Abstract
Objectives
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) produced good tumor response in cases with lung squamous cell carcinoma, one of the most difficult cancers to treat. Secreted protein acidic and rich in cysteine (SPARC) binds to albumin, suggesting that SPARC plays an important role in tumor uptake of nab-paclitaxel. There is as yet no predictive marker for cytotoxic agents against non-small-cell lung cancer (NSCLC), and hence we believed that SPARC expression might be associated with tumor response to nab-paclitaxel.
Patients and methods
We studied stromal SPARC reactivity and its association with clinicopathological characteristics in 200 cases of NSCLC using a custom tissue microarray fabricated in our laboratory by immunohistochemical staining. We also investigated the relationship between stromal SPARC reactivity and tumor response to nab-paclitaxel using biopsy or surgical specimens obtained from advanced or recurrent lung cancer patients.
Results
High SPARC stromal reactivity (>50% of optical fields examined) was detected in 16.5% of cases and intermediate SPARC reactivity (10%–50%) in 56% of cases. High expression in cancer cells was rare (five cases). Stromal SPARC level was correlated with smoking index, squamous cell carcinoma, and vessel invasion. Furthermore, patients with high stromal SPARC reactivity in biopsy specimens such as transbronchial lung biopsy or surgical specimens tended to respond better to nab-paclitaxel.
Conclusion
Stromal SPARC was detected by immunohistochemical staining in ∼70% of NSCLC cases, and good tumor response to nab-paclitaxel was correlated with high stromal SPARC reactivity. SPARC may be a useful predictive marker for selecting patients likely to respond favorably to nab-paclitaxel treatment.
Keywords: lung cancer, nab-paclitaxel, SPARC, predictive marker, squamous cell carcinoma
Introduction
Chemotherapy continues to occupy a leading role in the treatment of advanced and recurrent non-small-cell lung cancer (NSCLC), whereas most new chemotherapeutic agents and molecular targeted therapy are for lung adenocarcinoma. EGFR activating mutations or EML4-ALK fusion genes are established predictive markers in molecular targeted therapy.1,2 However, efficacy of anti-cancer agents for the treatment of lung squamous cell carcinoma is limited compared to adenocarcinoma, and molecular targeted therapy is not indicated for most patients with squamous cell carcinoma. Recently, Socinski et al3 reported that carboplatin plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as a first-line therapy in patients with advanced NSCLC demonstrated a significantly higher overall response rate than carboplatin plus solvent-based paclitaxel (33% vs 25%, P=0.005) and in patients with squamous histology compared to patients with non-squamous histology (41% vs 24%, P<0.001). An important property of nab-paclitaxel is an excellent tumor shrinkage effect, leading to relief from cancer-related symptoms. This antitumor effect is detected not only in squamous cell carcinoma but also in some patients with adenocarcinoma according to clinical observations. Based on these results, we thought that it would be useful to identify a predictive marker for nab-paclitaxel.
nab-Paclitaxel is a 130 nm albumin-bound formulation of paclitaxel particles (Celgene, Summit, NJ, USA). Albumin is the most abundant plasma protein, with a molecular weight of 66 kDa, and is emerging as a versatile carrier for targeted drug delivery.4 Secreted protein acidic and rich in cysteine (SPARC), also referred to as osteonectin or basement membrane protein 40 (BM-40), plays a crucial role in cell growth and angiogenesis through interaction with the extracellular matrix or cytokines.5–7 SPARC binds to albumin and the bound form co-localizes in cancer tissues, suggesting that SPARC plays an important role in promoting tumor uptake of nab-paclitaxel.8,9 Expression of SPARC is correlated with prognosis in breast cancer cases,10,11 and high SPARC stromal reactivity is correlated with improved survival in pancreatic cancer cases.12 However, there has been no investigation of the relationship between SPARC stromal reactivity and tumor response to nab-paclitaxel in NSCLCs. Lung squamous cell carcinoma, one of the most difficult cancers to treat, showed good tumor response to nab-paclitaxel.3 Although recent studies1,2 have described predictive markers for molecular targeted therapy, there have been few reports of studies focusing on predictive markers for chemotherapeutic agents. Since nab-paclitaxel is produced by the albumin transportation system, SPARC bound to albumin may be a predictive marker. If SPARC is useful as a predictive marker in the clinical setting, we expect that patient selection based on SPARC expression could lead to a tailor-made therapy for lung cancer patients, especially those with squamous histology. In this study, we present evidence to support the possibility that SPARC may indeed be a predictive marker for nab-paclitaxel.
Patients and methods
Tissue samples and tissue microarrays
Tissue samples were surgical specimens obtained from 200 successive lung cancer patients who underwent surgery at Saga Medical School Hospital between 2004 and 2009 without anticancer chemotherapy or thoracic irradiation before surgery. The study was approval by the Saga University Clinical Research Ethics Committee; informed consent was obtained from all patients for the use of surgical specimens in this study. The tissue microarray was constructed using a Tissue Microarrayer (Pathology Institute Corp., Toyama, Japan). Two 1 mm diameter cylinders were punched from each of the 200 specimens and arrayed in three tissue microarray blocks. The clinical stage of the cancer was determined according to the criteria of the 7th edition of the International Union Against Cancer.
Immunohistochemical staining
Deparaffinized 4 µm-thin tissue sections were microwaved in Tris-EDTA buffer (pH 9.0). After blocking the tissue sections in 0.3% hydrogen peroxide in phosphate-buffered saline for 30 minutes at room temperature, serially cut sections were incubated overnight at 4°C with anti-SPARC antibody (Abcam, Cambridge, MA, USA) at 1:200 dilution, anti-CK7 antibody at 1:200, or anti-vimentin antibody at 1:200. The Dako EnVision System (DAKO Corp., Carpinteria, CA, USA) was used for detection. Counter staining was performed with hematoxylin. Immunohistochemical examination with each antibody was performed by the following criteria and scored according to sequential arbitrary cutoffs. Staining of the cancer cell nuclei or cytoplasm with anti-SPARC antibody in whole tissue sections was taken as positive and scored as − (≤10% positive cells), ± (>10% but ≤30%), + (>30% but ≤60%), and ++ (>60%). SPARC stromal reactivity was defined as the percentage of reactive stromal area among the optical fields and scored as − (≤10%, low), + (>10% but ≤50%, intermediate), and ++ (>50%, high). We defined positive staining as intermediate or high stromal reactivity. All slides were scored by three researchers.
Treatment and assessment of efficacy
Forty-three patients had histologically or cytologically confirmed advanced or recurrent NSCLC and were treated with 100 mg/m2 30-minute infusion of nab-paclitaxel on days 1, 8, and 15, with nab-paclitaxel followed by carboplatin AUC 6 on day 1; this regimen was repeated every 3 weeks. Treatment of at least six cycles is encouraged in general clinical practice, but can continue beyond six cycles in the absence of progressive disease if there is no unacceptable toxicity. Tumor response was evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST) Version 1.1.
Statistical analysis
Associations between immunohistochemical staining results and clinicopathological characteristics were examined using the chi-square test for categorical data. The survival rate was calculated according to the Kaplan–Meier method, and the log-rank test was used for assessing differences. Cox proportional hazards regression analysis, with adjustment for potentially confounding variables gender, age, smoking index, and pathological stage, was used to calculate the mortality hazard rate (HR) and 95% confidence interval (CI). P-values <0.05 were regarded as statistically significant. Statistical analyses were conducted using SPSS 11.0J (SPSS Japan Inc., Tokyo, Japan).
Results
Expression of SPARC was correlated with smoking status, squamous cell carcinoma, vessel invasion, and poor prognosis in NSCLC patients
Table 1 summarizes the relationships between SPARC stromal reactivity and clinicopathological characteristics based on the immunohistochemical analysis using anti-SPARC antibody. A representative case of immunostaining with anti-SPARC antibody is shown in Figure 1. Positive staining for SPARC (+ or ++) was detected in stromal fibroblasts and endothelial cells of immature vessels of the lung cancer tissues. In contrast, positive staining of cytoplasmic or nuclear SPARC in cancer cells was rare, noted in five of 200 (2.5%) or 17 of 200 (8.5%) cases examined, respectively. Staining level in the adjacent noncancerous tissue was low. One hundred and forty-five patients (72.5%) showed positive staining for SPARC using immunohistochemistry. Immunostaining positivity was significantly higher in patients with Brinkman index (BI) ≥400 (80/98, 82%) compared to those with BI <400 (65/102, 64%; P=0.01). Immunostaining positivity was higher in squamous cell carcinoma (26/29, 90%) compared with adenocarcinoma (107/155, 69%; P=0.03) and was higher in vessel-invasion positive (45/53, 85%) compared with vessel-invasion negative (95/140, 68%) cases (P=0.03). Differences according to gender, pathological stage, EGFR mutation status, and lymphatic invasion were not statistically significant.
Table 1.
Relationship between SPARC stromal reactivity and clinicopathological characteristics
| SPARC stromal reactivity | <10% (−) | 10%–50% (+) | >50% (++) | P-value |
|---|---|---|---|---|
| Total (N=200) | 55 (27.5%) | 112 (56%) | 33 (16.5%) | |
| Sex | 0.08 | |||
| Male (n=116) | 25 | 69 | 22 | |
| Female (n=84) | 30 | 43 | 11 | |
| BI | 0.01 | |||
| <400 (n=102) | 37 | 53 | 12 | |
| ≥400 (n=98) | 18 | 59 | 21 | |
| Histology | 0.03a | |||
| Ad (n=155) | 48 | 86 | 21 | |
| Sq (n=29) | 3 | 18 | 8 | |
| Other (n=16) | 4 | 8 | 4 | |
| p-stage | 0.12 | |||
| I (n=144) | 44 | 78 | 22 | |
| II (n=30) | 7 | 14 | 9 | |
| III (n=18) | 2 | 14 | 2 | |
| IV (n=8) | 2 | 6 | 0 | |
| EGFR mutation | 0.13 | |||
| Ex19 or ex21 (n=73) | 25 | 40 | 8 | |
| Not mutated (n=127) | 30 | 72 | 25 | |
| Vessel invasionb | 0.03 | |||
| Positive (n=53) | 8 | 32 | 13 | |
| Negative (n=140) | 45 | 76 | 19 | |
| Lymphatic invasionb | 0.23 | |||
| Positive (n=33) | 5 | 22 | 6 | |
| Negative (n=159) | 47 | 86 | 26 |
Notes:
Ad vs Sq;
Evaluable cases only.
Abbreviations: SPARC, secreted protein acidic and rich in cysteine; BI, Brinkman index; Ad, adenocarcinoma; Sq, squamous cell carcinoma.
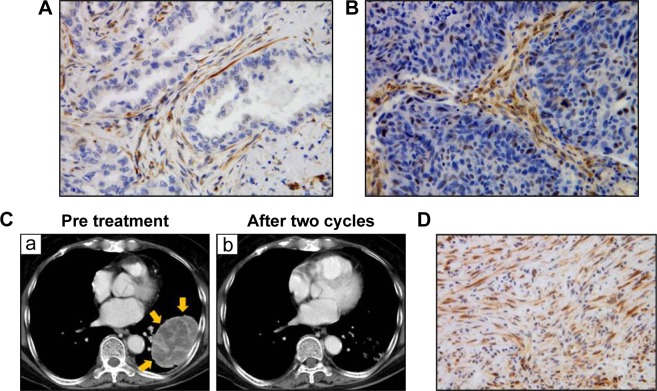
Figure 1.
Expression of SPARC in lung cancer tissues.
Notes: Immunohistochemical staining of lung adenocarcinoma tissue was performed using anti-SPARC antibody (A, ×400) and lung squamous cell carcinoma tissue (B, ×400). Counter staining was performed by hematoxylin. (C) (a) CT findings of the lung tumor in one case: a primary tumor was observed in the left lower lobe. The tumor position is indicated by the yellow arrows. After two cycles of chemotherapy, the tumor was markedly reduced (b). Immunohistochemical staining of TBLB samples from the primary lesion before treatment were performed using anti-SPARC antibody (D, ×400).
Abbreviations: SPARC, secreted protein acidic and rich in cysteine; CT, computed tomography; TBLB, transbronchial lung biopsy.
Because SPARC expression has been reported to be connected with poor prognosis in breast cancer, we investigated the prognosis associated with SPARC expression in patients with lung cancer. Table 2 shows that, based on the multivariate Cox proportional hazards model, pathological stage evidenced a significant effect on survival even with adjustment of one for the other. Stromal SPARC reactivity tended to be associated with poor survival, but the association was not statistically significant. Among the patients in stage I, those with positive staining for stromal SPARC had significantly shorter survival than negative staining (log-rank P=0.05, Figure 2).
Table 2.
Survival outcome by multivariate Cox proportional hazards analysis for NSCLC patients
| Factors | Hazard ratio (95% CI) |
P-value |
|---|---|---|
| Gender (male/female) | 2.45 (0.76–7.95) | 0.14 |
| Age | 1.03 (1.00–1.07) | 0.08 |
| Smoking index (≥400/<400) | 1.09 (0.38–3.09) | 0.88 |
| Pathological stage | 3.75 (1.89–7.42) | <0.01 |
| SPARC (positive/negative) | 1.58 (0.65–3.85) | 0.31 |
Abbreviations: CI; confidence interval; NSCLC, non-small-cell lung cancer; SPARC, secreted protein acidic and rich in cysteine.
Figure 2.
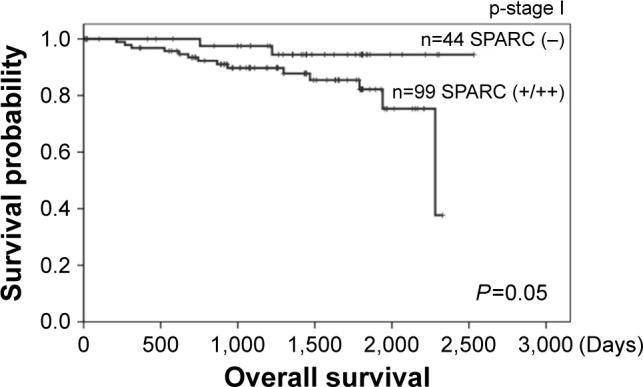
Cumulative Kaplan–Meier survival curves stratified according to SPARC immunostaining of specimens from patients in pathological stage I.
Abbreviation: SPARC, secreted protein acidic and rich in cysteine.
Stromal SPARC reactivity is a possible predictive marker for nab-paclitaxel in advanced or metastatic NSCLC patients
Table 3 shows the relationship between stromal SPARC reactivity in biopsy or surgical specimens from 43 lung cancer patients and tumor response to nab-paclitaxel in advanced or metastatic NSCLCs. Pretreatment biopsy specimens were obtained from nine transbronchial lung biopsies (TBLBs), three transbronchial biopsies (TBB), three computed tomography-guided lung biopsies, 13 endobronchial ultrasound-guided transbronchial needle aspirations (EBUS-TBNA), and 15 surgical specimens (12 primary lesions and three metastatic lesions). Tumor response evaluated according to RECIST Version 1.1 revealed that the best overall response to chemotherapy that could be achieved up to regression or recurrence was a partial response in 20 cases (47%), stable disease in 13 cases (30%), and progressive disease in 10 cases (23%; Table 4). Stromal SPARC reactivity was ++ (>50%) in 18 cases (42%), + (10%–50%) in 11 cases (25.5%), − (0%–10%) in five cases (11.5%), and not evaluable in nine cases (21%). Most nonevaluable cases had little tumor stroma in the biopsy specimens, which was often observed in EBUS-TBNA. Stromal SPARC reactivity was high (++) in almost all evaluable cases with partial response to nab-paclitaxel, intermediate (+) in seven of eleven of those with stable disease, and low (−) in three of eight of those with progressive disease, suggesting that stromal SPARC reactivity may be a predictive marker for nab-paclitaxel in NSCLC (Table 4). In particular, high stromal reactivity is a reliable marker for tumor shrinkage in response to nab-paclitaxel (P=0.002 by Fisher’s exact test). A representative case is described as follows. Figure 1 shows a case of inoperable stage IIIA squamous cell carcinoma in a 70-year-old woman with smoking index 900. After two cycles of carboplatin plus nab-paclitaxel as a first-line chemotherapy, primary tumor and lymph node metastasis were markedly reduced. Figure 1 shows that SPARC was overexpressed in tumor stroma in TBLB specimen obtained before treatment.
Table 3.
Clinicopathological characteristics of NSCLC patients who received chemotherapy
| Total (N=43) | |
|---|---|
| Age, years | 49–80 (mean 70) |
| Gender | Male (n=33) |
| Female (n=10) | |
| BI | <400 (n=10) |
| ≥400 (n=33) | |
| Histology | Ad (n=19) |
| Sq (n=18) | |
| Other (n=6) | |
| Stage | IIIA (n=5) |
| IIIB/IV (n=27) | |
| Post-operative recurrence (n=11) | |
| Specimen | TBB, TBLB (n=12) |
| CT-guided lung biopsy (n=3) | |
| EBUS-TBNA (LNs) (n=13) | |
| Surgical specimen (n=15) |
Abbreviations: NSCLC, non-small-cell lung cancer; BI, Brinkman index; Ad, adenocarcinoma; Sq, squamous cell carcinoma; TBB, transbronchial biopsy; TBLB, transbronchial lung biopsy; CT, computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; LNs, lymph nodes.
Table 4.
Relationship between stromal SPARC reactivity in biopsy or surgical specimens and tumor response to nab-paclitaxel in NSCLC
| Tumor response | Stromal SPARC reactivity
|
P-value | |||
|---|---|---|---|---|---|
| (−) | (+) | (++) | NE | ||
| PR (n=20) | 0 | 2 | 13 | 5 | 0.002* |
| SD (n=13) | 2 | 7 | 2 | 2 | 0.02** |
| PD (n=10) | 3 | 2 | 3 | 2 | |
Notes:
PR vs SD;
PR vs PD evaluable cases only.
Abbreviations: SPARC, secreted protein acidic and rich in cysteine; NSCLC, non-small-cell lung cancer; NE, not evaluated; PR, partial response; SD, stable disease; PD, progressive disease.
Discussion
We have demonstrated that stromal SPARC reactivity is high in patients with squamous cell carcinoma who are smokers and correlated with poor prognosis. Strong SPARC stroma reactivity was seen in lung cancer patients who evidenced tumor response to chemotherapy containing nab-paclitaxel. These results suggest that SPARC expression might be involved in tumor progression and response to nab-paclitaxel.
SPARC has an important role in tissue remodeling, angiogenesis, and embryonic development. In normal tissues and bones, SPARC is expressed during remodeling and repair, but not in a stationary state.13,14 However, SPARC has been reported to be overexpressed in, and correlated with poor prognosis with, various cancers, such as breast, pancreas, head and neck, bladder, and cervix uteri cancers.10–12,15–17 In breast cancer, overexpression of SPARC is associated with microcalcifications and bone metastases.18 Another study reported that SPARC was detected in 54.2% of 253 cases with resected infiltrating ductal carcinoma of the breast.19 According to our result, where stromal SPARC reactivity was correlated with vessel invasion, it is speculated to be associated with cancer invasion and metastasis. Because SPARC expression was observed in stromal tissue but not in cancer cells, we assume that SPARC may function as a tumor promoter mediated through a cancer–stromal interaction.
SPARC expression in the stroma, but not in the tumor cells, was correlated with improved survival of pancreatic cancer patients treated with nab-paclitaxel in conjunction with gemcitabine.12 Albumin acts as a drug carrier and accumulates in solid tumors due to leaky capillaries combined with an absent or defective lymphatic drainage system. SPARC has an ability to bind albumin, causing albumin accumulation in malignant tissues.20,21 This binding ability might be involved in the association between SPARC expression and nab-paclitaxel efficacy. We demonstrated a significant correlation between stromal SPARC reactivity in NSCLC and tumor response to nab-paclitaxel using biopsy or surgical specimens obtained from patients previously treated with chemotherapy. These results suggest that high stromal reactivity of SPARC has the potential to be a predictive factor for nab-paclitaxel efficacy in patients with lung cancer. Transcytosis from vessel to tumor tissue mediated through caveolae is well known to be a method of drug delivery for nab-paclitaxel.22 A high level of caveolin-1, a major component of caveolae, in tumor stroma was reported to be associated with improved response to nab-paclitaxel in conjunction with carboplatin and improved survival in NSCLC patients.23 Molecules related to drug delivery, such as SPARC and caveolin-1, may therefore be useful as predictive markers for nab-paclitaxel. There are few patients with progressive disease, so further investigation is necessary to increase the number of cases.
A limitation of this proposed assay system is the difficulty in evaluating SPARC expression in tumor stroma in advanced cases due to the small sizes of tissue specimens. Furthermore, aspiration biopsy of lymph node using EBUS-TBNA cannot be used for the evaluation because stromal tissue is not contained in lymph nodes. Recently, endobronchial ultrasonography with a guide sheath (EBUS-GS) enables us to perform biopsy of small lesions in the peripheral region.24 Using such new technology, biopsy of the primary lesion should be promoted for the evaluation of stroma. nab-Paclitaxel demonstrates a higher response rate in patients with squamous cell carcinoma, and in some cases they evidence marked tumor shrinkage as presented in Figure 1. If SPARC is available as a predictive marker in the clinical setting, we expect that nab-paclitaxel will occupy the forefront of tailor-made therapy for lung cancer, especially squamous cell carcinoma.
Conclusion
nab-Paclitaxel demonstrates a higher response rate in patients with squamous cell carcinoma, and in some cases they evidence marked tumor shrinkage as presented in Figure 1. If SPARC is available as a predictive marker in the clinical setting, we expect that nab-paclitaxel will occupy the forefront of tailor-made therapy for lung cancer, especially squamous cell carcinoma.
Acknowledgments
The authors would like to give heartfelt thanks to Dr Hironori Sadamatsu who contributed to the treatment of patients and collection of biopsy specimens, and Mr Fumihiro Mutoh for technical assistance with the experiments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 4.Guarneri V, Dieci MV, Conte P. Enhancing intracellular taxane delivery: current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert Opin Pharmacother. 2012;13(3):395–406. doi: 10.1517/14656566.2012.651127. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Wang M, Xi B, et al. SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer. PLoS One. 2012;7(8):e42413. doi: 10.1371/journal.pone.0042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JL, Chen GW, Liu YC, et al. Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One. 2012;7(9):e44618. doi: 10.1371/journal.pone.0044618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorantla B, Bhoopathi P, Chetty C, et al. Notch signaling regulates tumor-induced angiogenesis in SPARC-overexpressed neuroblastoma. Angiogenesis. 2013;16(1):85–100. doi: 10.1007/s10456-012-9301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiessling F, Fink C, Hansen M, et al. Magnetic resonance imaging of nude mice with heterotransplanted high-grade squamous cell carcinomas: use of a low-loaded, covalently bound Gd-Hsa conjugate as contrast agent with high tumor affinity. Invest Radiol. 2002;37(4):193–198. doi: 10.1097/00004424-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chuang VT, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm Res. 2002;19(5):569–577. doi: 10.1023/a:1015396825274. [DOI] [PubMed] [Google Scholar]
- 10.Jones C, Mackay A, Grigoriadis A, et al. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64(9):3037–3045. doi: 10.1158/0008-5472.can-03-2028. [DOI] [PubMed] [Google Scholar]
- 11.Nagai MA, Gerhard R, Fregnani JH, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2011;126(1):1–14. doi: 10.1007/s10549-010-0867-2. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins G, Douglas-Jones A, Bryce R, Mansel RE, Jiang WG. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72(4):267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43(8):791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 15.Chin D, Boyle GM, Williams RM, et al. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113(5):789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka M, Kanda K, Li NC, et al. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166(6):2495–2499. [PubMed] [Google Scholar]
- 17.Sova P, Feng Q, Geiss G, et al. Discovery of novel methylation biomarkers in cervical carcinoma by global demethylation and microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(1):114–123. doi: 10.1158/1055-9965.EPI-05-0323. [DOI] [PubMed] [Google Scholar]
- 18.Bellahcène A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146(1):95–100. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YW, Park YK, Lee J, Ko SW, Yang MH. Expression of osteopontin and osteonectin in breast cancer. J Korean Med Sci. 1998;13(6):652–657. doi: 10.3346/jkms.1998.13.6.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132(3):171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269(8):6072–6082. [PubMed] [Google Scholar]
- 22.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54(3):431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 23.Bertino EM, Williams TM, Nana-Sinkam SP, et al. Stromal Caveolin-1 is associated with response and survival in a phase II trial of nab-paclitaxel with carboplatin for advanced NSCLC patients. Clin Lung Cancer. 2015;16(6):466–474. doi: 10.1016/j.cllc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126(3):959–965. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]