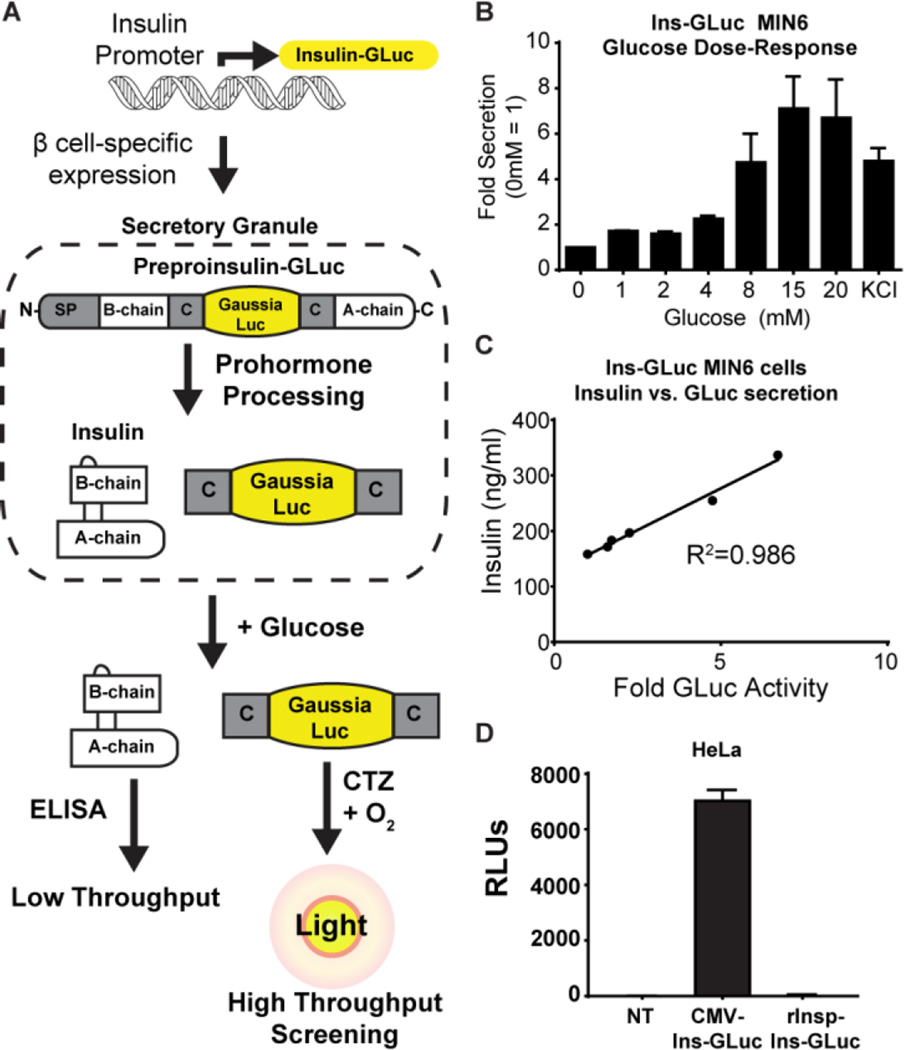
To generate the biosensor, luciferase from Gaussia princeps (GLuc, 18 kDa) was used because it is naturally secreted and is >2,000-fold more sensitive than firefly or Renilla luciferases. In addition, GLuc uses a single substrate (coelenterazine) in an ATP-independent reaction. GLuc is an optimal choice for high-throughput screening of small molecules, given its very low rate of off-target inhibition by chemicals.4 The GLuc gene was optimized for mammalian expression2 as well as mutated (M43I/M110I) to improve glow-like kinetics.5 This enhanced GLuc was inserted within the C-peptide of human insulin, similar to previous GFP insertions in C-peptide6 (Fig 1A), and expression was driven with the 414-bp proximal region of the rat insulin promoter (rInsp). Prior studies inserting GFP N- or C-terminal to insulin caused misfolding,7 while insertion within the C-peptide led to proper processing and GFP secretion.6b In the course of developing our biosensor, work from Burns, et al.3 was published using a similar sensor expressed from a CMV promoter and screening a relatively small library of known compounds (Pharmakon 1600). Our advancement of this system provides a reliable luciferase reporter of insulin secretion that 1) is amenable to high throughput screening without the need for any commercial kits and 2) has restricted expression in β cells for future human islet validation experiments in medium to high throughput. Here we validate our rat insulin promoter-driven Ins-GLuc biosensor in MIN6 cells and demonstrate a proof-of-concept that this system can be used to discover novel modifiers of glucose-mediated amplification of insulin secretion.
Figure 1.
A) Schematic for the creation and expression of the Ins-GLuc biosensor. B) Fold secreted luciferase activity in in MIN6 β cells expressing Ins-GLuc driven by the rat insulin promoter (rInsp). Raw RLUs were normalized to the activity at 0 mM glucose and expressed as fold ± SE. C) Correlation between GLuc luciferase activity and insulin secretion measured by ELISA from rInsp-Ins-GLuc-MIN6 subjected to a glucose dose-response curve (0, 1, 2, 4, 8, 20 mM). D) HeLa cells only express CMV-driven and not rInsp-driven reporter activity. Data are the average of 3 experiments ± SE.
We generated mouse MIN6 β cells stably-expressing the Ins-GLuc driven by the rat insulin promoter (Fig 1A). To validate that these cells faithfully secrete insulin in response to glucose, we measured secretion as a function of increasing concentrations of glucose from cells plated in a 96-well format. Samples were collected for luciferase assays. We observed substantial secretion of luciferase and insulin only in response to elevated glucose concentrations (≥8 mM) (Fig 1B). Use of the rat insulin promoter led to robust luciferase activity compared to the CMV-driven biosensor (Fig S1A), but the fold response compared to unstimulated cells was similar for both promoters (Fig S1B). Secreted luciferase activity from the rInsp-Ins-GLuc reporter also correlated well with insulin secretion measured by ELISA (Fig 1C), suggesting this assay is a reliable surrogate for insulin secretion for further high throughput studies. Of key importance to our future use of the assay is that the reporter expresses selectively in β cells. To demonstrate this, we transfected HeLa cells with the Ins-GLuc reporter driven by either the CMV or the rat insulin promoter. As expected, HeLa cells transfected with the CMV-driven construct had robust luciferase activity, while the rat insulin promoter-driven construct exhibited less than 1 % of the activity of that driven by CMV (Fig 1D). AlphaTC1-9 cells similarly expressed the CMV-based reporter selectively over the rInsp-driven reporter (Fig S3C).. In addition, dissociated human islets lentivirally-transduced with the rInsp-driven biosensor selectively expressed Gaussia in beta cells (Fig S3A) and luciferase was secreted in response glucose (Fig S3B). Therefore, we moved forward only with the rat insulin promoter-driven Ins-GLuc and all further assays are using this biosensor.
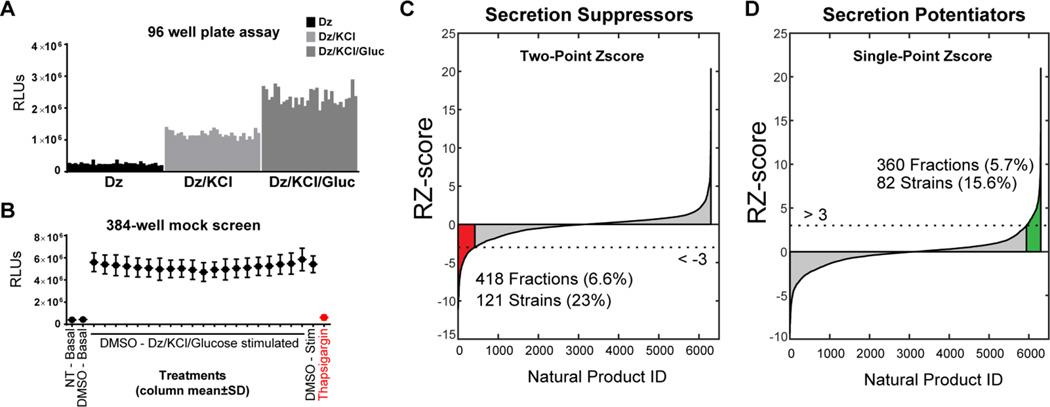
Under physiological conditions, the pancreatic islet is exposed to fasted glucose levels of <5 mM as well as fed levels of ~10 mM, usually for short durations (<1–2 h).8 When glucose enters the β cell, its metabolism increases the ratio of ATP to ADP. This change induces the closure of ATP-dependent potassium channels (KATP) leading to membrane depolarization and opening of voltage-gated calcium channels.9 The resultant calcium influx causes insulin secretion and is commonly referred to as the ‘triggering pathway’ (previously KATP-dependent secretion).10 Glucose also acts independently of further calcium influx in an ‘amplifying pathway’ of insulin secretion (previously KATP-independent secretion). The amplifying pathway is estimated to account for ~50% of glucose’s effect on insulin secretion.11 While some important molecular mechanisms have been identified as regulators of amplification such as purine12 and glutamine13 metabolism, we lack a complete understanding of this critical β cell function. The amplifying pathway was recently shown to contribute to diet-induced obesity in mice,14 highlighting the importance of gaining mechanistic understanding of this secretory pathway. It is possible to experimentally deconvolve the role of glucose in the triggering and amplifying pathways using the KATP-channel opening drug diazoxide. Using the diazoxide paradigm, while KATP channels are held open, a depolarizing concentration of KCl causes calcium influx and the KATP-dependent triggering pathway of insulin secretion. Further addition of glucose elicits amplification of insulin secretion without any further calcium influx. We exploited the diazoxide paradigm to specifically probe glucose-regulated amplification signaling pathways in a high throughput screen of marine bacterial natural products. We tested the diazoxide paradigm in our assay in a 96-well format and observed the expected triggering and amplifying responses (Fig 2A).
Figure 2.
A) Ins-GLuc MIN6 β cells respond as expected to the diazoxide paradigm; 200 µM diazoxide (Dz) (basal), Dz + 35 mM KCl (triggering), and Dz + 35 mM KCl + 20mM glucose (amplifying). Data are representative of an entire 96-well plate with 32 replicates for each condition and a Z-score >0.5. B) Mock screen of 24 h DMSO treatment of 384 well plates of Ins-GLuc MIN6 cells followed by the GLuc assay modified for high throughput screening. Data are the average of 3 mock plates ± SE for each column of the 384 well plate. C,D) Ins-GLuc MIN6 cells were treated overnight with the entire UTSW marine natural product library (~6400 fractions), DMSO (1%), or thapsigargin (1µM) (in red) and the next day cells were subjected to stimulation under Dz/KCl/glucose conditions for 1 h before assaying luciferase activity. Two-point normalized values were used to calculate the RZ score for suppressors of secretion (B) and RZ-score calculated from single-point normalized values were used for potentiators (C). Fractions with RZ scores > 3 or < -3 were flagged as hits.
In order to optimize the assay for high throughput screening in a 384-well format we determined the optimal cell number for glucose-responsive luciferase secretion as between 50,000 and 20,000 cells per well (Fig S2A). The DMSO sensitivity of the cell line was also acceptable as no major impact of DMSO on stimulated secretion was observed even up to 1% (Fig S2B). Mock screens in 384-well plates demonstrated that the assay gave a suitable 10-fold dynamic range in stimulation index and Z’-scores >0.5. Therefore, Ins-GLuc MIN6 cells were cultured in 384-well plates prior to a 24 h incubation with natural products or solvent controls. The dishes were subjected to washing, stimulation, and luciferase assays as described in the Supplemental Methods. After correction and normalization of the data we obtained a high-confidence (>3 or <-3 robust Z-score) list of hits that either suppressed (Fig 2C) or potentiated (Fig 2D) insulin secretion in the presence of diazoxide, KCl and glucose. Because thapsigargin was a positive control for suppression of secretion, the two-point normalized RZ-score was chosen as the most appropriate means to assess the inhibitory hits (Fig 2C). As there was no positive control for potentiation of insulin secretion, single-point normalization was used in the case of secretion-enhancing natural products (Fig 2D).
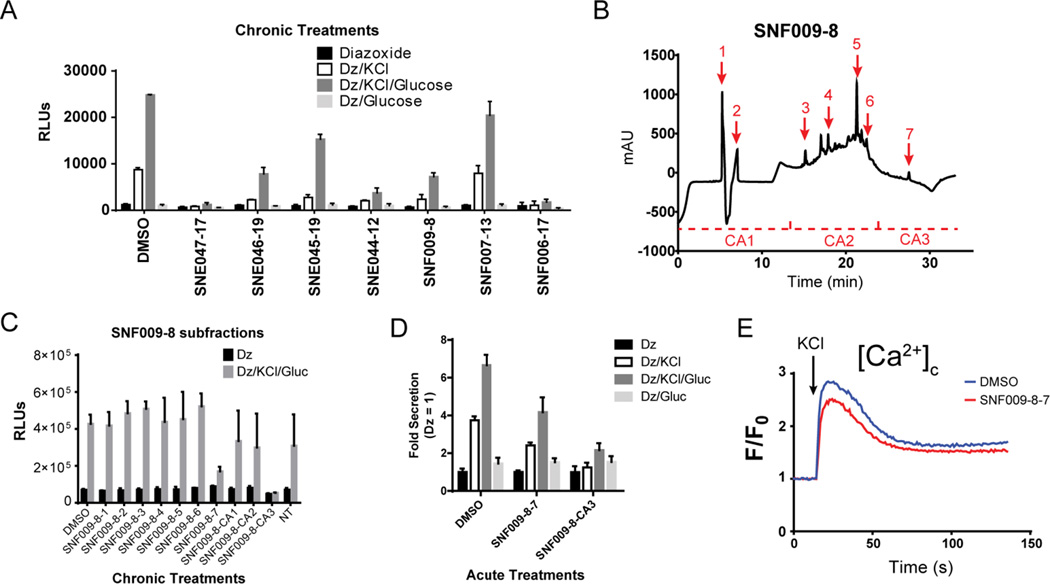
To follow up on a model example of a natural product hit from the screen, we selected a subset of hits from the SNF and SNE groups of fractions and validated their activities in Ins-GLuc MIN6 cells (Fig 3A). We found that SNF009-8 retained inhibitory activity and we observed no effect on cellular morphology or confluence after a 24-h treatment. SNF009-8 was selected for further subfractionation by reverse-phase chromatography into 10 fractions (Fig 3B). These subfractions were rescreened in 24-h exposures of Ins-GLuc MIN6 cells and SNF009-8-7 and –CA3 (catch-all: all material eluted in this range of time was collected, except for the peak for subfraction SNF009-8-7) were found to contain activity (Fig 3C). We also noted activity in shorter-term 2 h treatments of Ins-GLuc MIN6 cells (Fig 3D), suggesting the active component(s) exhibits its effects relatively rapidly without prolonged treatment. Finally, to begin delineating the mechanisms of SNF009-8 actions, we performed calcium influx assays on MIN6 cells lacking the reporter and treated overnight with either DMSO or SNF009-8-7 (Fig 3E). Only a minimal suppression of cytosolic calcium induced by depolarizing KCl (35mM) was observed. This suggests that SNF009-8-7 is able to suppress glucose-induced secretion largely independently of effects on calcium influx.
Figure 3.
A) A subset of inhibitory natural product hits were rescreened in Ins-GLuc MIN6 cells by treating 24 h (1 µg/ml), washing and preincubation in KRBH with 200 µM diazoxide (Dz), followed by stimulation with 35 mM KCl and 20 mM glucose for 1 h. Bar graph represents luciferase activity in supernatants ± S.D. B) Fraction SNF009-8 was selected for further subfractionation by reversed-phase HPLC yielding 10 isolates. C) The subfractions were assessed phenotypically for activity as in (A). D) To probe acute effects of the subfractions, Ins-GLuc MIN6 cells were pretreated for 1 h with SNF009-8-7 and –CA3 (1 µg/ml) in KRBH before 1 h stimulations in the diazoxide paradigm. E) MIN6 β cell calcium influx assays were performed on cells pretreated overnight with SNF009-8-7. Cells were loaded with Fura-2-AM (5 µM), stimulated with 35 mM KCl, and monitored for 2 min.
An interesting facet of our screening strategy is that the cells are incubated with chemical treatments overnight and then on the following day washed, preincubated, and stimulated in the absence of the treatment. This means that any suppression or enhancement of glucose-stimulated secretion is a long-lasting effect, perhaps due to altered gene expression, or altered protein levels or post-translational modifications. In the future we hope to expand the screening platform to include both chronic and acute chemical treatments. By combining different treatment durations and different phenotypic outputs (i.e. calcium influx, ERK1/2 activation, cAMP levels, gene expression) we hope to discover novel glucose-regulated pathways in the beta cell which regulate insulin secretion.
In conclusion, we have generated a useful model system to rapidly screen large libraries of compounds for chemicals that either suppress or potentiate glucose-stimulated insulin secretion. Additionally, once provided with either the construct or the cell line, beta cell research labs will be able to easily establish and perform the assay with home-made buffers and reagents to allow medium to high throughput testing of any chemical or genetic (siRNA/shRNA) library.
Supplementary Material
Acknowledgments
We would like to acknowledge Anwu Zhou, Hanspeter Nierderstrasser and the entire HTS core for assistance in experimental setup and data analysis. We would also like to thank John Hulleman for providing advice regarding Gaussia luciferase assays. Thanks to Curtis Thorne and the entire Cobb lab for many helpful conversations. This work was supported by a National Institutes of Health NRSA DK100113 (to M.A.K.), P30 CA142543 (to B.A.P), and U41 AT008718 (to J.B.M.). Early phases of this work were supported by DK55310 (to M.H.C.).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supporting Information Available: The following files are available free of charge. Kalwat_Supporting_Info.pdf. Supporting Materials and Methods, References, and Figure S1–3
Author Contributions
Conceptualization, M.A.K.; Software, C.W.; Formal Analysis, M.A.K., C.W., B.A.P.; Investigation, M.A.K., A.Y.N., M.M., I.H.; Writing- Original draft, M.A.K., M.H.C.; Supervision, J.B.M., B.A.P., M.H.C.; Funding Acquisition, M.A.K., J.B.M., B.A.P., M.H.C.
The authors declare no competing financial interests.
REFERENCES
- 1.Heyduk E, Moxley MM, Salvatori A, Corbett JA, Heyduk T. Homogeneous insulin and C-Peptide sensors for rapid assessment of insulin and C-peptide secretion by the islets. Diabetes. 2010;59(10):2360–2365. doi: 10.2337/db10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11(3):435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Burns SM, Vetere A, Walpita D, Dancik V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 2015;21(1):126–137. doi: 10.1016/j.cmet.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ho PI, Yue K, Pandey P, Breault L, Harbinski F, McBride AJ, Webb B, Narahari J, Karassina N, Wood KV, Hill A, Auld DS. Reporter enzyme inhibitor study to aid assembly of orthogonal reporter gene assays. ACS chemical biology. 2013;8(5):1009–1017. doi: 10.1021/cb3007264. [DOI] [PubMed] [Google Scholar]
- 5.(a) Welsh JP, Patel KG, Manthiram K, Swartz JR. Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochemical and biophysical research communications. 2009;389(4):563–568. doi: 10.1016/j.bbrc.2009.09.006. [DOI] [PubMed] [Google Scholar]; (b) Maguire CA, Deliolanis NC, Pike L, Niers JM, Tjon-Kon-Fat LA, Sena-Esteves M, Tannous BA. Gaussia luciferase variant for high-throughput functional screening applications. Anal Chem. 2009;81(16):7102–7106. doi: 10.1021/ac901234r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Rajan S, Eames SC, Park SY, Labno C, Bell GI, Prince VE, Philipson LH. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am J Physiol Endocrinol Metab. 2010;298(3):E403–E410. doi: 10.1152/ajpendo.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Watkins S, Geng X, Li L, Papworth G, Robbins PD, Drain P. Imaging Secretory Vesicles by Fluorescent Protein Insertion in Propeptide Rather Than Mature Secreted Peptide. Traffic. 2002;3(7):461–471. doi: 10.1034/j.1600-0854.2002.30703.x. [DOI] [PubMed] [Google Scholar]; (c) Hodish I, Absood A, Liu L, Liu M, Haataja L, Larkin D, Al-Khafaji A, Zaki A, Arvan P. In vivo misfolding of proinsulin below the threshold of frank diabetes. Diabetes. 2011;60(8):2092–2101. doi: 10.2337/db10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hodish I, Liu M, Rajpal G, Larkin D, Holz RW, Adams A, Liu L, Arvan P. Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. The Journal of biological chemistry. 2010;285(1):685–694. doi: 10.1074/jbc.M109.038042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouli AE, Kennedy HJ, Schofield JG, Rutter GA. Insulin targeting to the regulated secretory pathway after fusion with green fluorescent protein and firefly luciferase. Biochemical Journal. 1998;331(2):669–675. doi: 10.1042/bj3310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54(11):3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]; (b) (b) Cook DL, Ikeuchi M, Fujimoto WY. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]; (c) Rorsman P, Ashcroft FM, Trube G. Single Ca channel currents in mouse pancreatic B-cells. Pflugers Archiv : European journal of physiology. 1988;412(6):597–603. doi: 10.1007/BF00583760. [DOI] [PubMed] [Google Scholar]; (d) Satin LS, Cook DL. Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Archiv : European journal of physiology. 1985;404(4):385–387. doi: 10.1007/BF00585354. [DOI] [PubMed] [Google Scholar]
- 10.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52(5):739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 12.Gooding JR, Jensen MV, Dai X, Wenner BR, Lu D, Arumugam R, Ferdaoussi M, MacDonald PE, Newgard CB. Adenylosuccinate Is an Insulin Secretagogue Derived from Glucose-Induced Purine Metabolism. Cell Rep. 2015;13(1):157–167. doi: 10.1016/j.celrep.2015.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, Nakayama Y, Harada K, Hastoy B, Wu X, Takahashi H, Kimura K, Matsubara T, Hoshikawa R, Hatano N, Sugawara K, Shibasaki T, Inagaki N, Bamba T, Mizoguchi A, Fukusaki E, Rorsman P, Seino S. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;9(2):661–673. doi: 10.1016/j.celrep.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetterli L, Carobbio S, Frigerio F, Karaca M, Maechler P. The Amplifying Pathway of the beta-Cell Contributes to Diet-induced Obesity. The Journal of biological chemistry. 2016;291(25):13063–13075. doi: 10.1074/jbc.M115.707448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.